Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review
Abstract
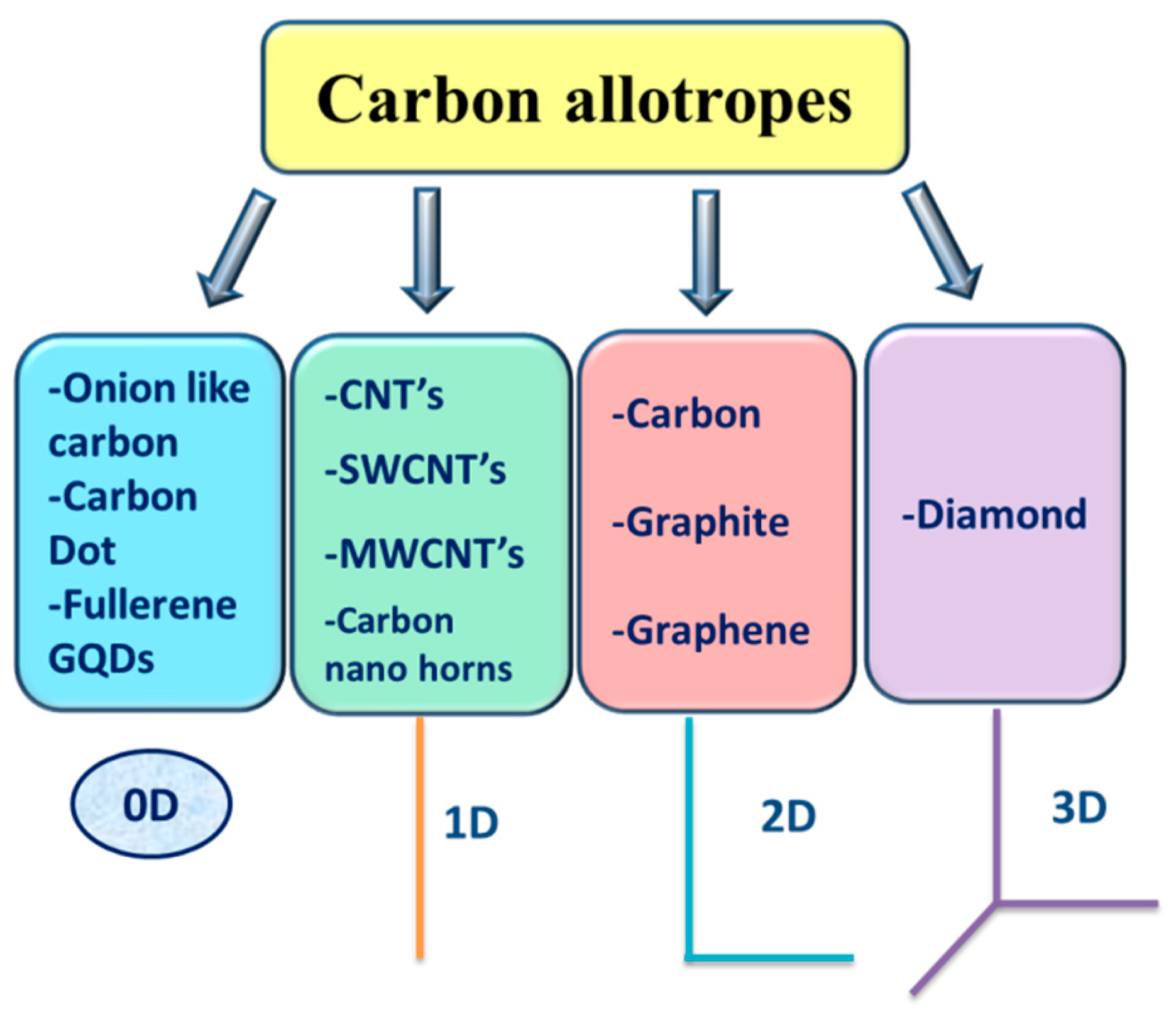
1. Introduction
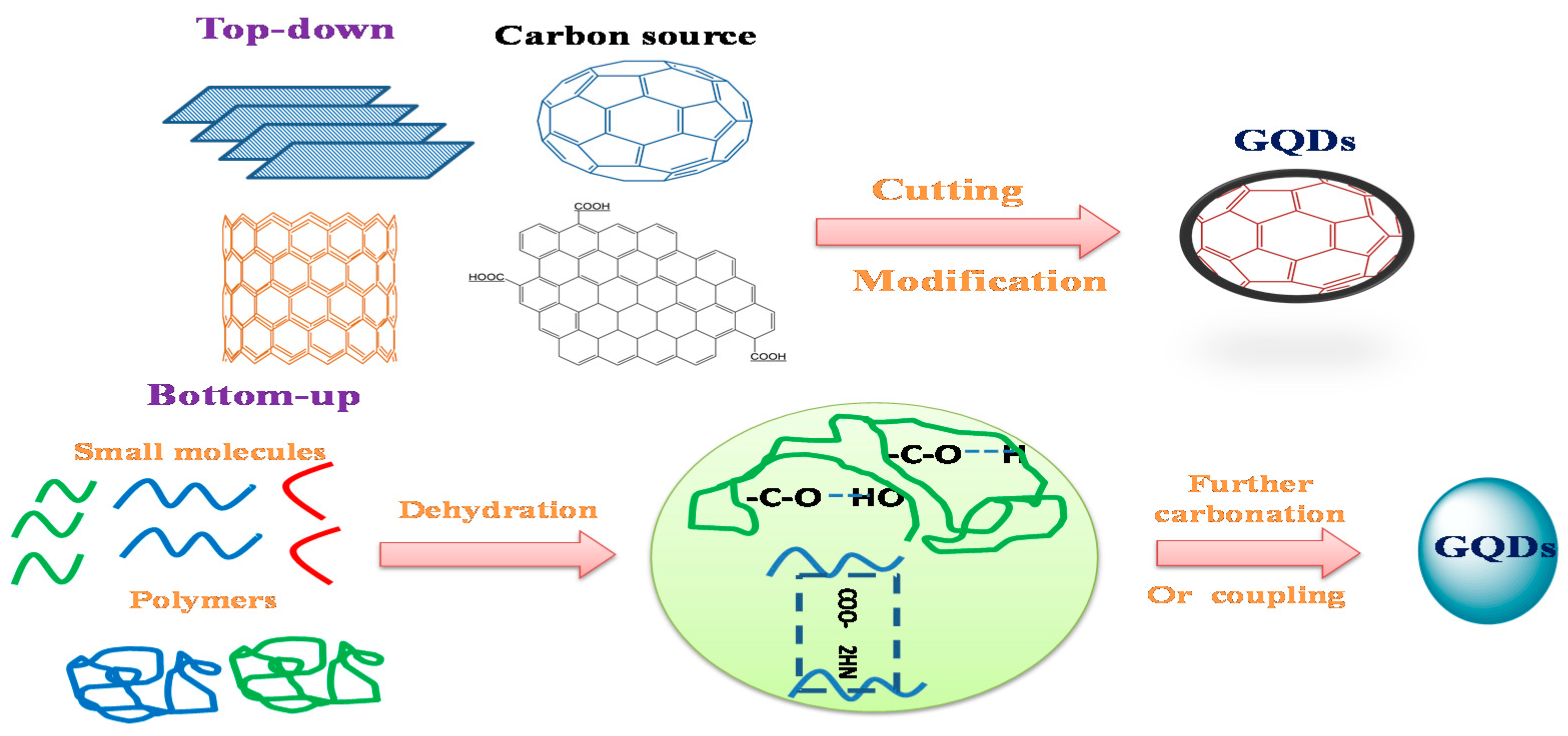
2. Synthetic Routes of GQDs
2.1. Top-Down Technique
2.1.1. Liquid Peeling Process
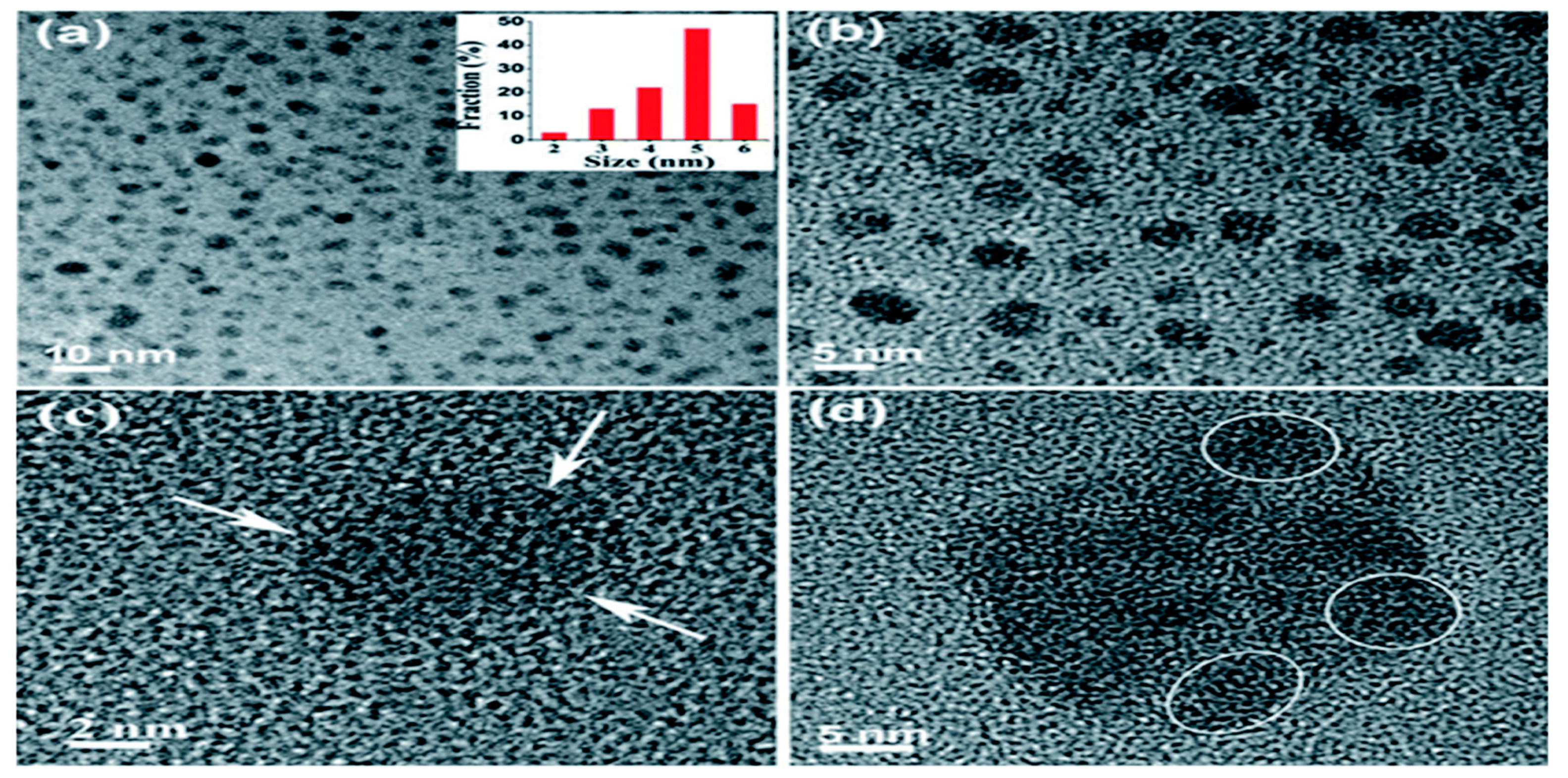
2.1.2. Hydrothermal Method
2.1.3. Electrochemical Method
2.2. Bottom-Up Methods
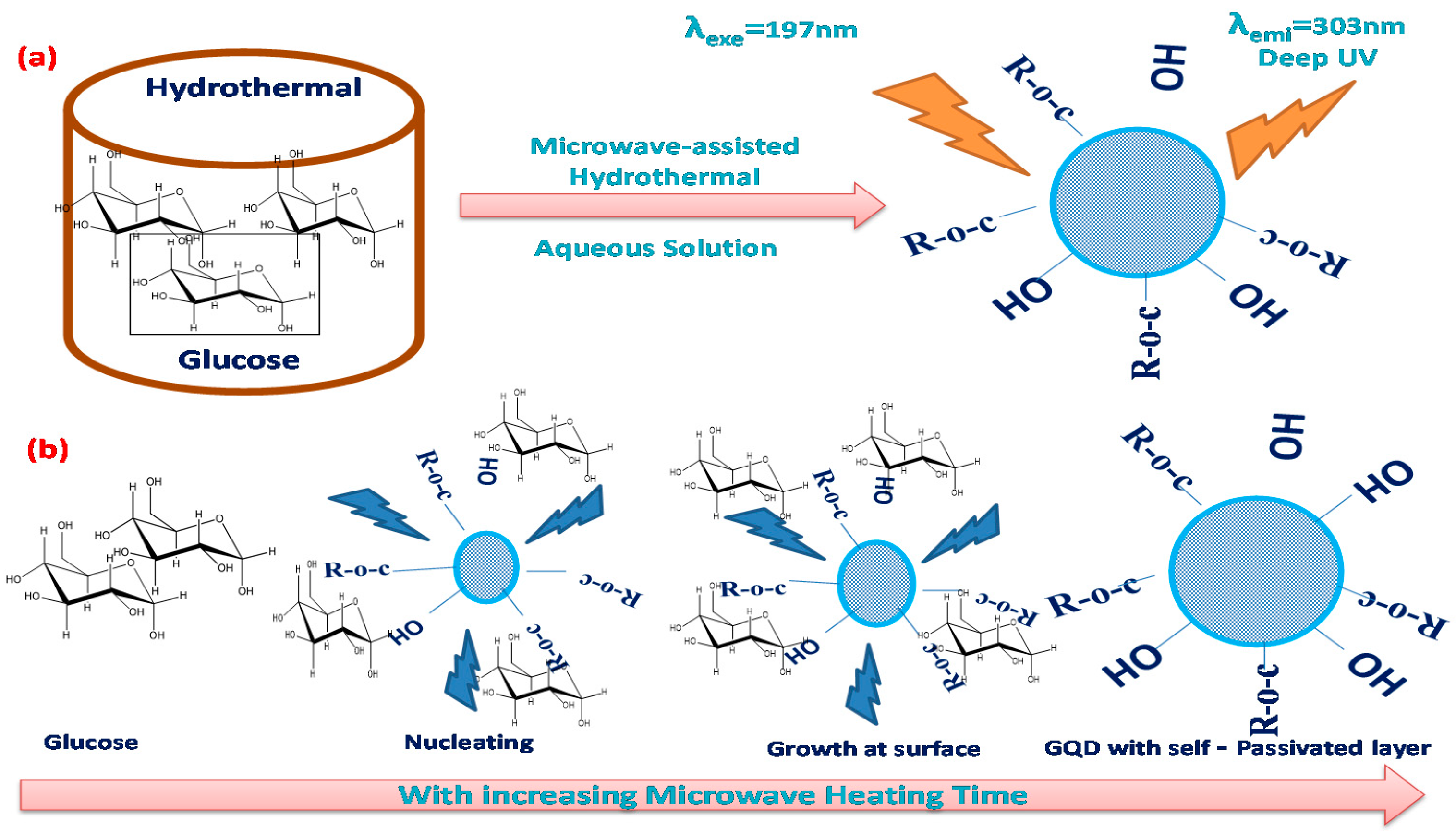
2.2.1. Hydrothermal Method Using Microwaves
2.2.2. Method of Soft Template
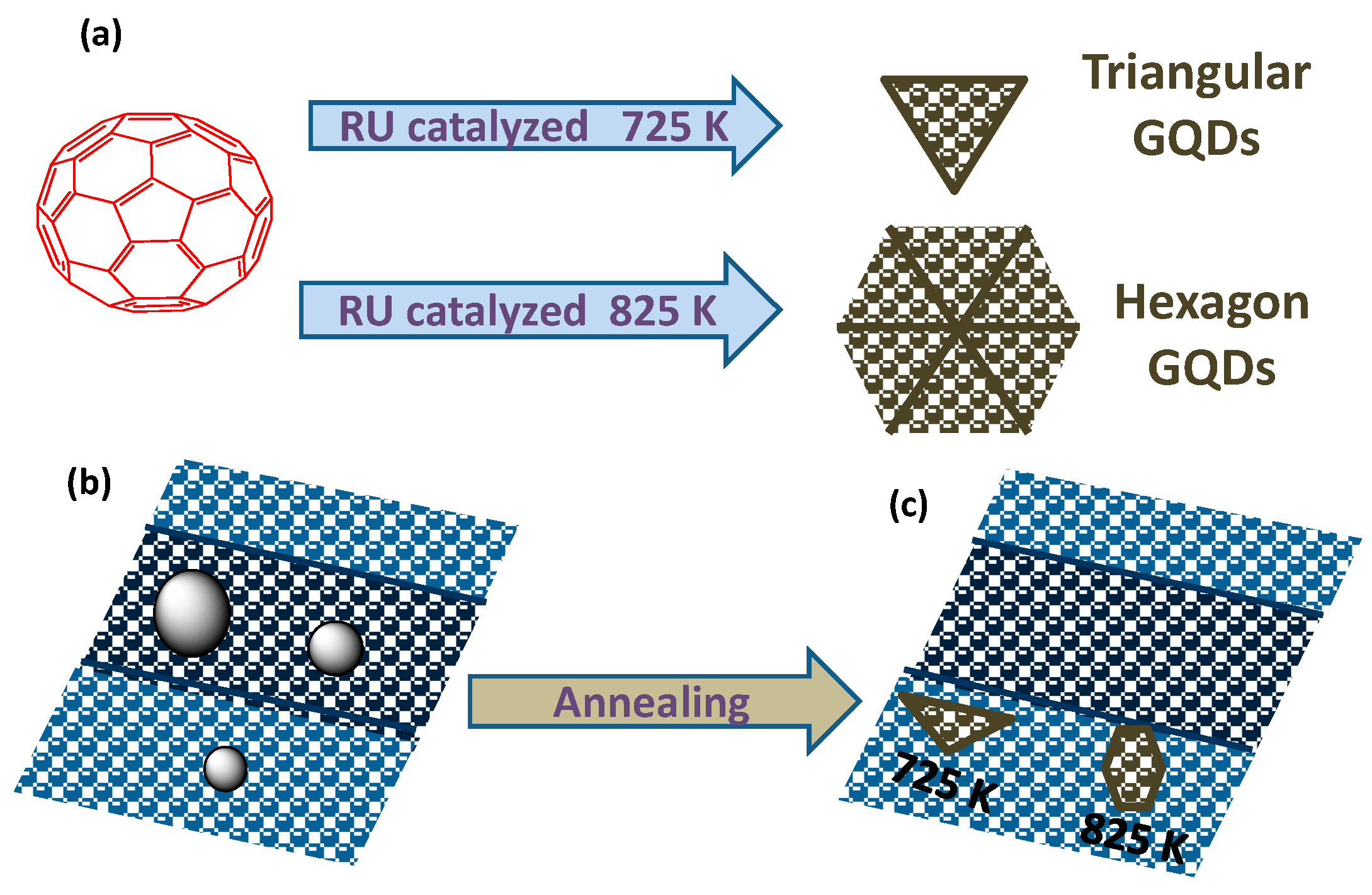
2.2.3. Metal Catalyzed Method
2.3. Green Synthesis
3. Photoluminescence (PL)
4. Application of GQDs
4.1. Sensors
4.1.1. Sensor Photoluminescence
4.1.2. Electrochemiluminescence (ECL) Based Sensor
4.1.3. Electrochemical Sensor
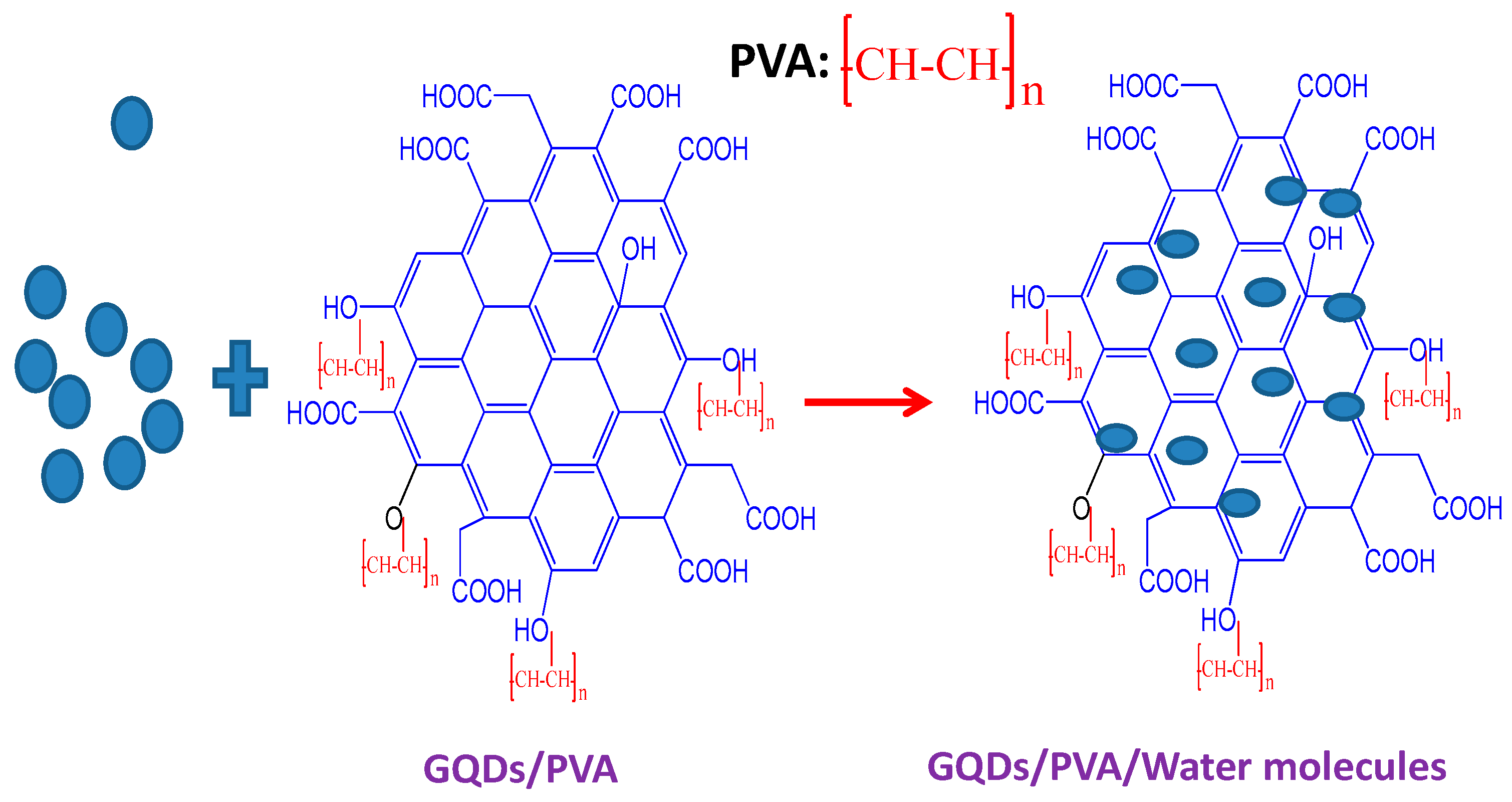
4.1.4. Humidity Sensor
4.1.5. Gas sensor Type
4.2. Biomedical Applications
4.2.1. Bio Images
4.2.2. Biosensors
4.2.3. Drug Delivery Formulating Systems
4.3. Energy Storage
4.3.1. Supercapacitor
4.3.2. Lithium-Ion Batteries
4.4. Corrosion Protection
4.4.1. Corrosion Protection by Functionalized GQDs
4.4.2. N-doping GQDs for Raising Corrosion Prevention
4.5. GQDs in Fuel Cell
5. Challenges and Potential Opportunities
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacob-Lopes, E.; Santos, A.B.; Severo, I.A.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q. Dual production of bioenergy in heterotrophic cultures of cyanobacteria: Process performance, carbon balance, biofuel quality and sustainability metrics. Biomass Bioenergy 2020, 142, 105756. [Google Scholar] [CrossRef]
- Mudusu, D.; Reddy, N.K.; Lee, S.; Hahn, Y.-B. Recent advances in graphene monolayers growth and their biological applications: A review. Adv. Colloid Interface Sci. 2020, 283, 102225. [Google Scholar] [CrossRef] [PubMed]
- Rajani, D.M.; Crasta, F.; Kottur, V.K.N. A Review on Carbon Nanotubes as Novel Drug Carriers in Cancer Therapy. In Proceedings of International Conference on Intelligent Manufacturing and Automation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 459–467. [Google Scholar]
- Chen, L.; Hou, Z.; Liu, Y.; Luan, C.; Zhu, L.; Li, W. High strength and high ductility copper matrix composite reinforced by graded distribution of carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106063. [Google Scholar] [CrossRef]
- Stieber, S.; Schröter, N.; Schiendorfer, A.; Hoffmann, A.; Reif, W. FlowFrontNet: Improving Carbon Composite Manufacturing with CNNs; University of Augsburg: Augsburg, Germany, 2020. [Google Scholar]
- Habert, G.; Röck, M.; Steininger, K.; Lupísek, A.; Birgisdottir, H.; Desing, H.; Chandrakumar, C.; Pittau, F.; Passer, A.; Rovers, R.; et al. Carbon budgets for buildings: Harmonising temporal, spatial and sectoral dimensions. Build. Cities 2020, 1, 429–452. [Google Scholar] [CrossRef]
- Guo, F.; Yang, H.; Liu, L.; Han, Y.; Al-Enizi, A.M.; Nafady, A.; Kruger, P.E.; Telfer, S.G.; Ma, S. Hollow capsules of doped carbon incorporating metal@metal sulfide and metal@metal oxide core–shell nanoparticles derived from metal–organic framework composites for efficient oxygen electrocatalysis. J. Mater. Chem. A 2019, 7, 3624–3631. [Google Scholar] [CrossRef]
- Rana, U.A.; Anis, A.; Nafady, A.; Al-Zahrani, S.M. Nitrogen and Phosphorus Co-Doped Crystalline Carbon Materials. U.S. Patent 10010866, 3 July 2018. [Google Scholar]
- Azadmanjiri, J.; Srivastava, V.K.; Kumar, P.; Sofer, Z.; Min, J.; Gong, J. Graphene-Supported 2D transition metal dichalcogenide van der waals heterostructures. Appl. Mater. Today 2020, 19, 100600. [Google Scholar] [CrossRef]
- Rajesh, J. Flexible Material in Solar Panel for Potential Efficiency; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 33, pp. 1116–1120. [Google Scholar]
- Adetayo, A.; Runsewe, D. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open J. Compos. Mater. 2019, 9, 207–229. [Google Scholar] [CrossRef]
- Tabandeh-Khorshid, M.; Kumar, A.; Omrani, E.; Kim, C.; Rohatgi, P. Synthesis, characterization, and properties of graphene reinforced metal-matrix nanocomposites. Compos. Part B Eng. 2020, 183, 107664. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review. J. Energy Chem. 2019, 30, 132–144. [Google Scholar] [CrossRef]
- Mohan, V.B. Handling and Risk Mitigation of Nanoscale Graphene and Related Materials: Some Considerations and Recommendations. C—J. Carbon Res. 2019, 5, 36. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene and its derivatives for Analytical Lab on Chip platforms. TrAC Trends Anal. Chem. 2019, 114, 326–337. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, L.; Chen, J.; Ma, F.; Yu, Y. Broad spectrum response flower spherical-like composites CQDs@ CdIn2S4/CdS modified by CQDs with up-conversion property for photocatalytic degradation and water splitting. Int. J. Hydrogen Energy 2020, 45, 1822–1836. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Huang, W.-L.; Chung, C.-J.; Chiang, I.-T.; Chen, L.-C.; Chang, H.-Y.; Su, W.-C.; Cheng, C.; Chen, S.-J.; Teng, H. Elucidating Quantum Confinement in Graphene Oxide Dots Based On Excitation-Wavelength-Independent Photoluminescence. J. Phys. Chem. Lett. 2016, 7, 2087–2092. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, P.; Barnych, B.; Zhao, C.; Sun, G.; Ge, M. Design and Synthesis of Core–Shell Carbon Polymer Dots with Highly Stable Fluorescence in Polymeric Materials. ACS Appl. Nano Mater. 2019, 2, 6503–6512. [Google Scholar] [CrossRef]
- Teaima, M.H.; ElAsaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm. J. 2020, 28, 859–868. [Google Scholar] [CrossRef]
