Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Bottom Ash Sampling and Sieving
2.2. X-ray Fluorescence Spectrometry (XRF)
2.3. X-ray Powder Diffraction (XRPD) Phase Analysis
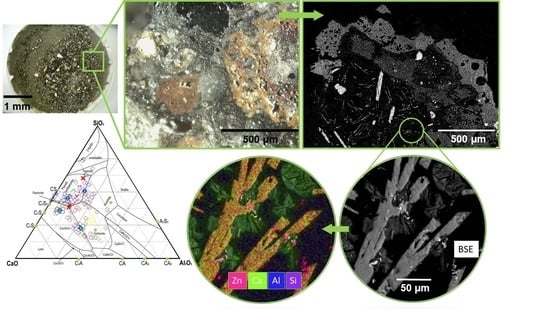
2.4. Optical and SEM-EDS Microscopy
2.5. Statistical Analysis
3. Results
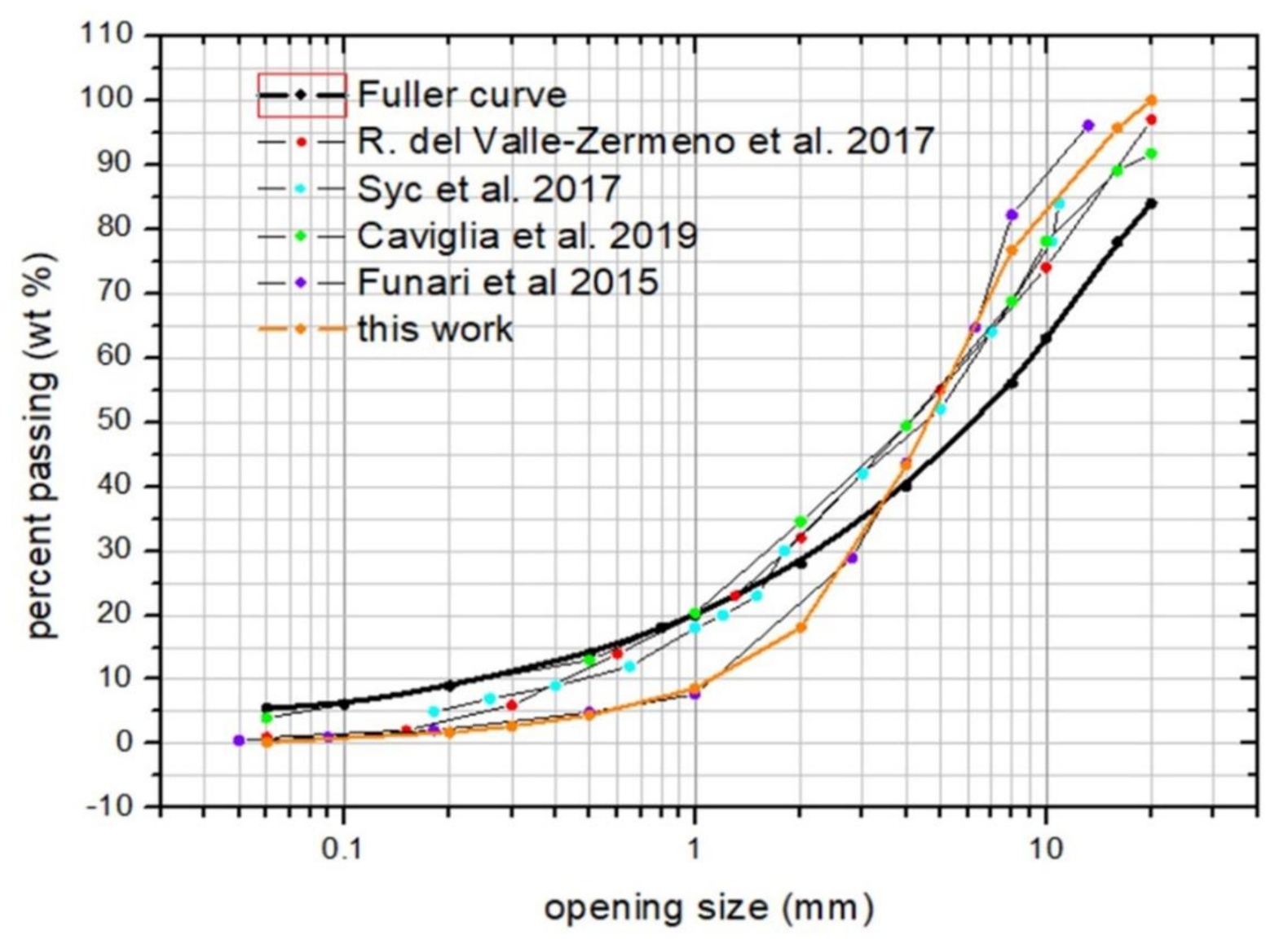
3.1. Grain Size Distribution
3.2. Chemical Analysis of MSWI Bottom Ash
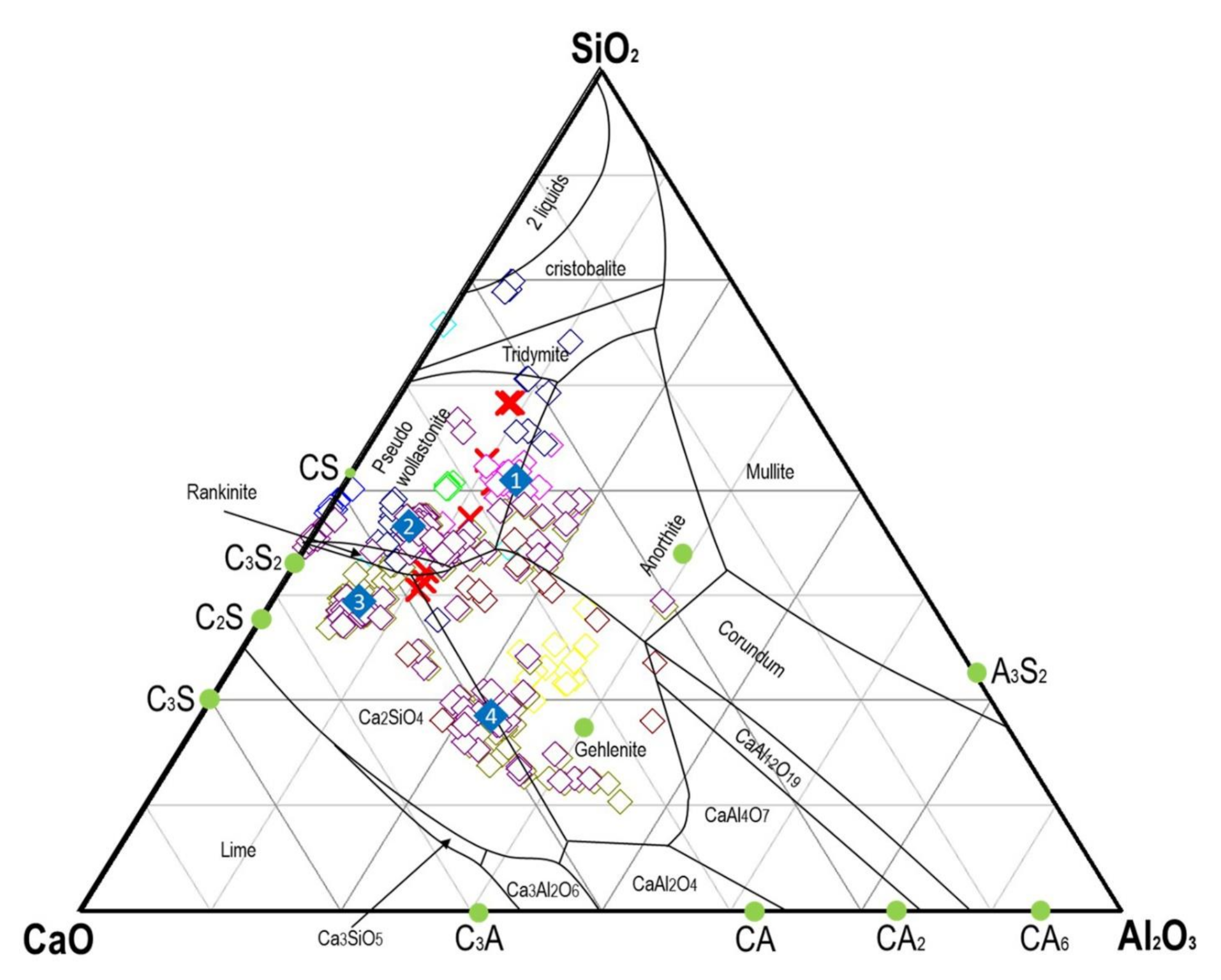
3.3. XRPD and Rietveld Analysis
3.4. Bottom Ash Morphology
