Swelling Properties and Permeability of GMZ Bentonite-Sand Mixtures during Different Solutions Infiltration
Abstract
1. Introduction
2. Experimental Investigation
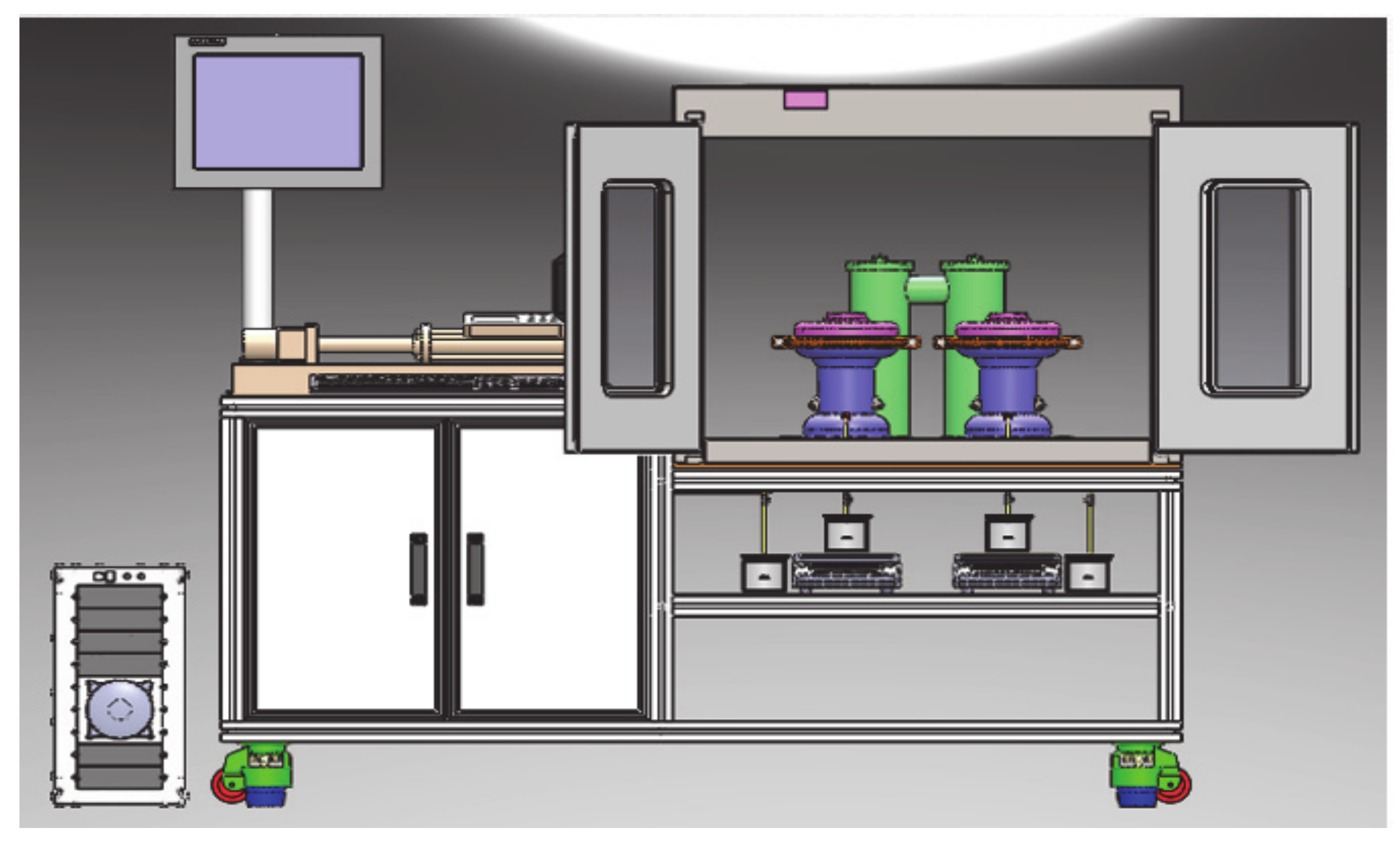
2.1. Apparatus
2.2. Testing Materials and Specimen Preparation
2.3. Test Procedures
