Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Samplings
2.2. Ultrasound-Assisted Extraction of Polyphenolic Compounds
2.3. Total Polyphenolic Content Assay (Folin)
2.4. Determination of Antioxidant Activity (ABTS and DPPH Assay)
2.5. Orbitrap High-Resolution Mass Spectrometry Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Yield in Waste Biomass and Polyphenols
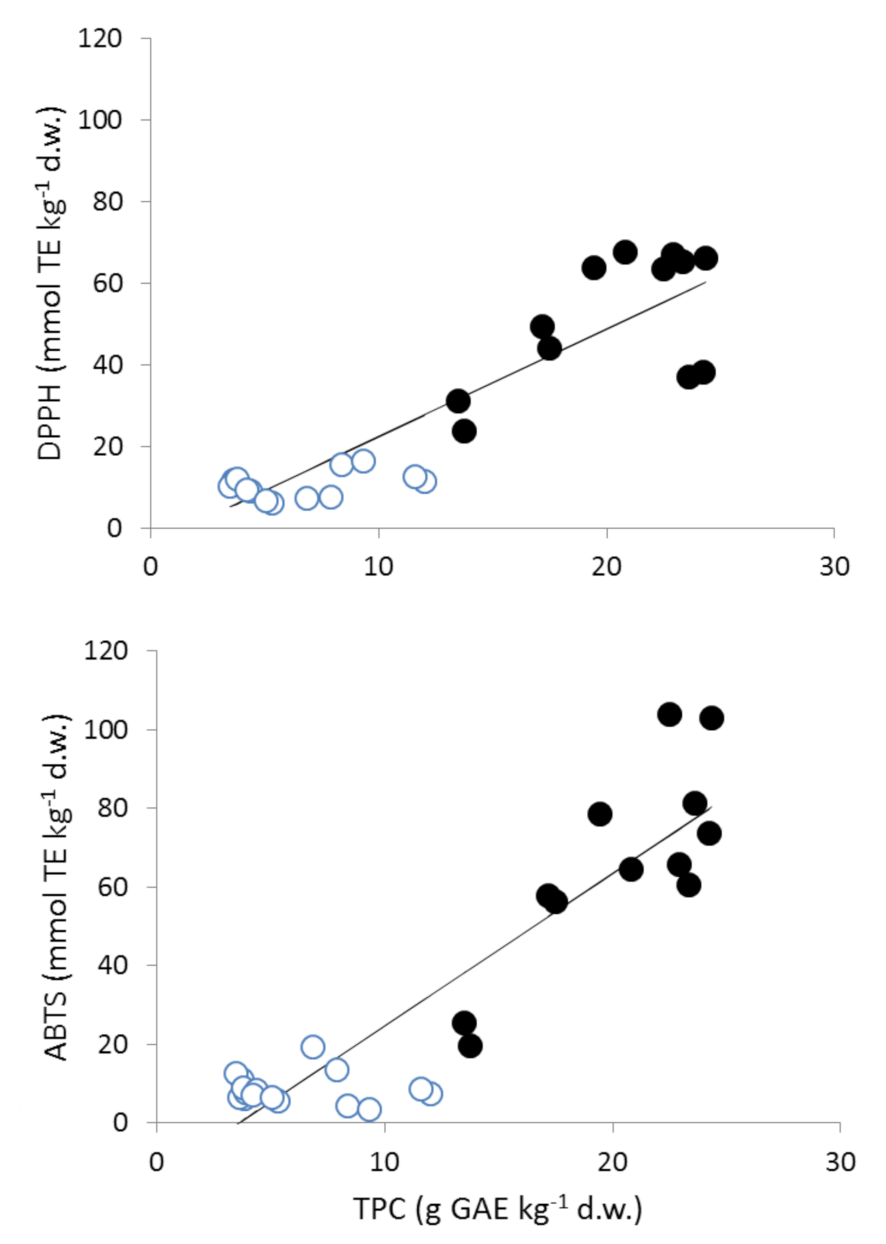
3.2. Radical Scavenging Activity (DPPH, ABTS, and % Inhibition)
3.3. Phenolic Profile: Phenolic Acids and Flavonoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 8 January 2021).
- FAO. The economic significance of tobacco. Econ. Soc. Dev. Pap. 1989, 85, 90. [Google Scholar]
- ISTAT. 2018. Available online: http://dati.istat.it/ (accessed on 8 January 2021).