3.4.1. Optical Observation
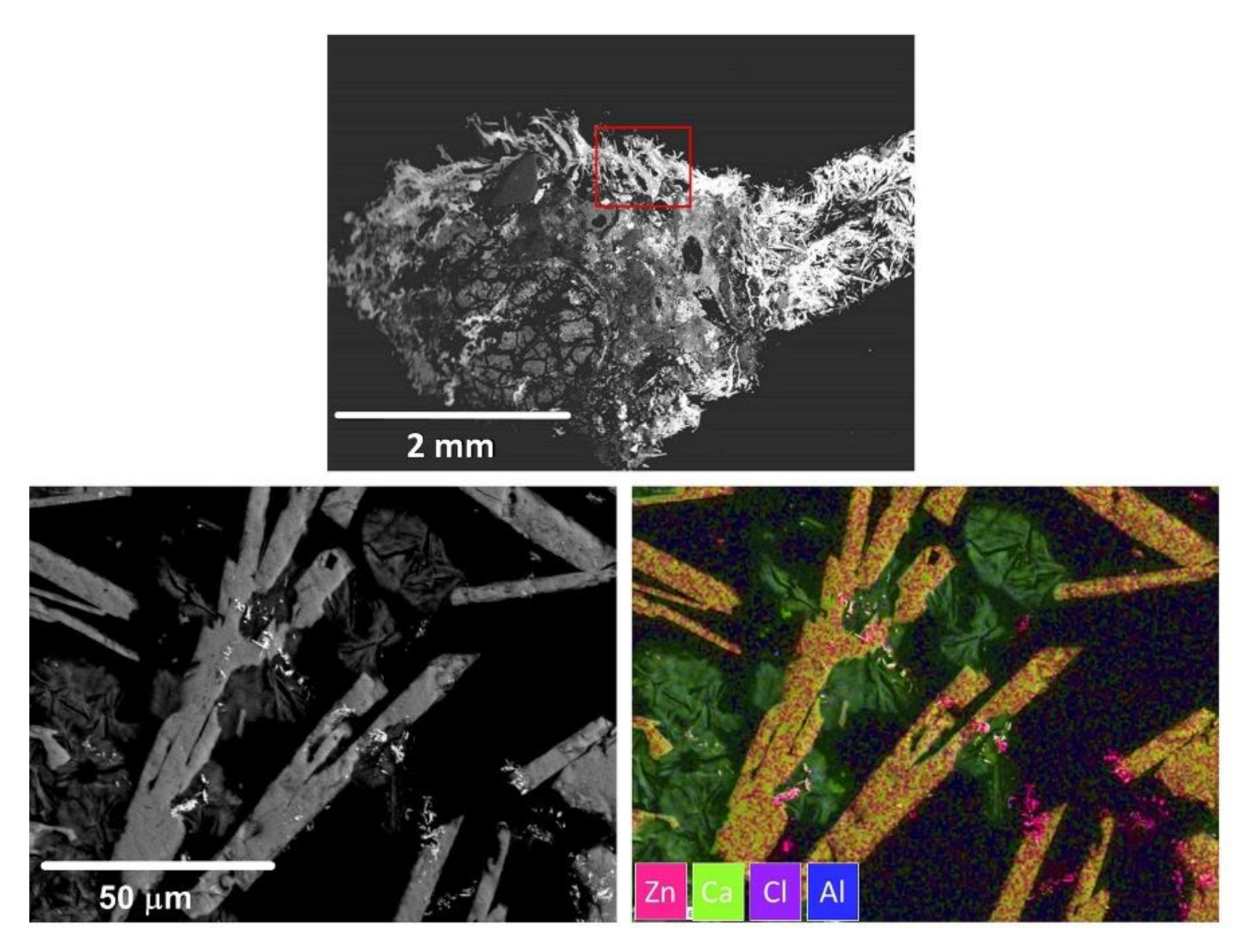
3.4.2. SEM-EDS Investigation
4. Discussion
- (1)
- In solid solution in the spinel oxide with a remarkable heterogeneity between different granules. Zn is substituted with Fe2+ between 2000 to 8000 mg/kg.