- Shoueir, K.; Wassel, A.R.; Ahmed, M.K.; El-Naggar, M.E. Encapsulation of extremely stable polyaniline onto Bio-MOF: Photo-activated antibacterial and depletion of ciprofloxacin from aqueous solutions. J. Photochem. Photobiol. A Chem. 2020, 400, 112703. [Google Scholar] [CrossRef]
- Goetz, K.P.; Taylor, A.D.; Paulus, F.; Vaynzof, Y. Shining Light on the Photoluminescence Properties of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 1910004. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Cirtoaje, C.; Petrescu, E.; Stan, C.; Rogachev, A. Electric Freedericksz transition in nematic liquid crystals with graphene quantum dot mixture. Appl. Surf. Sci. 2019, 487, 1301–1306. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Oh, H.M.; Duong, N.T.; Bang, S.H.; Yoon, S.J.; Jeong, M.S. Highly Enhanced Photoresponsivity of a Monolayer WSe2 Photodetector with Nitrogen-Doped Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2018, 10, 10322–10329. [Google Scholar] [CrossRef]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.; You, J.; Bando, Y.; Hossain, S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Maio, A.; Scaffaro, R.; Riccobono, A.; Pibiri, I. Functionalization of Graphene with Molecules and/or Nanoparticles for Advanced Applications. In Handbook of Graphene; Archivio Istituzionale della Ricerca dell’Università degli Studi di Palermo Prodotti della Ricerca; Scrivener Publishing: Austin, TX, USA, 2019; Volume 1, pp. 559–609. [Google Scholar]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/ Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Faridbod, F.; Sanati, A.L. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Anal. Chem. 2019, 15, 103–123. [Google Scholar] [CrossRef]
- Gu, H.; Tang, H.; Xiong, P.; Zhou, Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, G.K.; Flores-Moreno, R. Quantum chemical study of Triton X-100 modified graphene surface. Electrochim. Acta 2017, 248, 225–231. [Google Scholar] [CrossRef]
- Badıllıa, U.; Mollarasouli, F.; Bakirhan, N.K.; Ozkane, Y.; Özkan, S.A. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Anal. Chem. 2020, 131, 116013. [Google Scholar] [CrossRef]
- Bokare, A.; Nordlund, D.; Melendrez, C.; Robinson, R.; Keles, O.; Wolcott, A.; Erogbogbo, F. Surface functionality and formation mechanisms of carbon and graphene quantum dots. Diam. Relat. Mater. 2020, 110, 108101. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, e1808283. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Nejad, S.S.; Babaie, A.; Bagheri, M.; Rezaei, M.; Abbasi, F.; Shomali, A. Effects of graphene quantum dot (GQD) on photoluminescence, mechanical, thermal and shape memory properties of thermoplastic polyurethane nanocomposites. Polym. Adv. Technol. 2020. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Al-Radadi, N.S.; Ahmedef, M.K.; Shoueir, K.; El-Kemary, M. Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 2020, 13, 8626–8637. [Google Scholar] [CrossRef]
- Elsayed, M.T.; Hassanbc, A.A.; Abdelaal, S.A.; Taher, M.M.; Ahmed, M.; Shoueir, K.R. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936. [Google Scholar] [CrossRef]
- Temerov, F.; Belyaev, A.; Ankudze, B.; Pakkanen, T.T.; Beliaev, A. Preparation and photoluminescence properties of graphene quantum dots by decomposition of graphene-encapsulated metal nanoparticles derived from Kraft lignin and transition metal salts. J. Lumin. 2019, 206, 403–411. [Google Scholar] [CrossRef]
- Wang, R.; Fan, H.; Jiang, W.; Ni, G.; Qu, S. Amino-functionalized graphene quantum dots prepared using high-softening point asphalt and their application in Fe3+ detection. Appl. Surf. Sci. 2019, 467, 446–455. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Sadjadi, S.; Salehi, Z. Pd immobilized on hybrid of magnetic graphene quantum dots and cyclodextrin decorated chitosan: An efficient hydrogenation catalyst. Int. J. Biol. Macromol. 2020, 150, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, A.M.; Refaat, A.; Yahia, I.; Zahran, H.Y.; Elhaes, H.; Ibrahim, M.A.; Shkir, M. Functionalization of graphene quantum dots (GQDs) with chitosan biopolymer for biophysical applications. Opt. Quantum Electron. 2019, 52, 16. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Kumar, B.V.V.R.; Banik, B.K.; Borah, P. Polyaromatic Hydrocarbons (PAHs): Structures, Synthesis and their Biological Profile. Curr. Org. Synth. 2020, 17, 625–640. [Google Scholar] [CrossRef]
- Zhou, L.; Geng, J.; Liu, B. Graphene Quantum Dots from Polycyclic Aromatic Hydrocarbon for Bioimaging and Sensing of Fe3+ and Hydrogen Peroxide. Part. Part. Syst. Charact. 2013, 30, 1086–1092. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Z.; Shi, X.; Kai, Z.; Zhang, H. Recent progress in black phosphorus and black-phosphorus-analogue materials: Properties, synthesis and applications. Nanoscale 2019, 11, 14491–14527. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin Solid Films 2019, 673, 19–25. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Wu, Z.; Du, Z.; Tang, Z.; Yang, J. Graphene Quantum Dots with High Yield and High Quality Synthesized from Low Cost Precursor of Aphanitic Graphite. Nanomaterials 2020, 10, 375. [Google Scholar] [CrossRef]
- Naumov, A.V.; Hasan, T.; Campbell, E.; Lin, C.-W.; Belcher, A.M. (Invited) In Vitro and In Vivo Near-Infrared Imaging with Biocompatible Bottom-up and Top-Down-Synthesized Graphene Quantum Dots. ECS Meet. Abstr. 2020, 648. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Wu, Y.; Wang, T.; Sun, L.; Gao, P.; Sun, Y.; Huang, H.; Zhou, G.; Hu, J. Bottom-up synthesis and structural design strategy for graphene quantum dots with tunable emission to the near infrared region. Carbon 2019, 142, 673–684. [Google Scholar] [CrossRef]