- Nomisma. La Filiera del Tabacco in Italia. Impatto Socioeconomico ed Aspetti di Politica Fiscale; XV Rapporto; Nomisma: Bologna, Italy, 2012; pp. 83–84. [Google Scholar]
- Rawat, A.; Mali, R.R.; Saini, A.K.; Chauhan, P.K.; Singh, V.; Sharma, P. Phytochemical Properties and Pharmcological Activities of Nicotiana Tabacum: A Review. Indian J. Pharm. Biol. Res. 2013, 1, 74–82. [Google Scholar] [CrossRef]
- Novotny, T.E.; Slaughter, E. Tobacco Product Waste: An Environmental Approach to Reduce Tobacco Consumption. Curr. Environ. Heal. Rep. 2014, 1, 208–216. [Google Scholar] [CrossRef]
- Sharma, Y.; Nagar, A.; Srivastava, N.S.; Bala, K. Antioxidant Activity of Polyphenolic Flavonoid of Stem of Nicotiana tabacum. Am. J. Drug Discov. Dev. 2016, 7, 25–32. [Google Scholar] [CrossRef]
- Barla, F.G.; Kumar, S. Tobacco biomass as a source of advanced biofuels. Biofuels 2016, 10, 335–346. [Google Scholar] [CrossRef]
- Zi, W.; Zhang, X.; Peng, J.; Zhang, L.; Long, M.; Zuo, J. Optimization of Microwave Drying Biomass Material of Stem Granules from Waste Tobacco Using Response Surface Methodology. Dry. Technol. 2013, 31, 1234–1244. [Google Scholar] [CrossRef]
- Andrianov, V.; Borisjuk, N.; Pogrebnyak, N.; Brinker, A.; Dixon, J.; Spitsin, S.; Flynn, J.; Matyszczuk, P.; Andryszak, K.; Laurelli, M.; et al. Tobacco as a production platform for biofuel: Overexpression ofArabidopsis DGATandLEC2genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 2010, 8, 277–287. [Google Scholar] [CrossRef]
- Leffingwell, J.C. Leaf Chemistry—Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types In Tobacco: Production, Chemistry, And Technology; Davis, D.L., Nielsen, M.T., Eds.; Blackwell Science: Hoboken, NJ, USA, 1999; pp. 265–284. [Google Scholar]
- Tremblay, R.; Wang, D.; Jevnikar, A.M.; Ma, S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010, 28, 214–221. [Google Scholar] [CrossRef]
- Oeung, S.; Nov, V.; Ung, H.; Roum, K.; Yin, V.; Keo, S.; Chea, S. Phytochemical analysis of different extracts of leaves of Nicotiana tabacum L. of Cambodia. 2017. Asian J. Pharmacogn. 2017, 1, 18–26. [Google Scholar]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind. Crop. Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Charlton, A. Medicinal uses of tobacco in history. J. R. Soc. Med. 2004, 97, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I. Nicotine and Other Insecticidal Alkaloids. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Springer International Publishing: Berlin/Heidelberg, Germany, 1999; pp. 29–69. [Google Scholar]
- Fusetto, R.; O’Hair Richard, A.J. Nicotine as an Insecticide in Australia: A Short History. Available online: https://search.informit.org/doi/epdf/10.3316/ielapa.531770497153501 (accessed on 8 January 2021).
- López-Arrieta, J.; Sanz, F.J.F.J.S. Nicotine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2001, 2, CD001749. [Google Scholar] [CrossRef] [PubMed]
- Ebarreto, G.E.; Eiarkov, A.; Moran, V.E. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front. Aging Neurosci. 2015, 6, 340. [Google Scholar] [CrossRef]
- Akyazi, R.; Soysal, M.; Altunc, Y.E.; Lisle, A.; Hassan, E.; Akyol, D. Acaricidal and sublethal effects of tobacco leaf and garlic bulb extract and soft soap on Tetranychus urticae Koch. (Acari: Trombidiformes: Tetranychidae). Syst. Appl. Acarol. 2018, 23, 2054–2069. [Google Scholar] [CrossRef]
- Raveen, R.; Kumar, L.; Sugirtha, A.; Karthikeyan, N.; Tennyson, S.; Arivoli, S.; Jayakumar, M. Efficacy of Tobacco Leaf Extracts on the Larva of the Dengue Vector Mosquito. J. Sci. Front. Res. 2019, 19, 7. [Google Scholar]
- Choudhary, A.; Ashraf, S.; Musheer, N. Screening of phytoextracts to control of Fusarium oxysporum f.sp. vigni incitant of mungbean (Vigna radiata) wilt.2017. Int. J. Acad. Res. Dev. 2017, 2, 1181–1184. [Google Scholar]