- (2)
- As a secondary constituent in glasses of probable cement origin with silicates or aluminates. There is substantial heterogeneity in the chemical composition of the glass, more or less rich in Al or Si, as well as Ca and Fe. Zn is present in quantities at the limit of detection with punctual microprobe analyses, always less than 2000 mg/kg. Given the widespread presence of glass, it may account for a background value, always present at about 1000–2000 mg/kg. It may be the prevailing form in larger grains.
- (3)
- The presence of Zn in large amounts was observed only in a single sample as a hydroxide, pure or with Ca (up to 74 wt.% in the pure hydroxide and 32 wt.% in that with Ca). As this mineralogical phase needs to be supported by other cases, its presence is not found here (Figure 7).
5. Conclusions
- (a)
- In bottom ashes, the same crystal phases are observed at any grain size, albeit in different amounts. However, at the electron microscope scale, we observed a wealth of local situations, including residual material made of silicate and metallic inclusions, silicate melts of different composition, droplets of metal phases, and neo-formed minerals, with a variety of quenching structures.
- (b)
- The higher presence of amorphous phases at any grain size suggests that most of the observed compositional changes occur within the amorphous phases.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decreto Legislativo 3 Aprile 2006, n. 152, Norme in Materia Ambientale; Presidente della Repubblica: Rome, Italy, 2006.
- Commission of European Communities. Towards a Circular Economy: A Zero Waste Programme for Europe; Communication No. 398; (COM (2014), 398); Commission of European Communities: Brussels, Belgium, 2014; Available online: http://ec.europa.eu/environment/circular-economy/pdf/circular-economy-communication.pdf (accessed on 15 December 2020).
- EUROSTAT. Municipal Waste by Waste Management Operations (1995–2019). Online data code: env_wasmun. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasmun (accessed on 16 December 2020).
- Izquierdo, M.; López-Soler, A.; Ramonich, E.V.; Barra, M.; Querol, X. Characterisation of bottom ash from municipal solid waste incineration in Catalonia. J. Chem. Technol. Biotechnol. 2002, 77, 576–583. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Segarra, M.; Fernández, M.A.; Espiell, F. Characterization of the bottom ash in municipal solid waste incinerator. J. Hazard. Mater. 1999, 64, 211–222. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.I.; Miralles, L.; Segarra, M.; Espiell, F. Short-term natural weathering of MSWI bottom ash as a function of particle size. Waste Manag. 2003, 23, 887–895. [Google Scholar] [CrossRef]
- Speiser, C.; Baumann, T.; Niessner, R. Characterization of municipal solid waste incineration (MSWI) bottom ash by scanning electron microscopy and quantitative energy dispersive X-ray microanalysis (SEM/EDX). Fresenius’ J. Anal. Chem. 2001, 370, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Šyc, M.; Krausová, A.; Kameníková, P.; Šomplák, R.; Pavlas, M.; Zach, B.; Pohořelý, M.; Svoboda, K.; Punčochář, M. Material analysis of Bottom ash from waste-to-energy plants. Waste Manag. 2018, 73, 360–366. [Google Scholar] [CrossRef]