- Raeyani, D.; Shojaei, S.; Kandjani, S.A.; Wlodarski, W. Synthesizing Graphene Quantum Dots for Gas Sensing Applications. Procedia Eng. 2016, 168, 1312–1316. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- León, I.N.-D.; Johny, J.; Vázquez-Rodríguez, S.; García-Gómez, N.; Carranza-Bernal, S.; Mendivil, I.; Shaji, S.; Sepulveda-Guzman, S. Tuning the luminescence of nitrogen-doped graphene quantum dots synthesized by pulsed laser ablation in liquid and their use as a selective photoluminescence on–off–on probe for ascorbic acid detection. Carbon 2019, 150, 455–464. [Google Scholar] [CrossRef]
- Park, J.; Bazylewski, P.; Wong, V.; Shah, H.; Fanchini, G. Solvent-free growth of carbon dots by sputter-plasma assisted chemical vapour deposition over large areas. Carbon 2019, 146, 28–35. [Google Scholar] [CrossRef]
- Kalita, H.; Palaparthy, V.S.; Baghini, M.S.; Aslam, M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon 2020, 165, 9–17. [Google Scholar] [CrossRef]
- Chen, J.; Long, Z.; Wang, S.; Meng, Y.; Zhang, G.; Nie, S. Biodegradable blends of graphene quantum dots and thermoplastic starch with solid-state photoluminescent and conductive properties. Int. J. Biol. Macromol. 2019, 139, 367–376. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A.M. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C 2021, 120, 111756. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Sangam, S.; Gupta, A.; Shakeel, A.; Bhattacharya, R.; Sharma, A.K.; Suhag, D.; Chakrabarti, S.; Garg, S.K.; Chattopadhyay, S.; Basu, B.; et al. Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green Chem. 2018, 20, 4245–4259. [Google Scholar] [CrossRef]
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D.; Joseph, K. Simple and Cost-Effective Synthesis of Fluorescent Graphene Quantum Dots from Honey: Application as Stable Security Ink and White-Light Emission. Part. Part. Syst. Charact. 2015, 33, 70–74. [Google Scholar] [CrossRef]
- Kalita, H.; Mohapatra, J.; Pradhan, L.; Mitra, A.; Bahadur, D.; Aslam, M. Efficient synthesis of rice based graphene quantum dots and their fluorescent properties. RSC Adv. 2016, 6, 23518–23524. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Sun, J.; Huang, H.; Su, X.; Wang, Z. Sodium hexametaphosphate modulated fluorescence responsive biosensor based on self-assembly/disassembly mode of reduced-graphene quantum dots / chitosan system for alkaline phosphatase. Talanta 2020, 207, 120341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xia, G.; Du, M.; Lu, Y.; Xu, H. Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl. Surf. Sci. 2019, 483, 52–59. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Z.; Yi, M. Liquid-exfoliated graphene as highly efficient conductive additives for cathodes in lithium ion batteries. Carbon 2019, 153, 156–163. [Google Scholar] [CrossRef]
- Kadian, S.; Sethi, S.K.; Manik, G. Recent advancements in synthesis and property control of graphene quantum dots for biomedical and optoelectronic applications. Mater. Chem. Front. 2020. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Qin, J.; Li, S.; Yuan, N.; Chen, Y.; Lei, B.; Hu, C.; Liu, Y. Rapid hydrothermal method for the preparation of carbon quantum dots in the presence of hydrogen peroxide. Micro Nano Lett. 2020, 15, 465–468. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Joffrion, J.B.; Clower, W.; Wilson, C.G. Tunable excitation-independent emissions from graphene quantum dots through microplasma-assisted electrochemical synthesis. Nano-Struct. Nano-Objects 2019, 19, 100341. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Zdrazil, L.; Zahradnicek, R.; Mohan, R.; Sedlacek, P.; Nejdl, L.; Schmiedova, V.; Pospisil, J.; Horák, M.; Weiter, M.; Zmeskal, O.; et al. Preparation of graphene quantum dots through liquid phase exfoliation method. J. Lumin. 2018, 204, 203–208. [Google Scholar] [CrossRef]
- Hoang, T.T.; Phuong, P.H.; Tran, Q.T. A Facile Microwave-Assisted Hydrothermal Synthesis of Graphene Quantum Dots for Organic Solar Cell Efficiency Improvement. J. Nanomater. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Valappil, M.O.; Pillai, V.K.; Alwarappan, S. Spotlighting graphene quantum dots and beyond: Synthesis, properties and sensing applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Gervasoni, J.; Primiano, A.; Rinaldi, M.; Fioretti, D.; Prampolini, C.; Tiberio, F.; et al. Graphene Quantum Dots’ Surface Chemistry Modulates the Sensitivity of Glioblastoma Cells to Chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6301. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.; Zhao, J.; Jiang, P.; Jiang, F.-L.; Li, X. Bridge between Temperature and Light: Bottom-Up Synthetic Route to Structure-Defined Graphene Quantum Dots as a Temperature Probe In Vitro and in Cells. ACS Appl. Mater. Interfaces 2020, 12, 22002–22011. [Google Scholar] [CrossRef]