- Sharma, Y.; Dua, D.; Nagar, A.; Srivastava, N.S. Antibacterial activity, phytochemical screening and antioxidant activity of stem of Nicotiana tabacum. Int. J. Pharm. Sci. Res. 2016, 7, 1156–1167. [Google Scholar] [CrossRef]
- Rymerson, R.T.; Menassa, R.; Brandle, J.E. Tobacco, a Platform for the Production of Recombinant Proteins. In Molecular Farming of Plants and Animals for Human and Veterinary Medicine; Erickson, L., Yu, W.-J., Brandle, J., Rymerson, R., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2002; pp. 1–31. [Google Scholar]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive Roles of Polyphenol Oxidase in Plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antiox. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Docheva, M.H.; Dagnon, S. Polyphenols in tobacco extracts obtained by macroporous resin. Compt. Rend. Acad. Bulg. Sci. 2015, 68, 183–190. [Google Scholar]
- Docheva, M.H.; Staykova, M.B.; Stoilova, A.B.; Dimanov, D. Basic chemical components and radical scavenging activity of tobacco extracts obtained by macroporous resin. Bulg. Chem. Commun. 2017, 49, 212–216. [Google Scholar]
- Dagnon, S.; Ivanov, I.; Bojilov, D.; Docheva, M.; Statkova, S. Evaluation of the Main Polyphenolic Compounds in Aromatic Plants of Asteraceae and Solanaceae Families of Bulgarian Origin. J. Pharmacogn. Phytochem. 2013, 1, 76–84. [Google Scholar]
- Karabegović, I.T.; Veljković, V.B.; Lazić, M.L. Ultrasound-assisted Extraction of Total Phenols and Flavonoids from Dry Tobacco (Nicotiana tabacum) Leaves. Nat. Prod. Commun. 2011, 6, 1855–1856. [Google Scholar] [CrossRef] [PubMed]
- Docheva, M.H.; Popova, V.T.; Ivanova, T.A.; Nikolova, V.V.; Hristeva, T.H.; Nikolov, N.N. Polyphenol content and anti-oxidant activity of aqueous/methanol extracts from different tobacco species (Nicotiana). Bulg. Chem. Commun. 2018, 50, 553–559. [Google Scholar]
- Wang, X.; Liu, P.; Wang, F.; Fu, B.; He, F.; Zhao, M. Influence of altitudinal and latitudinal variation on the composition and antioxidant activity of polyphenols in Nicotiana tabacum L. leaf. Emir. J. Food Agric. 2017, 29, 359–366. [Google Scholar] [CrossRef]
- Fu, B.; Ji, X.; Zhao, M.; He, F.; Wang, X.; Wang, Y.; Liu, P.; Niu, L. The influence of light quality on the accumulation of flavonoids in tobacco (Nicotiana tabacum L.) leaves. J. Photochem. Photobiol. B Biol. 2016, 162, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Re, G.A.; Piluzza, G.; Sanna, F.; Molinu, M.G.; Sulas, L. Polyphenolic composition and antioxidant capacity of legume-based swards are affected by light intensity in a Mediterranean agroforestry system. J. Sci. Food Agric. 2019, 99, 191–198. [Google Scholar] [CrossRef]
- Nasr, S.B.; Aazza, S.; Mnif, W.; Miguel, M. Phenol content and antioxidant activity of different young and adult plant parts of tobacco from Tunisia, dried at 40 and 70 °C. J. Appl. Pharm. Sci. 2014, 4, 023–031. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Ren, K.; He, X.; Gong, J.; Hu, X.; Su, J.; Jin, Y.; Zhao, Z.; Zhu, Y.; Zou, C. Dynamic changes in physiological and biochemical properties of flue-cured tobacco of different leaf ages during flue-curing and their effects on yield and quality. BMC Plant Biol. 2019, 19, 1–21. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jáuregui, O.; Codina, C.; Tiburcio, A.F.; Bastida, J.; Viladomat, F. Analysis of phenolic compounds by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry in senescent and water-stressed tobacco. Plant Sci. 2012, 182, 71–78. [Google Scholar] [CrossRef]