- Zevenbergen, C.; Van Reeuwijk, L.; Bradley, J.P.; Comans, R.N.J.; Schuiling, R.D. Weathering of MSWI bottom ash with emphasis on the glassy constituents. J. Geochem. Explor. 1998, 62, 293–298. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W. Understanding the chemical and mineralogical properties of the inorganic portion of MSWI bottom ash. Waste Manag. 2010, 30, 1509–1520. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W. Characterization of MSWI fly ash through mineralogy and water extraction. Resour. Conserv. Recycl. 2011, 55, 524–534. [Google Scholar] [CrossRef]
- Eusden, J.D.; Eighmy, T.T.; Hockert, K.; Holland, E.; Marsella, K. Petrogenesis of municipal solid waste combustion bottom ash. Appl. Geochem. 1999, 14, 1073–1091. [Google Scholar] [CrossRef]
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F. Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. J. Hazard. Mater. 2011, 187, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Alam, Q.; Schollbach, K.; Rijnders, M.; Van Hoek, C.; Van Der Laan, S.; Brouwers, H.J.H. The immobilization of potentially toxic elements due to incineration and weathering of bottom ash fines. J. Hazard. Mater. 2019, 379, 120798. [Google Scholar] [CrossRef]
- Caviglia, C.; Confalonieri, G.; Corazzari, I.; Destefanis, E.; Mandrone, G.; Pastero, L.; Boero, R.; Pavese, A. Effects of particle size on properties and thermal inertization of bottom ashes (MSW of Turin’s incinerator). Waste Manag. 2019, 84, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Inkaew, K.; Saffarzadeh, A.; Shimaoka, T. Modeling the formation of the quench product in municipal solid waste incineration (MSWI) bottom ash. Waste Manag. 2016, 52, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, L.; Carsana, M.; Cassago, D.; Curzio, A.Q.; Collepardi, M. MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 2004, 34, 1899–1906. [Google Scholar] [CrossRef]
- Forteza, R.; Far, M.; Seguı, C.; Cerdá, V. Characterization of bottom ash in municipal solid waste incinerators for its use in road base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Hjelmar, O.; Holm, J.; Crillesen, K. Utilisation of MSWI bottom ash as sub-base in road construction: First results from a large-scale test site. J. Hazard. Mater. 2007, 139, 471–480. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: First results about characterization and reuse. Waste Manag. 2020, 116, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Council Regulation (EU) 2017/997 of 8 June 2017 amending Annex III to Directive 2008/98/EC of the European Parliament and of the Council as regards the hazardous property HP 14 ‘Ecotoxic’ (Text with EEA relevance); Official Journal of the European Union: Luxembourg, Luxembourg, 14 June 2017.
- Wiles, C.C. Municipal solid waste combustion ash: State-of-the-knowledge. J. Hazard. Mater. 1996, 47, 325–344. [Google Scholar] [CrossRef]