- Jin, Q.-Q.; Zhang, C.-Y.; Wang, W.-N.; Chen, B.-J.; Ruan, J.; Qian, H.-S. Recent Development on Controlled Synthesis of Metal Sulfides Hollow Nanostructures via Hard Template Engaged Strategy: A Mini-review. Chem. Rec. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Prabhu, S.A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene quantum dots synthesis and energy application: A review. Carbon Lett. 2020, 1–12. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Bottom-Up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright Green Synthesis of Fluorescent Carbon Dots from Eutrophic Algal Blooms for In Vitro Imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443. [Google Scholar]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Shahid, S.A.; Nafady, A.; Ullah, I.; Taufiq-Yap, Y.H.; Shakir, I.; Anwar, F.; Rashid, U. Characterization of Newly Synthesized ZrFe 2 O 5 Nanomaterial and Investigations of Its Tremendous Photocatalytic Properties under Visible Light Irradiation. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Shoueir, K.R. Green microwave synthesis of functionalized chitosan with robust adsorption capacities for Cr(VI) and/or RHB in complex aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 33020–33031. [Google Scholar] [CrossRef]
- Wassel, A.R.; El-Naggar, M.E.; Shoueir, K.R. Recent advances in polymer/metal/metal oxide hybrid nanostructures for catalytic applications: A review. J. Environ. Chem. Eng. 2020, 8, 104175. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Shouir, K.R.; El-Kemary, M. Activated H2O2 on Ag/SiO2–SrWO4 surface for enhanced dark and visible-light removal of methylene blue and p-nitrophenol. J. Alloys Compd. 2020, 155848. [Google Scholar] [CrossRef]
- El-Shabasy, R.; Yosri, N.; El-Seedi, H.; Shoueir, K.; El-Kemary, M. A green synthetic approach using chili plant supported Ag/Ag2O@P25 heterostructure with enhanced photocatalytic properties under solar irradiation. Optik 2019, 192, 162943. [Google Scholar] [CrossRef]
- Shoueir, K.; Kandil, S.; El-hosainy, H.; El-Kemary, M. Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J. Clean. Prod. 2019, 230, 383–393. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; El-Hosainy, H.M.; Shoueir, K.R.; El-Mehasseb, I.M.; El-Kemary, M. Facile immobilization of Ag nanoparticles on g-C3N4/V2O5 surface for enhancement of post-illumination, catalytic, and photocatalytic activity removal of organic and inorganic pollutants. Appl. Surf. Sci. 2019, 467, 268–276. [Google Scholar] [CrossRef]
- Salama, A.; Aljohani, H.A.; Shoueir, K.R. Oxidized cellulose reinforced silica gel: New hybrid for dye adsorption. Mater. Lett. 2018, 230, 293–296. [Google Scholar] [CrossRef]
- Shoueir, K.; El-Sheshtawy, H.; Misbah, M.; El-Hosainy, H.; El-Mehasseb, I.; El-Kemary, M. Fenton-like nanocatalyst for photodegradation of methylene blue under visible light activated by hybrid green DNSA@Chitosan@MnFe2O4. Carbohydr. Polym. 2018, 197, 17–28. [Google Scholar] [CrossRef]
- Abdelbar, M.F.; El-Sheshtawy, H.S.; Shoueir, K.; El-Mehasseb, I.; Ebeid, E.-Z.M.; El-Kemary, M. Halogen bond triggered aggregation induced emission in an iodinated cyanine dye for ultra sensitive detection of Ag nanoparticles in tap water and agricultural wastewater. RSC Adv. 2018, 8, 24617–24626. [Google Scholar] [CrossRef]
- Shoueir, K.R.; Atta, A.M.; Sarhan, A.A.; Akl, M.A. Synthesis of monodisperse core shell PVA@P(AMPS-co-NIPAm) nanogels structured for pre-concentration of Fe(III) ions. Environ. Technol. 2016, 38, 967–978. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Al-Muhtaseb, A.H.; Khan, A.A.; Khan, A. Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Lee, H.; Anwer, H.; Park, J. Graphene quantum dots on stainless-steel nanotubes for enhanced photocatalytic degradation of phenanthrene under visible light. Chemosphere 2020, 246, 125761. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Anooj, E.; Praseetha, P. Synthesis and characterization of graphene quantum dots from nutmeg seeds and its biomedical application. Int. J. Recent Technol. Eng. 2019, 7, 144–151. [Google Scholar]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang-, L.; et al. Large-Scale and Controllable Synthesis of Graphene Quantum Dots from Rice Husk Biomass: A Comprehensive Utilization Strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, J.; Lv, G.; Li, D.; Hu, Y.; Zhou, C.; Liu, X.; Dai, Z. Green Synthesis of Graphene Quantum Dots from Cotton Cellulose. ChemistrySelect 2019, 4, 2898–2902. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Encapsulation of graphene quantum dot-crosslinked chitosan by carboxymethylcellulose hydrogel beads as a pH-responsive bio-nanocomposite for the oral delivery agent. Int. J. Biol. Macromol. 2019, 123, 389–397. [Google Scholar] [CrossRef]
- Lai, S.; Jin, Y.; Shi, L.; Zhou, R.; Zhou, Y.; An, D. Mechanisms behind excitation- and concentration-dependent multicolor photoluminescence in graphene quantum dots. Nanoscale 2020, 12, 591–601. [Google Scholar] [CrossRef]
- Feng, J.; Guo, Q.; Liu, H.; Chen, D.; Tian, Z.; Xia, F.; Ma, S.; Yu, L.; Dongab, L. Theoretical insights into tunable optical and electronic properties of graphene quantum dots through phosphorization. Carbon 2019, 155, 491–498. [Google Scholar] [CrossRef]
- Rajender, G.; Goswami, U.; Giri, P.K. Solvent dependent synthesis of edge-controlled graphene quantum dots with high photoluminescence quantum yield and their application in confocal imaging of cancer cells. J. Colloid Interface Sci. 2019, 541, 387–398. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, H.; Sun, L. Graphene quantum dots: Versatile photoluminescence for energy, biomedical, and environmental applications. J. Mater. Chem. C 2015, 3, 1157–1165. [Google Scholar] [CrossRef]
- Deng, X.; Sun, J.; Yang, S.; Shen, H.; Zhou, W.; Lu, J.; Ding, G.; Wang, Z. The emission wavelength dependent photoluminescence lifetime of the N-doped graphene quantum dots. Appl. Phys. Lett. 2015, 107, 241905. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, W.; Wu, T.; Wen, J.; Zhang, Y.; Wang, L.; He, Y.; Chu, H.; Hu, M. Coumarin-Modified Graphene Quantum Dots as a Sensing Platform for Multicomponent Detection and Its Applications in Fruits and Living Cells. ACS Omega 2020, 5, 7369–7378. [Google Scholar] [CrossRef]