- Sifola, M.I.; Postiglione, L. The effect of nitrogen fertilization and irrigation on dry matter partitioning, yield and quality of tobacco (Nicotiana tabacum L.) Burley type. Agric. Mediterr. 2002, 132, 33–43. [Google Scholar]
- Sifola, M.I.; Carrino, L.; Cozzolino, E.; Ianuario, S.; Lucibelli, A.; Coppola, A. A Survey of Fertility Program Responses of Kentucky Dark Fire-Cured Tobacco (Nicotiana tabacum L.) Yield and Quality for Cigars Manufacture in the Benevento Province (Southern Italy). Beiträge Tab. Contrib. Tob. Res. 2018, 28, 14–29. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef]
- MSTAC. A Microcomputer Program for Design Management and Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, MI, USA, 1991. [Google Scholar]
- Zhang, X.; Gao, H.; Zhang, L.; Liu, D.; Ye, X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Ind. Crop. Prod. 2012, 39, 162–169. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Galanakis, C.M. Handbook of Grape Processing By-Products: Sustainable Solutions; Academic Press: Cambridge, MA, USA, 2017; p. 293. [Google Scholar]
- Van Zonneveld, M.; Ramirez, M.; Williams, D.E.; Petz, M.; Meckelmann, S.; Avila, T.; Bejarano, C.; Ríos, L.; Peña, K.; Jäger, M.; et al. Screening Genetic Resources of Capsicum Peppers in Their Primary Center of Diversity in Bolivia and Peru. PLoS ONE 2015, 10, e0134663. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Putnik, P.; Bursac Kovacevic, D. Agri-Food Industry Strategies for Healthy Diets and Sustainability: New Challenges in Nutrition and Public Health; Academic Press: Cambridge, MA, USA, 2020; p. 267. [Google Scholar]
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and Pathogen Survival in Crop Residues, an Ecotone Between Plant and Soil. Phytobiomes J. 2019, 3, 246–255. [Google Scholar] [CrossRef]
- Alibaba. Available online: https://www.alibaba.com/premium/+extract_+polyphenol.html?src=sem_ggl&cmpgn=10415283818&adgrp=106519962474&fditm=&tgt=kwd-914849910957&locintrst=&locphyscl=1008311&mtchtyp=b&ntwrk=g&device=c&dvcmdl=&creative=446020226116&plcmnt=&plcmntcat=&p1=&p2=&aceid=&position=&gclid=EAIaIQobChMIgs3EuIiM7gIVl7p3Ch2brQXdEAMYAyAAEgKK6_D_BwE (accessed on 8 January 2021).
- Ru, Q.-M.; Wang, L.-J.; Li, W.-M.; Wang, J.-L.; Ding, Y.-T. In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves. Molecules 2012, 17, 11281–11291. [Google Scholar] [CrossRef]
- Sheen, S. The distribution of polyphenols, chlorogenic acid oxidase and peroxidase in different plant parts of tobacco, Nicotiana tabacum L. Phytochemistry 1969, 8, 1839–1847. [Google Scholar] [CrossRef]
- Rodu, B.; Ou, B. The antioxidant properties of tobacco. Tob. Sci. 2000, 44, 71–73. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the Antioxidant Capacity and Phenolic Content of Three Thymus Species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Docheva, M.; Dagnon, S.; Statkova-Abeghe, S. Flavonoid content and radical scavenging potential of extracts prepared from tobacco cultivars and waste. Nat. Prod. Res. 2014, 28, 1328–1334. [Google Scholar] [CrossRef]
- Shifflett, J.R.; Watson, L.; McNally, D.J.; Bezabeh, D.Z. Analysis of the Polyphenols of Tobacco Using Pressurized Liquid Extraction (PLE) and Ultra Performance Liquid Chromatography With Electrospray Ionization—Tandem Mass Spectometric Detection (UPLC-ESI-MS/MS). Beiträge Tab. Contrib. Tob. Res. 2017, 27, 195–207. [Google Scholar] [CrossRef]
- Dagnon, S.; Edreva, A. Application of Pattern Recognition Method for Color Assessment of Oriental Tobacco based on HPLC of Polyphenols. Beiträge Tab. Contrib. Tob. Res. 2003, 20, 356–359. [Google Scholar] [CrossRef]
- Sofic, E.; Copra-Janicijevic, A.; Salihovic, M.; Tahirovic, I.; Kroyer, G. Screening of medicinal plant extracts for querce-tin-3rutinoside (rutin) in Bosnia and Herzegovina. Med. Plants Int. J. Phytomedicines Relat. Ind. 2010, 2, 97. [Google Scholar] [CrossRef]