- Loginova, E.; Volkov, D.S.; Van De Wouw, P.M.F.; Florea, M.V.A.; Brouwers, H.J.H. Detailed characterization of particle size fractions of municipal solid waste incineration bottom ash. J. Clean. Prod. 2019, 207, 866–874. [Google Scholar] [CrossRef]
- Alam, Q.; Lazaro, A.; Schollbach, K.; Brouwers, H.J.H. Chemical speciation, distribution and leaching behavior of chlorides from municipal solid waste incineration bottom ash. Chemosphere 2020, 241, 124985. [Google Scholar] [CrossRef]
- Šyc, M.; Simon, F.G.; Hyks, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Bourtsalas, A.; Vandeperre, L.J.; Grimes, S.M.; Themelis, N.; Cheeseman, C.R. Production of pyroxene ceramics from the fine fraction of incinerator bottom ash. Waste Manag. 2015, 45, 217–225. [Google Scholar] [CrossRef]
- Hyks, J.; Astrup, T. Influence of operational conditions, waste input and ageing on contaminant leaching from waste incinerator bottom ash: A full-scale study. Chemosphere 2009, 76, 1178–1184. [Google Scholar] [CrossRef]
- Ente Nazionale Italiano di Unificazione. Tests to Determine the Geometric Characteristics of Aggregates—Part 2: Determination of the Particle Size Distribution—Control Sieves, Nominal Dimensions of the Openings, UNI EN 933-2: 2020; Ente Nazionale Italiano di Unificazione: Milan, Italy, 2020. [Google Scholar]
- Funari, V.; Braga, R.; Bokhari, S.N.H.; Dinelli, E.; Meisel, T. Solid residues from Italian municipal solid waste incinerators: A source for “critical” raw materials. Waste Manag. 2015, 45, 206–216. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Gómez-Manrique, J.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Material characterization of the MSWI bottom ash as a function of particle size. Effects of glass recycling over time. Sci. Total. Environ. 2017, 581, 897–905. [Google Scholar]
- Fuller, W.B.; Thompson, S.E. The laws of proportioning concrete. Trans. Am. Soc. Civ. Eng. 1907, 33, 222–298. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Accuracy of XRPD QPA using the combined Rietveld–RIR method. J. Appl. Cryst. 2000, 33, 267–278. [Google Scholar] [CrossRef]
- Pagani, A.; Francescon, F.; Pavese, A.; Diella, V. Sanitary-ware vitreous body characterization method by optical microscopy, elemental maps, image processing and X-ray powder diffraction. J. Eur. Ceram. Soc. 2010, 30, 1267–1275. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows, version 25.0; IBM Corporation: Armonk, NY, USA, 2017.
- Rankin, G.A. The ternary system CaO–Al2 O3–SiO2, with optical study by F. E. Wright. Am. J. Sci. 1915, 39, 1–79. [Google Scholar] [CrossRef]
- Perugini, D.; De Campos, C.P.; Ertel-Ingrisch, W.; Dingwell, D.B. The space and time complexity of chaotic mixing of silicate melts: Implications for igneous petrology. Lithos 2012, 155, 326–340. [Google Scholar] [CrossRef]