- Emamian, R.; Ebrahimi, M.; Karimi-Maleh, H.J.J.o.N. A Sensitive Sensor for Nano-Molar Detection of 5-Fluorouracil by Modifying a Paste Sensor with Graphene Quantum Dots and an Ionic Liquid. J. Nanostruct. 2020, 10, 230–238. [Google Scholar]
- Chen, S.; Ullah, N.; Wang, T.; Dou, K.P. Tuning the optical properties of graphene quantum dots by selective oxidation: A theoretical perspective. J. Mater. Chem. C 2018, 6, 6875–6883. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Rashid, S.A.; Jamaludin, N.; Noor, A.S.M.; Isloor, A.M. Surface plasmon resonance sensor using polypyrrole-chitosan/graphene quantum dots layer for detection of sugar. Mater. Res. Express 2019, 6, 075028. [Google Scholar] [CrossRef]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene quantum dots prepared from glucose as optical sensor for glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Hong, G.-L.; Deng, H.-H.; Zhao, H.-L.; Zou, Z.-Y.; Huang, K.-Y.; Peng, H.-P.; Liu, Y.-H.; Chen, W. Gold nanoclusters/graphene quantum dots complex-based dual-emitting ratiometric fluorescence probe for the determination of glucose. J. Pharm. Biomed. Anal. 2020, 189, 113480. [Google Scholar] [CrossRef]
- Mirzaie, A.; Hasanzadeh, M.; Jouyban, A. Cross-linked chitosan/thiolated graphene quantum dots as a biocompatible polysaccharide towards aptamer immobilization. Int. J. Biol. Macromol. 2019, 123, 1091–1105. [Google Scholar] [CrossRef]
- Pandey, A.; Raja, A.N. Recent development in chitosan-based electrochemical sensors and its sensing application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar]
- Raj, S.K.; Yadav, V.; Bhadu, G.R.; Patidar, R.; Kumar, M.; Kulshrestha, V. Synthesis of highly fluorescent and water soluble graphene quantum dots for detection of heavy metal ions in aqueous media. Environ. Sci. Pollut. Res. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boonta, W.; Talodthaisong, C.; Sattayaporn, S.; Chaicham, C.; Chaicham, A.; Sahasithiwat, S.; Kangkaew, L.; Kulchat, S. The synthesis of nitrogen and sulfur co-doped graphene quantum dots for fluorescence detection of cobalt (ii) ions in water. Mater. Chem. Front. 2020, 4, 507–516. [Google Scholar] [CrossRef]
- Ge, Q.; Kong, W.-H.; Liu, X.-Q.; Wang, Y.-M.; Wang, L.-F.; Ma, N.; Li, Y. Hydroxylated graphene quantum dots as fluorescent probes for sensitive detection of metal ions. Int. J. Miner. Met. Mater. 2020, 27, 91–99. [Google Scholar] [CrossRef]
- Prasad, S. Novel synthesis of graphene quantum dots using L-aspartic acid. In Quantum Dots, Nanostructures, and Quantum Materials: Growth, Characterization, and Modeling XVII; P SPIE OPTO: San Francisco, CA, USA, 2020. [Google Scholar]
- Xie, N.; Tan, L.; Li, H.-F.; Hu, H.-Y.; Wang, C.; Pan, M.; Wu, F.; Wu, P.; Wang, X.-D.; Zeng, Z.; et al. Manipulation of 3D nanocarbon hybrids toward synthesis of N-doped graphene quantum dots with high photoluminescence quantum yield. J. Lumin. 2020, 219, 116827. [Google Scholar] [CrossRef]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Liu, H.; Na, W.; Liu, Z.; Chen, X.; Su, X. A novel turn-on fluorescent strategy for sensing ascorbic acid using graphene quantum dots as fluorescent probe. Biosens. Bioelectron. 2017, 92, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Dinda, D.; Saha, S.K. Nitrogen, sulphur co-doped graphene quantum dot: An excellent sensor for nitroexplosives. Sens. Actuators B Chem. 2018, 257, 586–593. [Google Scholar] [CrossRef]
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Xu, T.; Yang, J.-X.; Song, J.-M.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y. Synthesis of high fluorescence graphene quantum dots and their selective detection for Fe3+ in aqueous solution. Sens. Actuators B Chem. 2017, 243, 863–872. [Google Scholar] [CrossRef]
- Zhang, R.; Adsetts, J.R.; Nie, Y.; Sun, X.; Ding, Z. Electrochemiluminescence of nitrogen- and sulfur-doped graphene quantum dots. Carbon 2018, 129, 45–53. [Google Scholar] [CrossRef]
- Chen, X.-M.; Su, B.-Y.; Song, X.-H.; Chen, Q.-A.; Chen, X.; Wang, X.-R. Recent advances in electrochemiluminescent enzyme biosensors. TrAC Trends Anal. Chem. 2011, 30, 665–676. [Google Scholar] [CrossRef]
- Han, A.; Yang, Y.; Zhang, Q.; Tu, Q.; Fang, G.; Liu, J.; Wang, S.; Li, R. Electrochemistry and electrochemiluminescence of copper metal cluster. J. Electroanal. Chem. 2017, 795, 116–122. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Wu, H.; Chen, C.; Chi, Y.; Chen, G. Electrochemiluminescence sensor for hexavalent chromium based on the graphene quantum dots/peroxodisulfate system. Electrochim. Acta 2015, 151, 552–557. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Xia, T.; Ma, Q. A novel electrochemiluminescence sensor for the detection of nitroaniline based on the nitrogen-doped graphene quantum dots. Biosens. Bioelectron. 2016, 85, 903–908. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Sai-Dan, X.; Yang, L.; Zhao-Yang, W.; Guo-Li, S.; Ru-Qin, Y. Application of inorganic layered materials in electrochemical sensors. Chin. J. Anal. Chem. 2015, 43, 1648–1655. [Google Scholar]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, L.; Wen, W.; Zhang, X.; Wang, S. Enzyme catalytic amplification of miRNA-155 detection with graphene quantum dot-based electrochemical biosensor. Biosens. Bioelectron. 2016, 77, 451–456. [Google Scholar] [CrossRef]