- Herrling, T.; Jung, K. The Radical Status Factor (RSF): A novel metric to characterize skin products. Int. J. Cosmet. Sci. 2012, 34, 285–290. [Google Scholar] [CrossRef]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
| Waste Biomass (kg d.w. ha−1) | Polyphenols Content 1 (g kg−1 d.w.) | Polyphenols Yield (kg ha−1) | |||||
|---|---|---|---|---|---|---|---|
| Burley | Kentucky | Burley | Kentucky | Burley | Kentucky | ||
| Topping materials | Leaf | 482.7 B | 191.6 | 12.6 a | 15.7 | 6.1 B | 3.0 |
| Stalk | 254.5 B | – | 5.2 b | – | 1.3 B | – | |
| Residual biomass 2 | Stalk | 3219.7 A | 1112.8 | 4.6 b | 8.1 | 14.7 A | 9.0 |
| Significance | ** | NS | * | NS | ** | NS | |
| TPC (g GAE kg−1 d.w.) | |||
|---|---|---|---|
| Burley | Kentucky | ||
| Topping materials | Leaf | 18.1 a | 22.4 A |
| Stalk | 5.6 b | – | |
| Residual biomass 1 | Stalk | 3.8 b | 8.1 B |
| Significance | * | ** | |
| DPPH (mmol TE kg−1 d.w.) | ABTS (mmol TE kg−1 d.w.) | ||||
|---|---|---|---|---|---|
| Burley | Kentucky | Burley | Kentucky | ||
| Topping materials | Leaf | 46.3 a | 56.4 a | 61.0 | 70.7 A |
| Stalk | 7.7 b | – | 10.0 | – | |
| Residual biomass 1 | Stalk | 11.2 b | 13.1 b | 7.8 | 7.5 B |
| Significance | * | * | NS | ** | |
| Burley | Quinic Acid | Chlorogenic Acid | Caffeic Acid | 5-O-p-Coumaroyl Quinic Acid | Feruloyl Quinic Acid Isomer 1 | 3-O-p-coumaroyl Quinic Acid | Feruloyl Quinic Acid Isomer 2 | |
| Topping materials | Leaf | 3926.2 | 2339.9 | 6.87 | 53.5 A | 45.3 a | 64.0 a | 20.8 a |
| Stalk | 4398.0 | 491.8 | 1.31 | 5.8 B | 2.2 b | 4.6 b | 1.9 b | |
| Residual biomass 1 | Stalk | 3153.0 | 1198.0 | 3.58 | 5.5 B | 2.1 b | 5.0 b | 2.0 b |
| Significance | NS | NS | NS | ** | * | * | * | |
| Kentucky | Quinic Acid | Chlorogenic Acid | Caffeic Acid | 5-O-p-Coumaroyl Quinic Acid | Feruloyl Quinic Acid Isomer 1 | 3-O-p-Coumaroyl Quinic Acid | Feruloyl Quinic Acid Isomer 2 | |
| Topping materials | Leaf | 1980.0 | 4777.5 a | 14.2 a | 88.7 a | 69.8 | 63.3 a | 23.7 a |
| Residual biomass 1 | Stalk | 6925.5 | 1998.6 b | 1.7 b | 9.5 b | 4.7 | 6.5 b | 3.9 b |
| Significance | NS | * | * | * | NS | * | * |
| Burley | Scopoletin | Isoscopoletin | Rutin | Isoquercetin | Luteolin Rutinoside | |
| Topping materials | Leaf | 15.2 | 10.7 | 4830.3 | 44.6 | 1250.0 a |
| Stalk | 108.2 | 48.3 | 150.5 | 1.4 | 14.3 b | |
| Residual biomass 1 | Stalk | 45.1 | 15.9 | 117.1 | 0.8 | 9.2 b |
| Significance | NS | NS | NS | NS | * | |
| Kentucky | Scopoletin | Isoscopoletin | Rutin | Isoquercetin | Luteolin Rutinoside | |
| Topping materials | Leaf | 48.0 | 30.0 | 7401.1 A | 75.3 A | 1152.2 A |
| Residual biomass 1 | Stalk | 177.7 | 108.3 | 127.8 B | 1.2 B | 16.8 B |
| Significance | NS | NS | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifola, M.I.; Carrino, L.; Cozzolino, E.; del Piano, L.; Graziani, G.; Ritieni, A. Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability 2021, 13, 2087. https://doi.org/10.3390/su13042087
Sifola MI, Carrino L, Cozzolino E, del Piano L, Graziani G, Ritieni A. Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability. 2021; 13(4):2087. https://doi.org/10.3390/su13042087
Chicago/Turabian StyleSifola, Maria Isabella, Linda Carrino, Eugenio Cozzolino, Luisa del Piano, Giulia Graziani, and Alberto Ritieni. 2021. "Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids)" Sustainability 13, no. 4: 2087. https://doi.org/10.3390/su13042087
APA StyleSifola, M. I., Carrino, L., Cozzolino, E., del Piano, L., Graziani, G., & Ritieni, A. (2021). Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability, 13(4), 2087. https://doi.org/10.3390/su13042087