- Qiao, X.C.; Tyrer, M.; Poon, C.S.; Cheeseman, C.R. Novel cementitious materials produced from incinerator bottom ash. Resour. Conserv. Recycl. 2008, 52, 496–510. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Josa, A.; Vázquez, E.; López-Soler, Á. Comparison between laboratory and field leachability of MSWI bottom ash as a road material. Sci. Total. Environ. 2008, 389, 10–19. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Kuo, J.-J.; Lin, S.-H. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Lau, R. Conversion of municipal solid waste incineration bottom ash to sorbent material for pollutants removal from water. J. Taiwan Inst. Chem. Eng. 2016, 60, 275–286. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, S.; Liu, L.; Wang, X.; Zhang, Z. Recycling ground MSWI bottom ash in cement composites: Long-term environmental impacts. Waste Manag. 2018, 78, 841–848. [Google Scholar] [CrossRef]
- Funari, V.; Mantovani, L.; Vigliotti, L.; Dinelli, E.; Tribaudino, M. Understanding room-temperature magnetic properties of anthropogenic ashes from municipal solid waste incineration to assess potential impacts and resources. J. Clean. Prod. 2020, 262, 121209. [Google Scholar] [CrossRef]
- Meima, A.J.; Van Der Weijden, R.D.; Eighmy, T.T.; Comans, R.N.J. Carbonation processes in municipal solid waste incinerator bottom ash and their effect on the leaching of copper and molybdenum. Appl. Geochem. 2002, 17, 1503–1513. [Google Scholar] [CrossRef]




| Major Elements (g/100 g) | Grain Size (mm) | CRM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >16 | 8–16 | 4–8 | 2–4 | 1–2 | 0.5–1 | 0.3–0.5 | 0.2–0.3 | 0.063–0.2 | <0.063 | m.v | c.v. | |
| SiO2 | 46.23 | 45.82 | 38.95 | 37.75 | 35.91 | 31.72 | 29.20 | 25.71 | 24.85 | 23.71 | n.a | n.a |
| CaO | 21.60 | 21.78 | 24.79 | 24.66 | 24.63 | 26.67 | 28.41 | 29.67 | 30.12 | 30.05 | n.a | n.a |
| Al2O3 | 8.59 | 8.26 | 8.92 | 10.39 | 10.05 | 9.63 | 8.96 | 8.27 | 8.46 | 8.27 | n.a | n.a |
| MgO | 3.71 | 3.95 | 3.64 | 4.33 | 4.42 | 3.91 | 3.59 | 3.26 | 3.15 | 3.23 | n.a | n.a |
| Fe2O3 | 3.04 | 3.01 | 3.60 | 3.63 | 4.05 | 3.74 | 3.48 | 2.64 | 2.49 | 2.21 | 1.6 | 1.3 |
| Na2O | 3.92 | 3.69 | 2.78 | 2.49 | 2.36 | 2.20 | 2.18 | 2.14 | 2.26 | 2.20 | 2.6 | 3.5 |
| P2O5 | 1.52 | 1.30 | 1.59 | 2.22 | 2.11 | 2.10 | 2.19 | 2.00 | 1.91 | 1.82 | n.a | n.a |
| K2O | 1.21 | 1.29 | 1.26 | 1.36 | 1.45 | 1.35 | 1.29 | 1.23 | 1.19 | 1.11 | n.a | n.a |
| TiO2 | 0.69 | 0.64 | 0.78 | 0.86 | 0.90 | 0.97 | 0.92 | 0.90 | 0.90 | 0.90 | n.a | n.a |
| MnO | 0.09 | 0.08 | 0.11 | 0.09 | 0.10 | 0.10 | 0.10 | 0.09 | 0.09 | 0.10 | 0.08 | 0.08 |
| LOI | 9.40 | 10.19 | 13.60 | 12.22 | 14.02 | 17.62 | 19.69 | 24.08 | 24.58 | 26.40 | n.a | n.a |
| Minor and trace elements (mg/Kg) | ||||||||||||
| S | 8070 | 7780 | 9600 | 9500 | 10,900 | 12,840 | 14,780 | 16,910 | 17,740 | 16,420 | n.a | n.a |
| Cl | 5430 | 5430 | 7420 | 8050 | 8236 | 9780 | 10,120 | 10,530 | 10,960 | 11,600 | n.a | n.a |
| Cu | 1261 | 1413 | 1104 | 1669 | 1335 | 1640 | 1885 | 1664 | 1637 | 2041 | 980 | 1050 |
| Zn | 1380 | 4400 | 1660 | 3630 | 3830 | 4270 | 5750 | 5940 | 6830 | 8740 | 15,714 | 16,800 |
| Ba | 955 | 907 | 1098 | 1684 | 1461 | 1681 | 1618 | 1631 | 1529 | 1879 | 4141 | 4650 |
| Pb | 1354 | 409 | 312 | 545 | 676 | 800 | 802 | 913 | 981 | 1172 | 5216 | 5000 |
| Cr | 880 | 629 | 573 | 434 | 651 | 644 | 697 | 592 | 621 | 627 | 794 | 810 |
| Sr | 429 | 378 | 436 | 681 | 499 | 485 | 517 | 571 | 568 | 572 | n.a | n.a |