- Sikarwar, S.; Yadav, B.; Sonker, R.K.; Dzhardimalieva, G.I.; Rajput, J.K. Synthesis and characterization of highly porous hexagonal shaped CeO2-Gd2O3-CoO nanocomposite and its opto-electronic humidity sensing. Appl. Surf. Sci. 2019, 479, 326–333. [Google Scholar] [CrossRef]
- Jawaher, K.R.; Indirajith, R.; Krishnan, S.; Robert, R.; Pasha, S.K.; Deshmukh, K.; Sastikumar, D.; Das, S.J. A High Sensitivity Isopropanol Vapor Sensor Based on Cr2O3–SnO2 Heterojunction Nanocomposites via Chemical Precipitation Route. J. Nanosci. Nanotechnol. 2018, 18, 5454–5460. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Genovese, L.; Giubileo, F.; Iemmo, L.; Luongo, G.; Foller, T.; Schleberger, M. Hysteresis in the transfer characteristics of MoS2 transistors. 2D Mater. 2017, 5, 015014. [Google Scholar] [CrossRef]
- Urban, F.; Martucciello, N.; Peters, L.; McEvoy, N.; Di Bartolomeo, A. Environmental Effects on the Electrical Characteristics of Back-Gated WSe2 Field-Effect Transistors. Nanomaterials 2018, 8, 901. [Google Scholar] [CrossRef]
- Pasha, S.K.; Chidambaram, K.; Kennedy, L.J.; Vijaya, J.J. Lead Oxide-PbO Humidity Sensor. Sens. Transducers 2010, 122, 113. [Google Scholar]
- Long, L.M.; Dinh, N.N.; Trung, T.Q. Synthesis and Characterization of Polymeric Graphene Quantum Dots Based Nanocomposites for Humidity Sensing. J. Nanomater. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Zad, A.I.; Ghiass, M.A.; Fardindoost, S.; Hatamie, S. A new approach to flexible humidity sensors using graphene quantum dots. J. Mater. Chem. C 2017, 5, 8966–8973. [Google Scholar] [CrossRef]
- Liang, R.; Luo, A.; Zhang, Z.; Li, Z.; Han, C.; Wu, W. Research Progress of Graphene-Based Flexible Humidity Sensor. Sensors 2020, 20, 5601. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Tong, R.-J.; Chen, M.-Q.; Xia, F. Relative humidity sensor based on hollow core fiber filled with GQDs-PVA. Sens. Actuators B Chem. 2019, 284, 96–102. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Q.; Zhou, M.; Liu, F.; Shen, H.; Wei, Z.; Wang, F.; Tan, C.; Meng, H. Humidity sensor based on a graphene oxide-coated few-mode fiber Mach-Zehnder interferometer. Opt. Express 2020, 28, 24682. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.-X.; Zhang, W.-G.; Kong, L.-X.; Yue, Y.; Yan, T.-Y. Ultra-Compact Optical Thermo-Hygrometer Based on Bilayer Micro-cap on Fiber Facet. IEEE Photon. Technol. Lett. 2020, 32, 1089–1092. [Google Scholar] [CrossRef]
- Alizadeh, T.; Shokri, M. A new humidity sensor based upon graphene quantum dots prepared via carbonization of citric acid. Sens. Actuators B Chem. 2016, 222, 728–734. [Google Scholar] [CrossRef]
- Yang, J.; Guan, C.; Yu, Z.; Yang, M.; Shi, J.; Wang, P.; Yang, J.; Yuan, L.J.S.; Chemical, A.B. High sensitivity humidity sensor based on gelatin coated side-polished in-fiber directional coupler. Sens. Actuators B Chem. 2020, 305, 127555. [Google Scholar] [CrossRef]
- Stackhouse, C.; Ren, J.; Shan, C.; Nafady, A.; Al-Enizi, A.M.; Ubaidullah, M.; Niu, Z.; Ma, S. Microporous Cyclen-Based Octacarboxylate Hydrogen-Bonded Organic Framework Exhibiting Selective Gas Adsorption. Cryst. Growth Des. 2019, 19, 6377–6380. [Google Scholar] [CrossRef]
- Urban, F.; Giubileo, F.; Grillo, A.; Iemmo, L.; Luongo, G.; Passacantando, M.; Foller, T.; Madauß, L.; Pollmann, E.; Geller, M.P.; et al. Gas dependent hysteresis in MoS2 field effect transistors. 2D Mater. 2019, 6, 045049. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Pelella, A.; Grillo, A.; Urban, F.; Giubileo, F. Air Pressure, Gas Exposure and Electron Beam Irradiation of 2D Transition Metal Dichalcogenides. Appl. Sci. 2020, 10, 5840. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Ooi, P.C.; Ye, Y.; Kim, T.W.; Guo, T. Room temperature pH-dependent ammonia gas sensors using graphene quantum dots. Sens. Actuators B Chem. 2016, 222, 763–768. [Google Scholar] [CrossRef]
- Arunragsa, S.; Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Hydroxyl edge-functionalized graphene quantum dots for gas-sensing applications. Diam. Relat. Mater. 2020, 105, 107790. [Google Scholar] [CrossRef]
- Raeyani, D.; Shojaei, S.; Kandjani, S.A. Optical graphene quantum dots gas sensors: Experimental study. Mater. Res. Express 2020, 7, 015608. [Google Scholar] [CrossRef]
- Kortel, M.; Mansuriya, B.D.; Santana, N.V.; Altintas, Z. Graphene Quantum Dots as Flourishing Nanomaterials for Bio-Imaging, Therapy Development, and Micro-Supercapacitors. Micromachines 2020, 11, 866. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Liu, W. Motif-Designed Peptide Nanofibers Decorated with Graphene Quantum Dots for Simultaneous Targeting and Imaging of Tumor Cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Aboelsaad, A.M.; Shoueir, K.R.; Abdulmalek, S.A.; Awad, D.; Shaban, S.Y.; Mansour, H. Chitosan-based dithiophenolato nanoparticles: Preparation, mechanistic information of DNA binding, antibacterial and cytotoxic activities. J. Mol. Liq. 2020, 318, 114252. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Xu, M.; Wang, J.; Hu, X.; Anwar, S.; Tedesco, A.C.; Morais, P.C.; Bi, H. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mater. Chem. B 2020, 8, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Xu, A.; He, P.; Huang, T.; Li, J.; Hu, X.; Xiang, P.; Chen, D.; Yang, S.; Wang, G.; Ding, G. Selective supramolecular interaction of ethylenediamine functionalized graphene quantum dots: Ultra-sensitive photoluminescence detection for nickel ion in vitro. Synth. Met. 2018, 244, 106–112. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Lan, C.; Zhao, S. Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sens. Actuators B Chem. 2016, 223, 246–251. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd Nanoparticles Decorated N-Doped Graphene Quantum Dots@N-Doped Carbon Hollow Nanospheres with High Electrochemical Sensing Performance in Cancer Detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef]