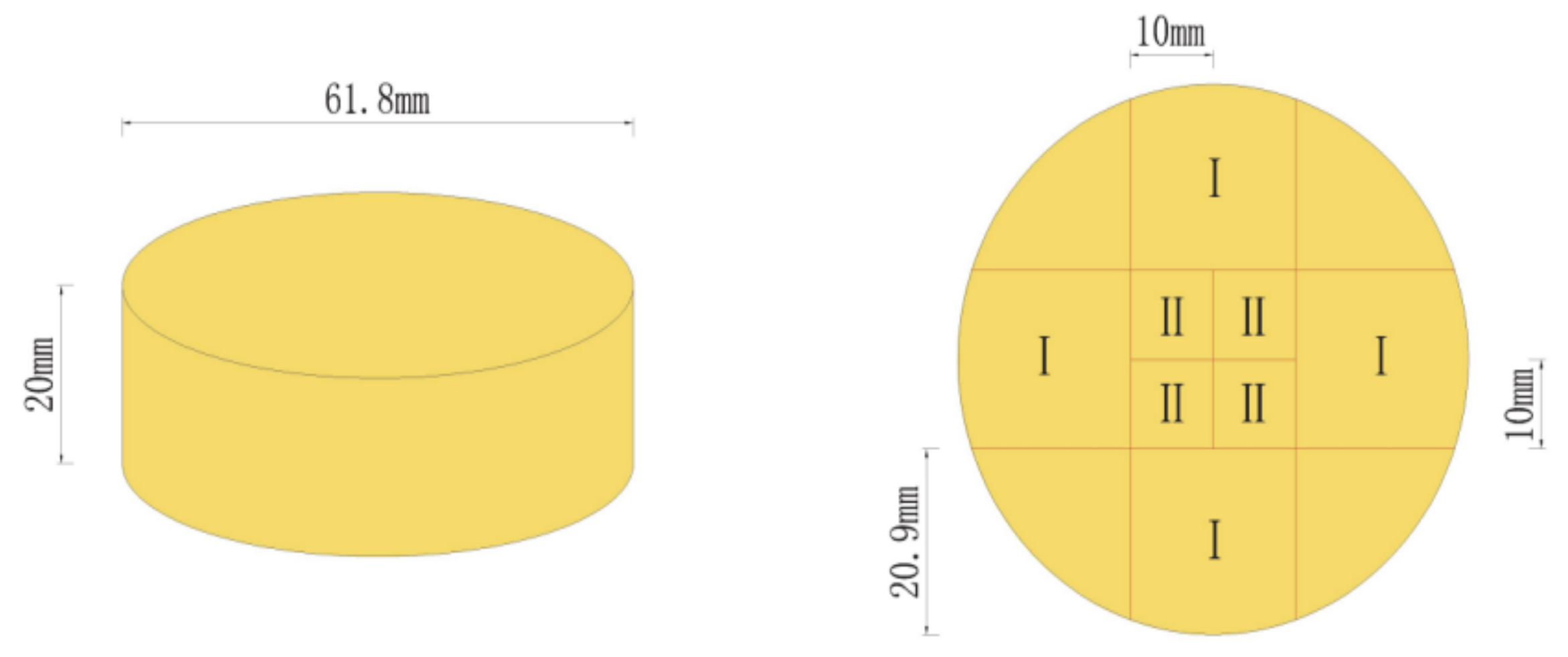
2.4. Specimen Cutting
3. Experimental Results and Discussion
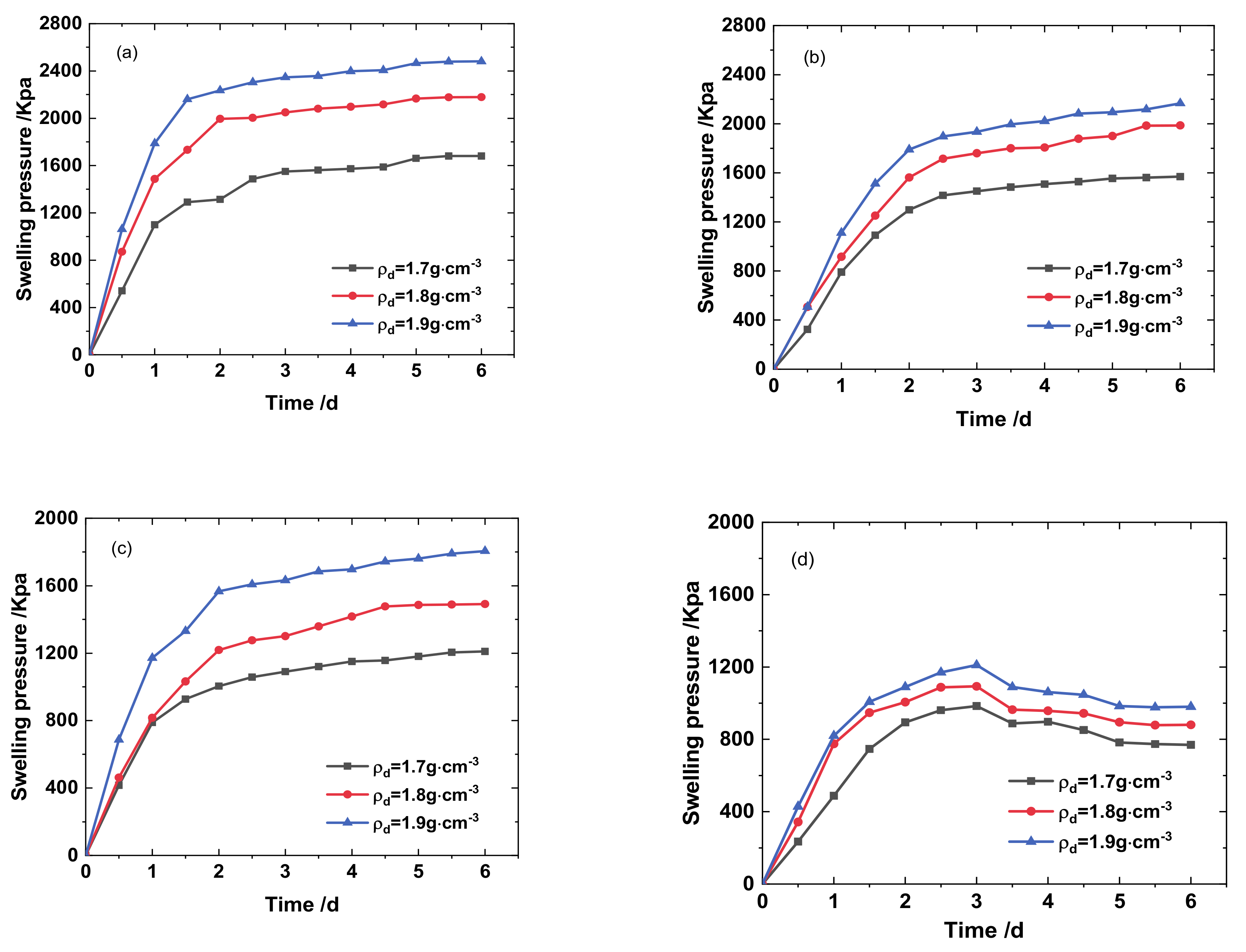
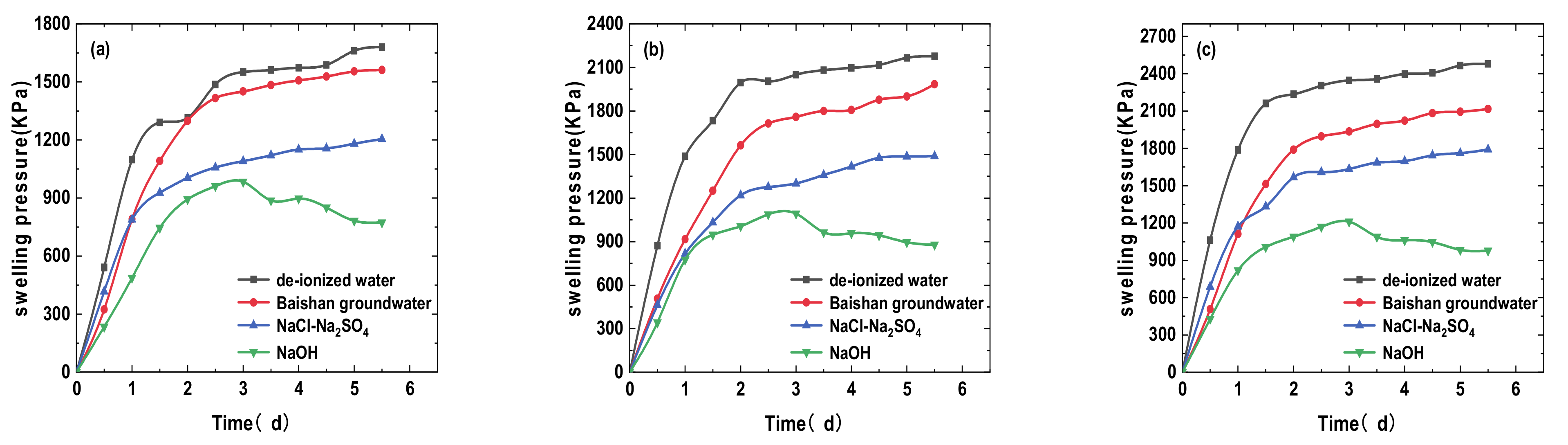