| Zr | 256 | 212 | 186 | 207 | 196 | 173 | 169 | 155 | 156 | 143 | n.a | n.a |
| Ni | 119 | 183 | 140 | 135 | 174 | 144 | 179 | 132 | 134 | 160 | 123 | 117 |
| Co | 27 | 27 | 32 | 25 | 62 | 51 | 57 | 44 | 44 | 43 | 29 | 26.7 |
| V | 61 | 69 | 73 | 84 | 84 | 90 | 79 | 78 | 78 | 79 | 113 | 35 |
| As | 54 | 26 | 41 | 36 | 47 | 52 | 56 | 59 | 59 | 74 | 50 | 54 |
| Ce | 37 | 44 | 41 | 46 | 47 | 48 | 36 | 29 | 38 | 51 | 41 | 47.7 |
| Sn | 19 | 17 | 19 | 44 | 36 | 53 | 43 | 49 | 58 | 91 | n.a | n.a |
| Rb | 32 | 34 | 31 | 30 | 32 | 30 | 27 | 27 | 25 | 24 | 85 | 102 |
| La | 18 | 35 | 13 | 8 | 25 | 30 | 21 | 6 | 14 | 16 | 28 | 30.2 |
| Y | 14 | 16 | 16 | 17 | 19 | 15 | 14 | 12 | 12 | 11 | n.a | n.a |
| Nd | 18 | 19 | 10 | 10 | 22 | 18 | 20 | 2 | 19 | 5 | n.a | n.a |
| Mo | 15 | 10 | 15 | 11 | 17 | 14 | 13 | 15 | 14 | 14 | n.a | n.a |
| Ga | 14 | 13 | 12 | 13 | 13 | 13 | 14 | 14 | 13 | 14 | n.a | n.a |
| Nb | 11 | 10 | 11 | 12 | 12 | 11 | 10 | 10 | 11 | 10 | n.a | n.a |
| Sc | <3 | 6 | 10 | 15 | 10 | 6 | 13 | 14 | 18 | 14 | 3.4 | 2.91 |
| Th | 13 | 7 | 6 | 5 | 7 | 7 | 7 | 8 | 9 | 9 | 6 | 5.28 |
| Hf | <3 | 7 | 6 | 4 | 3 | <3 | <3 | <3 | <3 | <3 | 7 | 4.85 |
| U | <3 | 3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | n.a | n.a |
| Phases | Grain Size (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <0.063 | 0.063–0.2 | 0.2–0.3 | 0.3–0.5 | 0.5–1 | 1–2 | 2–4 | 4–8 | 8–16 | >16 | |
| Calcite CaCO3 | 10 | 11 | 10 | 9 | 8 | 8 | 5 | 7 | 5 | 7 |
| Quartz SiO2 | 3 | 4 | 2 | 6 | 3 | 5 | 6 | 6 | 4 | 5 |
| Strätlingite Ca2Al2SiO7·8H2O | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 |
| Ettringite Ca6Al2(SO4)3(OH)12·26H2O | 4 | 6 | 5 | 3 | 3 | 2 | 1 | 1 | 3 | 3 |
| Hydrocalumite Ca4Al2(OH)12(Cl,CO3,OH)2·4H2O | 3 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 2 |
| Anorthite CaAl2Si2O8 | 2 | 2 | 2 | 7 | 2 | 3 | 3 | 2 | 1 | 2 |
| Vaterite CaCO3 | 3 | 3 | 4 | 4 | 2 | 3 | 1 | 2 | 2 | 2 |
| Gehlenite Ca2Al(AlSiO7) | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Hematite Fe2O3 | 1 | 1 | 1 | 1 | 3 | 1 | <1 | <1 | <1 | <1 |
| Magnetite Fe3O4 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Crystalline | 30 | 36 | 33 | 36 | 28 | 27 | 23 | 24 | 20 | 25 |
| Amorphous | 70 | 64 | 67 | 64 | 72 | 73 | 77 | 76 | 80 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovani, L.; Tribaudino, M.; Matteis, C.D.; Funari, V. Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash. Sustainability 2021, 13, 1911. https://doi.org/10.3390/su13041911
Mantovani L, Tribaudino M, Matteis CD, Funari V. Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash. Sustainability. 2021; 13(4):1911. https://doi.org/10.3390/su13041911
Chicago/Turabian StyleMantovani, Luciana, Mario Tribaudino, Chiara De Matteis, and Valerio Funari. 2021. "Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash" Sustainability 13, no. 4: 1911. https://doi.org/10.3390/su13041911
APA StyleMantovani, L., Tribaudino, M., Matteis, C. D., & Funari, V. (2021). Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash. Sustainability, 13(4), 1911. https://doi.org/10.3390/su13041911