- Salama, A.; Diab, M.A.; Abou-Zeid, R.; Aljohani, H.A.; Shoueir, K.R. Crosslinked alginate/silica/zinc oxide nanocomposite: A sustainable material with antibacterial properties. Compos. Commun. 2018, 7, 7–11. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Yehia, S.A.; Shoueir, K.R.; Saleh, S.R.; Awad, D.; Shaban, S.Y. Design, DNA binding and kinetic studies, antibacterial and cytotoxic activities of stable dithiophenolato titanium(IV)-chitosan Nanocomposite. J. Mol. Liq. 2019, 287, 111002. [Google Scholar] [CrossRef]
- Abdelbar, M.F.; Shams, R.S.; Morsy, O.M.; Hady, M.A.; Shoueir, K.; Abdelmoneme, R. Highly ordered functionalized mesoporous silicate nanoparticles reinforced poly (lactic acid) gatekeeper surface for infection treatment. Int. J. Biol. Macromol. 2020, 156, 858–868. [Google Scholar] [CrossRef] [PubMed]
- El-Bindary, A.A.; Toson, E.A.; Shoueir, K.R.; Aljohani, H.A.; Abo-Ser, M.M. Metal–organic frameworks as efficient materials for drug delivery: Synthesis, characterization, antioxidant, anticancer, antibacterial and molecular docking investigation. Appl. Organomet. Chem. 2020, 34, 5905. [Google Scholar] [CrossRef]
- Chen, L.; Alrbyawi, H.; Poudel, I.; Arnold, R.D.; Babu, R.J. Co-delivery of Doxorubicin and Ceramide in a Liposomal Formulation Enhances Cytotoxicity in Murine B16BL6 Melanoma Cell Lines. AAPS PharmSciTech 2019, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Ozkan, S.A.; Jouyban, A. Targeting and sensing of some cancer cells using folate bioreceptor functionalized nitrogen-doped graphene quantum dots. Int. J. Biol. Macromol. 2018, 118, 1021–1034. [Google Scholar] [CrossRef]
- Kadian, S.; Manik, G.; Das, N.; Roy, P. Targeted bioimaging and sensing of folate receptor-positive cancer cells using folic acid-conjugated sulfur-doped graphene quantum dots. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.-R. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int. J. Nanomed. 2015, 10, 6709–6724. [Google Scholar] [CrossRef]
- Al Nahain, A.; Lee, J.E.; In, I.; Lee, H.; Lee, K.D.; Jeong, J.H.; Park, S.Y. Target Delivery and Cell Imaging Using Hyaluronic Acid-Functionalized Graphene Quantum Dots. Mol. Pharm. 2013, 10, 3736–3744. [Google Scholar] [CrossRef]
- Nigam, P.; Waghmode, S.; Louis, M.; Wangnoo, S.; Chavan, P.; Sarkar, D. Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J. Mater. Chem. B 2014, 2, 3190–3195. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, T.; Gonzalez-Rodriguez, R.; Green, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Quantum Dot Formulation for Cancer Imaging and Redox-Based Drug Delivery. ECS Meet. Abstr. 2020, 662. [Google Scholar] [CrossRef]
- Qian, C.; Yan, P.; Wan, G.; Liang, S.; Dong, Y.; Wang, J. Facile synthetic Photoluminescent Graphene Quantum dots encapsulated β-cyclodextrin drug carrier system for the management of macular degeneration: Detailed analytical and biological investigations. J. Photochem. Photobiol. B Biol. 2018, 189, 244–249. [Google Scholar] [CrossRef]
- Shakir, I.; Almutairi, Z.A.; Shar, S.S.; Nafady, A. Nickel hydroxide nanoparticles and their hybrids with carbon nanotubes for electrochemical energy storage applications. Results Phys. 2020, 17, 103117. [Google Scholar] [CrossRef]
- Chen, C.; Qin, H.; Cong, H.-P.; Yu, S.-H. A Highly Stretchable and Real-Time Healable Supercapacitor. Adv. Mater. 2019, 31, e1900573. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Zadeh, M.H.R.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Kaur, G.; Pulagara, N.V.; Lahiri, I. Three-Dimensional Graphene Materials for Supercapacitors. Graphene Energy Storage Mater. Supercapacit. 2020, 64, 77. [Google Scholar]
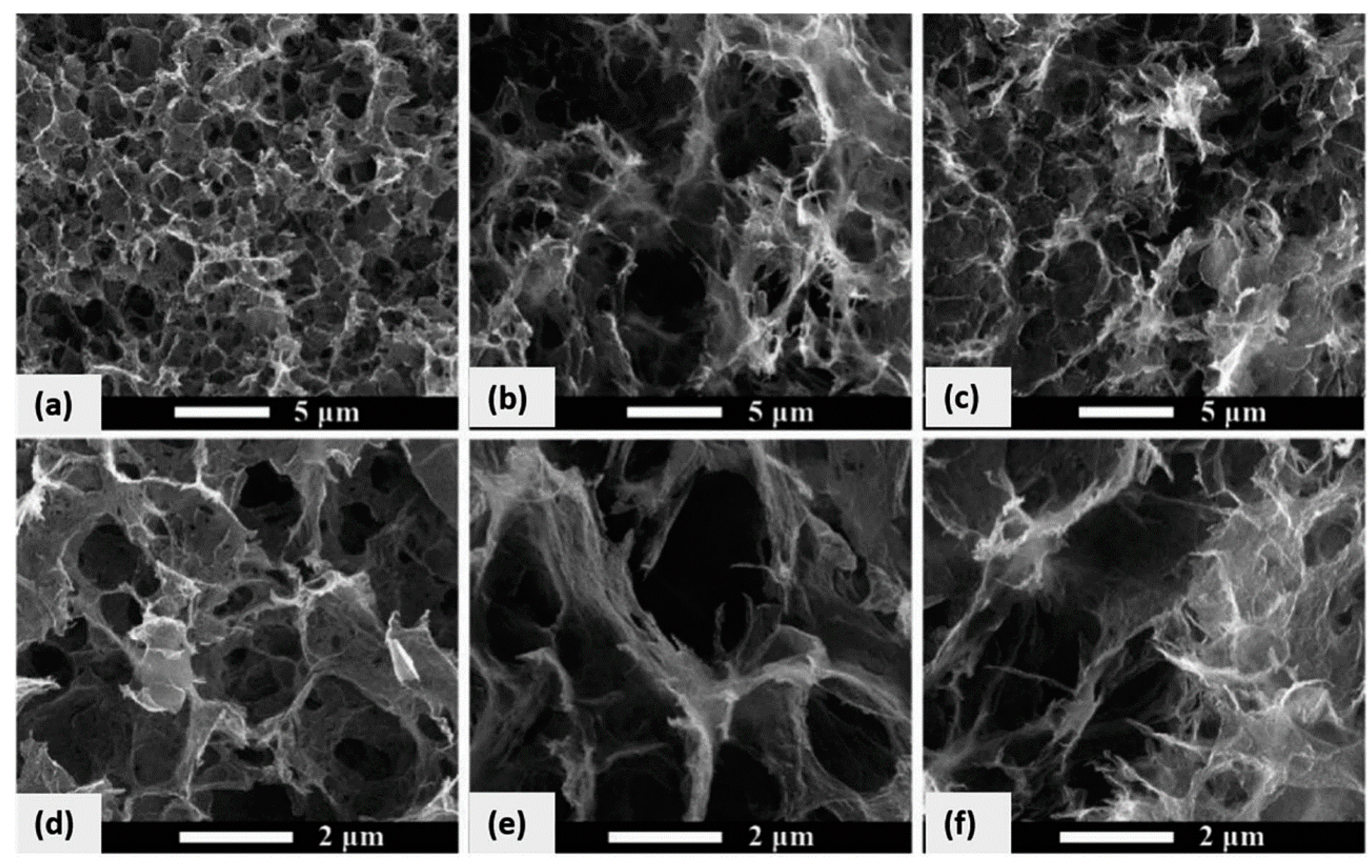
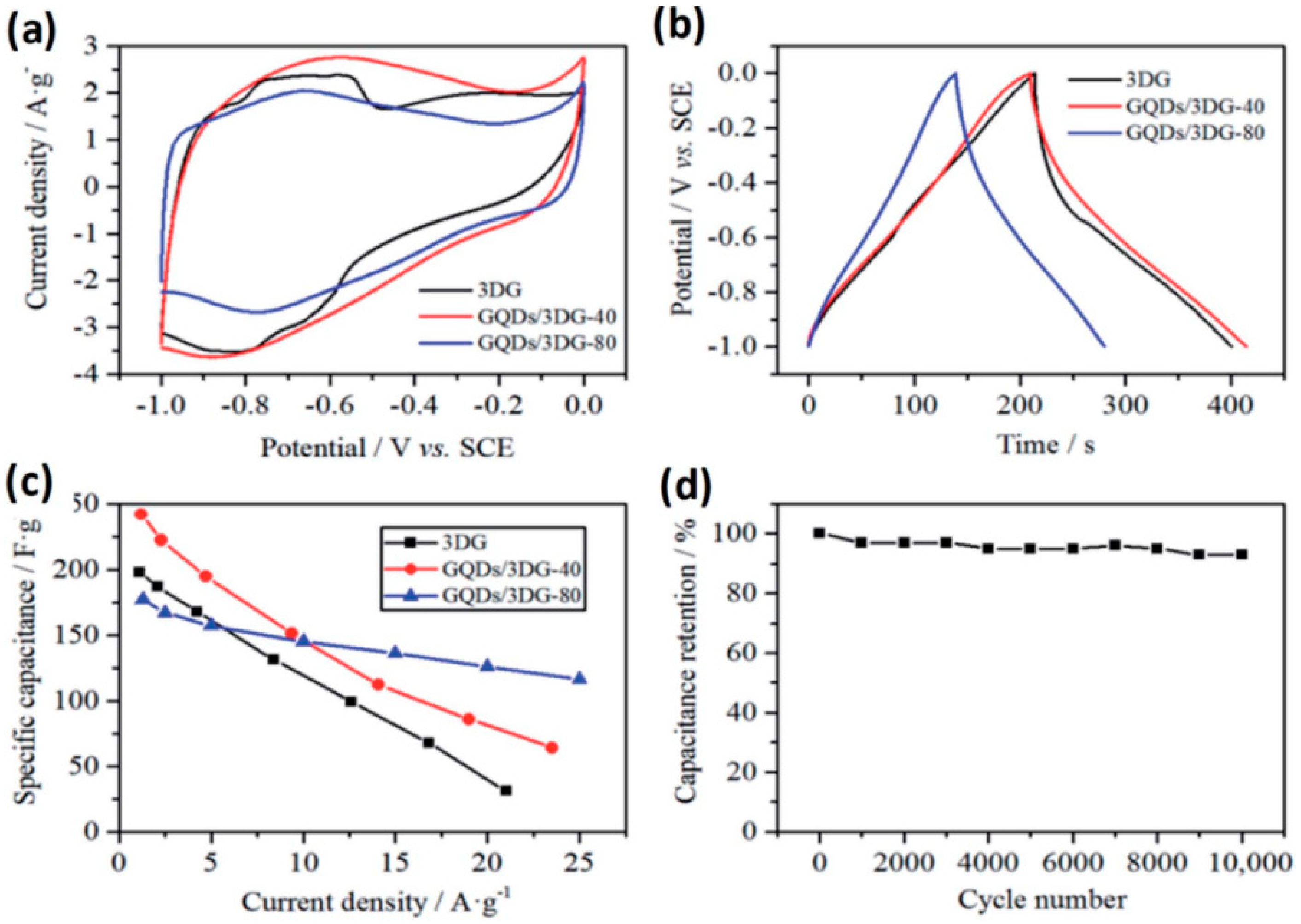
- Luo, P.; Guan, X.; Yu, Y.; Li, X.; Yan, F. Hydrothermal Synthesis of Graphene Quantum Dots Supported on Three-Dimensional Graphene for Supercapacitors. Nanomaterials 2019, 9, 201. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, Y.; Xiong, Z.; Guo, G.; Sun, Y.; Li, D.; Liu, Y. Stable GQD@PANi nanocomposites based on benzenoid structure for enhanced specific capacitance. Int. J. Hydrogen Energy 2018, 43, 8426–8439. [Google Scholar] [CrossRef]
- Abidin, S.N.J.S.Z.; Mamat, S.; Rasyid, S.A.; Zainal, Z.; Sulaiman, Y. Fabrication of poly(vinyl alcohol)-graphene quantum dots coated with poly(3,4-ethylenedioxythiophene) for supercapacitor. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 50–58. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Park, J.; Moon, J.; Kim, C.; Kang, J.H.; Lim, E.; Park, J.; Lee, K.J.; Yu, S.-H.; Seo, J.-H.; Lee, J. Graphene quantum dots: Structural integrity and oxygen functional groups for high sulfur/sulfide utilization in lithium sulfur batteries. NPG Asia Mater. 2016, 8, e272. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Xia, X.; Liu, J.; Zhang, X.; Wang, J.; Liang, P.; Lin, J.; Zhang, H.; Shen, Z.X.; et al. Graphene Quantum Dots Coated VO2Arrays for Highly Durable Electrodes for Li and Na Ion Batteries. Nano Lett. 2015, 15, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Radhamani, A.; Lau, H.C.; Ramakrishna, S. Nanocomposite coatings on steel for enhancing the corrosion resistance: A review. J. Compos. Mater. 2020, 54, 681–701. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Shoueir, K.R. Electrochemical behavior of smart N-isopropyl acrylamide copolymer nanogel on steel for corrosion protection in acidic solution. Int. J. Electrochem. Sci. 2015, 10, 870–882. [Google Scholar]
- Miorin, E.; Montagner, F.; Zin, V.; Giuranno, D.; Ricci, E.; Pedroni, M.; Spampinato, V.; Vassallo, E.; DeAmbrosis, S.M. Al rich PVD protective coatings: A promising approach to prevent T91 steel corrosion in stagnant liquid lead. Surf. Coat. Technol. 2019, 377, 124890. [Google Scholar] [CrossRef]
- Morozov, Y.; Calado, L.; Shakoor, R.; Raj, R.; Kahraman, R.; Taryba, M.; Montemor, M. Epoxy coatings modified with a new cerium phosphate inhibitor for smart corrosion protection of steel. Corros. Sci. 2019, 159, 108128. [Google Scholar] [CrossRef]
- Anagri, A.; Baitukha, A.; Chouvy, C.D.-; Lucas, I.T.; Pulpytel, J.; Tran, T.M.; Tabibian, S.; Khonsari, F.A. Nanocomposite coatings based on graphene and siloxane polymers deposited by atmospheric pressure plasma. Application to corrosion protection of steel. Surf. Coat. Technol. 2019, 377, 124928. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Gupta, R.K.; Malviya, M.; Ansari, K.; Lgaz, H.; Chauhan, D.; Quraishi, M. Functionalized graphene oxide as a new generation corrosion inhibitor for industrial pickling process: DFT and experimental approach. Mater. Chem. Phys. 2019, 236, 121727. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; A George, G.; Motta, N.; Waclawik, E.R.; Yan, C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J. Appl. Phys. 2014, 116, 053518. [Google Scholar] [CrossRef]
- Ding, X. Direct synthesis of graphene quantum dots on hexagonal boron nitride substrate. J. Mater. Chem. C 2014, 2, 3717–3722. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, C.; Li, Y.; Yu, D. Shining luminescent graphene quantum dots: Synthesis, physicochemical properties, and biomedical applications. TrAC Trends Anal. Chem. 2019, 116, 109–121. [Google Scholar] [CrossRef]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. Corrosion protection properties of novel epoxy nanocomposite coatings containing silane functionalized graphene quantum dots. J. Alloys Compd. 2018, 731, 1112–1118. [Google Scholar] [CrossRef]
- Lee, C.Y.; Bae, J.-H.; Kim, T.-Y.; Chang, S.-H.; Kim, S.Y. Using silane-functionalized graphene oxides for enhancing the interfacial bonding strength of carbon/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2015, 75, 11–17. [Google Scholar] [CrossRef]
- Wei, X.; Liu, P.; Ma, S.; Li, Z.; Peng, X.; Deng, R.; Zhao, Q. Improvement on corrosion resistance and biocompability of ZK60 magnesium alloy by carboxyl ion implantation. Corros. Sci. 2020, 173, 108729. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C 2019, 104, 109947. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, V.; Azambuja, D.S.; Alemán, C.; Armelin, E. Hybrid organophosphonic-silane coating for corrosion protection of magnesium alloy AZ91: The influence of acid and alkali pre-treatments. Surf. Coat. Technol. 2019, 357, 728–739. [Google Scholar] [CrossRef]
- Yao, Q.-S.; Li, Z.-C.; Qiu, Z.-M.; Zhang, F.; Chen, X.-B.; Chen, D.-C.; Guan, S.-K.; Zeng, R.-C. Corrosion resistance of Mg(OH)2/Mg–Al-layered double hydroxide coatings on magnesium alloy AZ31: Influence of hydrolysis degree of silane. Rare Met. 2019, 38, 629–641. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef]
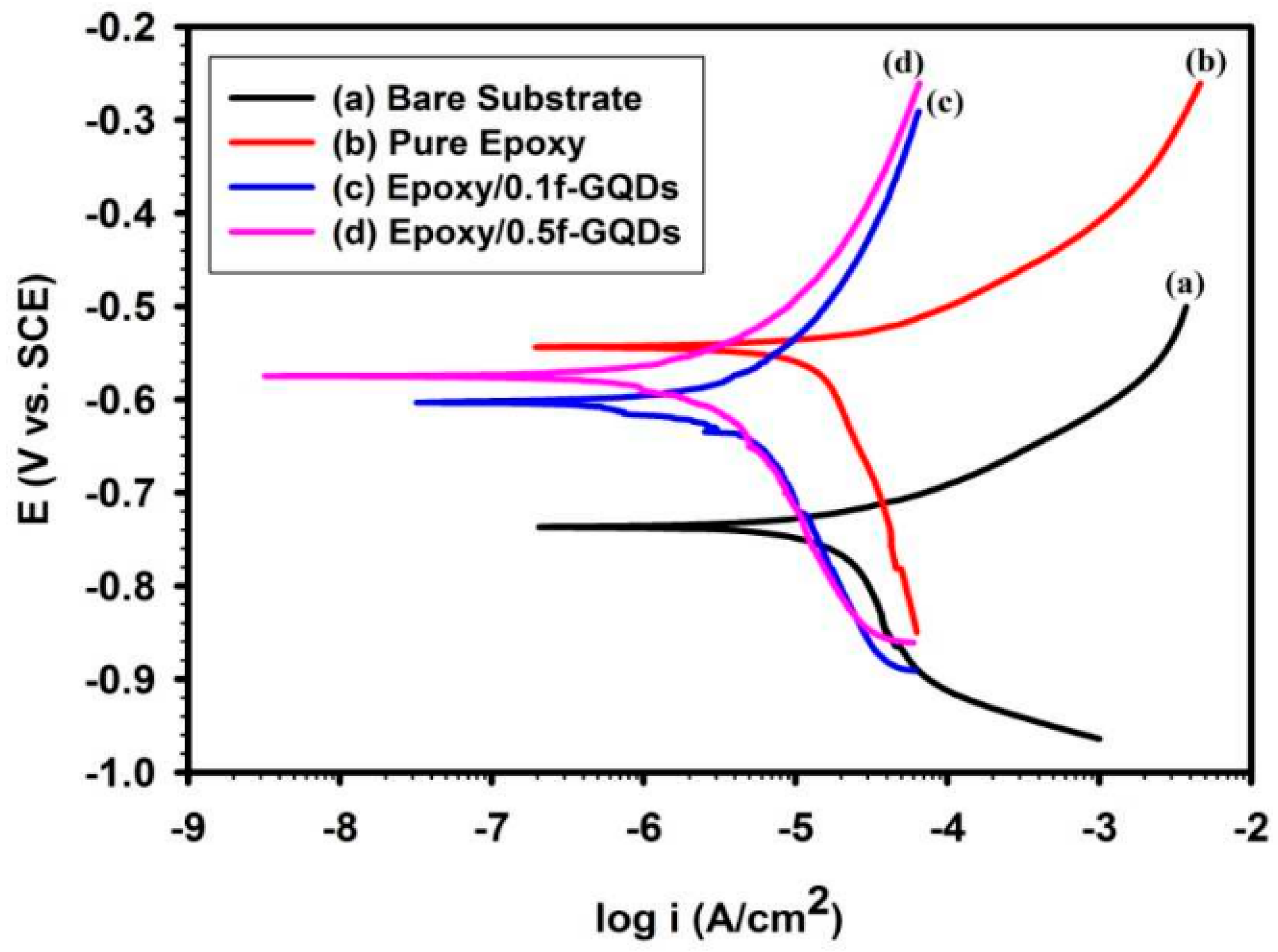
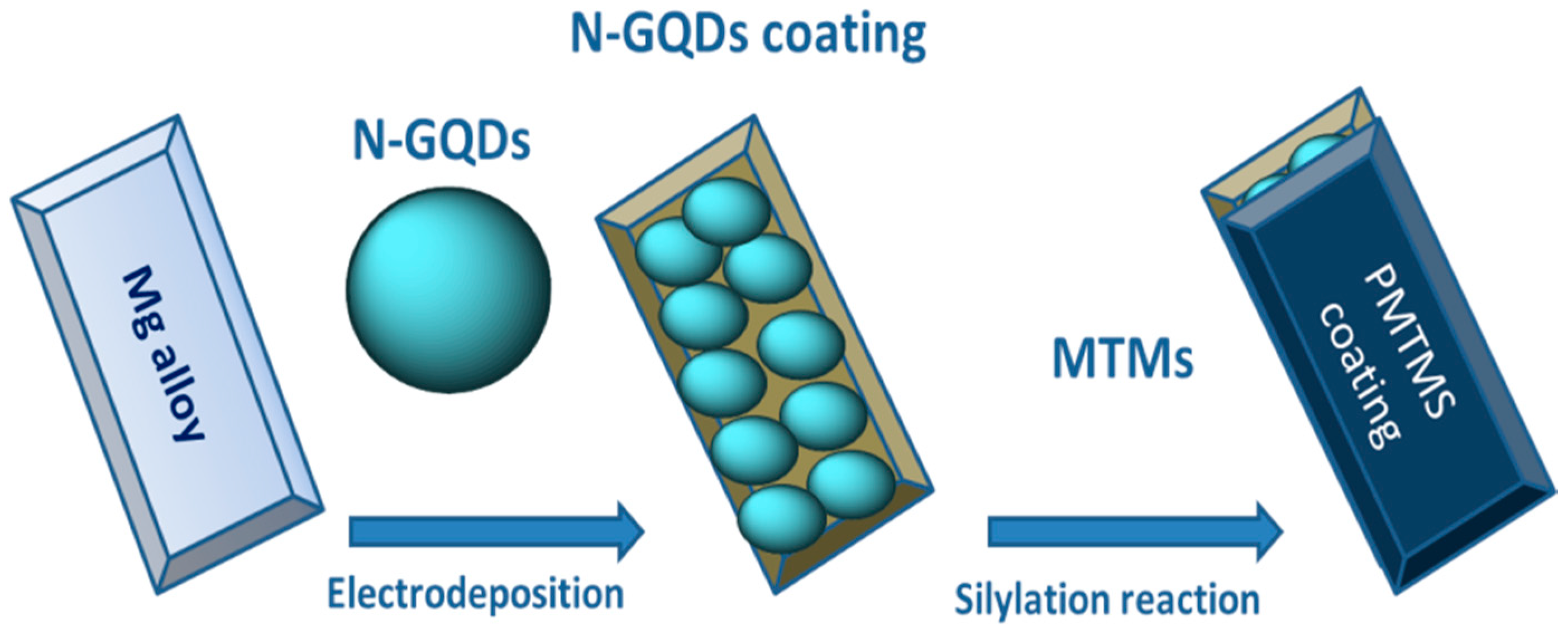
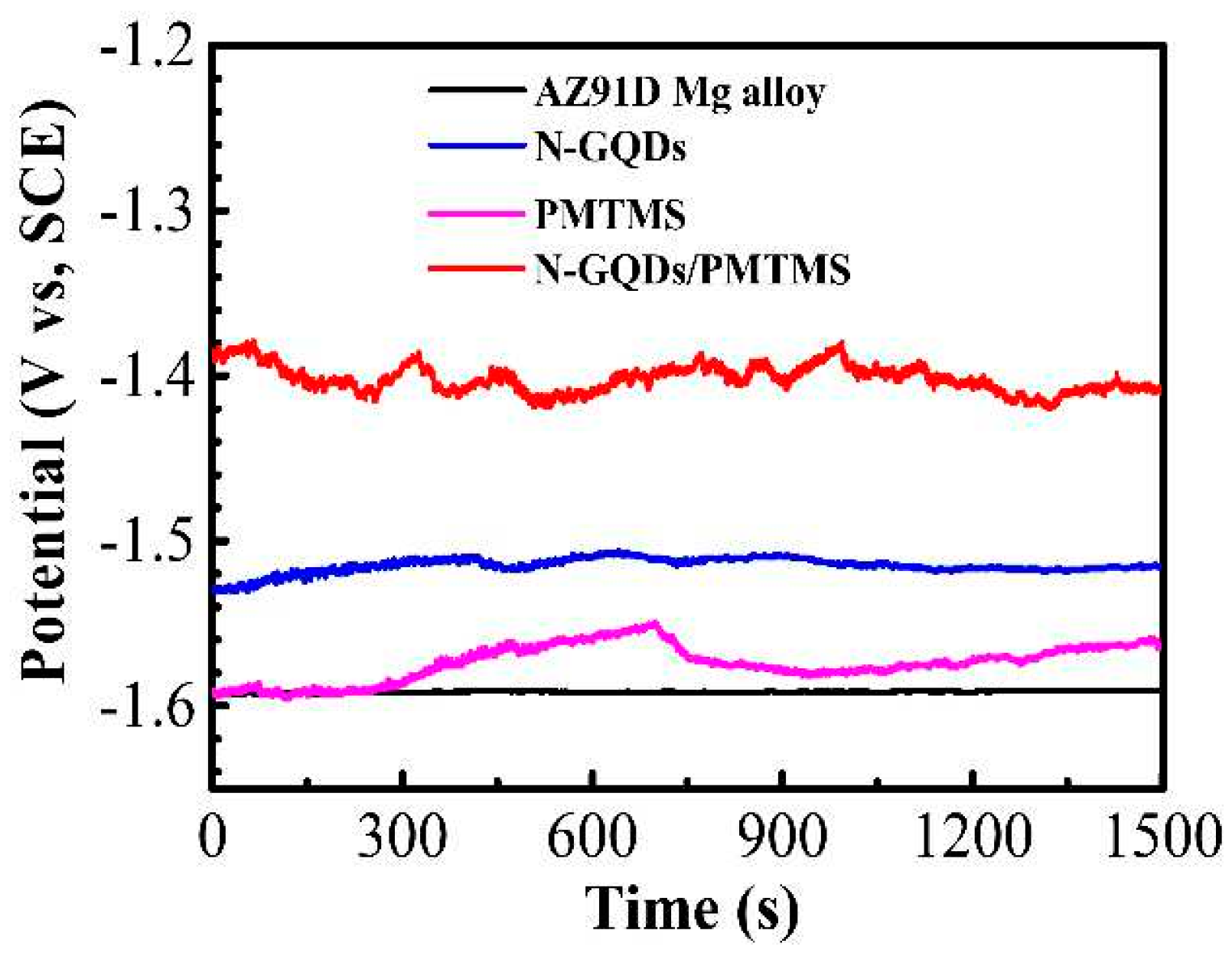
- Jiang, B.; Chen, A.; Gu, J.; Fan, J.; Liu, Y.; Wang, P.; Li, H.-J.; Sun, H.; Yang, J.; Wang, X. Corrosion resistance enhancement of magnesium alloy by N-doped graphene quantum dots and polymethyltrimethoxysilane composite coating. Carbon 2020, 157, 537–548. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.; Bahru, R. Carbon and graphene quantum dots in fuel cell application: An overview. Int. J. Energy Res. 2020, 45, 1396–1424. [Google Scholar] [CrossRef]


| Basis for Comparison | Top-Down Approach | Bottom-Up Approach |
|---|---|---|
| Basic | Successive cutting or grinding of bulk material to get nanoparticles | The buildup of material from bottom: atom or molecule to get nanoparticles |
| Starting materials | Solid-state | The starting material is either gaseous or liquid |
| Processing method | Physical method | Physical and chemical methods |
| Advantages |
|
|
| Disadvantages |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Jahdaly, B.A.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Taher, M.M.; Khalil, A.M. Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review. Sustainability 2021, 13, 2127. https://doi.org/10.3390/su13042127
Al Jahdaly BA, Elsadek MF, Ahmed BM, Farahat MF, Taher MM, Khalil AM. Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review. Sustainability. 2021; 13(4):2127. https://doi.org/10.3390/su13042127
Chicago/Turabian StyleAl Jahdaly, Badreah Ali, Mohamed Farouk Elsadek, Badreldin Mohamed Ahmed, Mohamed Fawzy Farahat, Mohamed M. Taher, and Ahmed M. Khalil. 2021. "Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review" Sustainability 13, no. 4: 2127. https://doi.org/10.3390/su13042127
APA StyleAl Jahdaly, B. A., Elsadek, M. F., Ahmed, B. M., Farahat, M. F., Taher, M. M., & Khalil, A. M. (2021). Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review. Sustainability, 13(4), 2127. https://doi.org/10.3390/su13042127