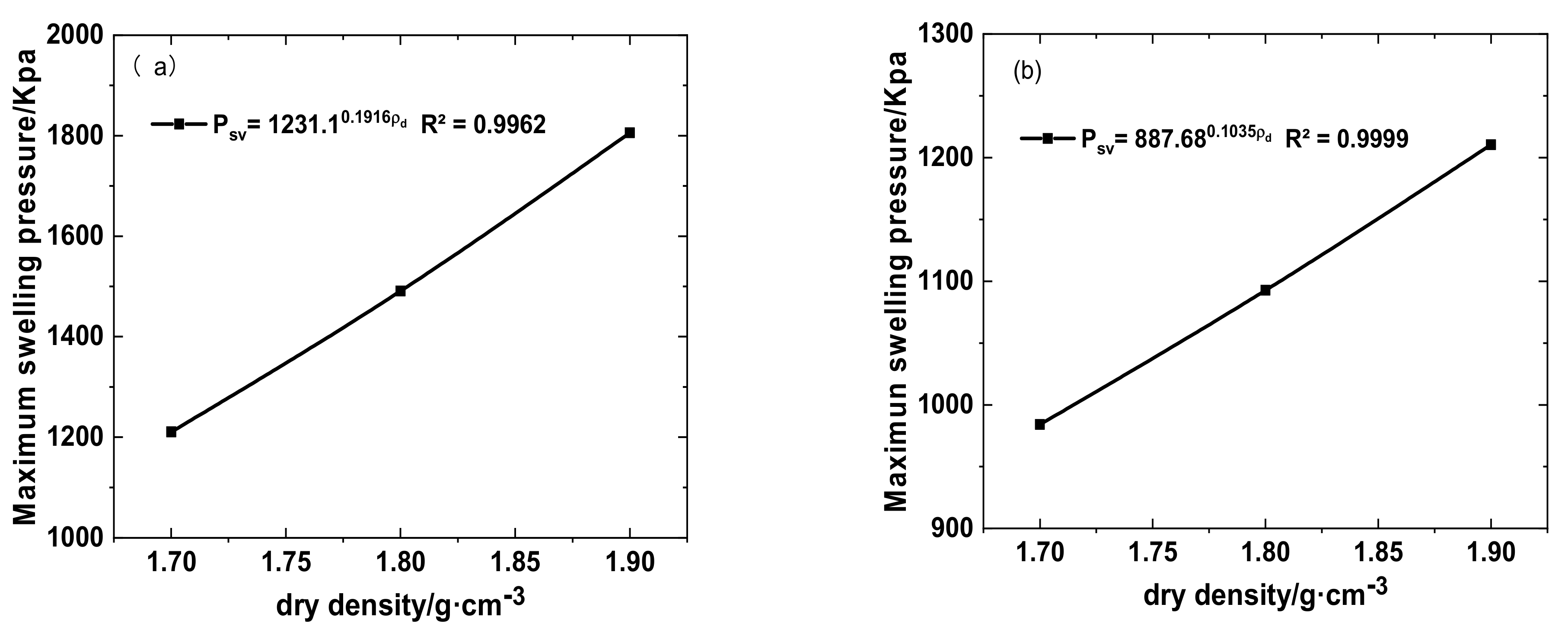
3.1. Effects of Different Solution Infiltration on Swelling Pressure of Bentonite-Sand Mixtures
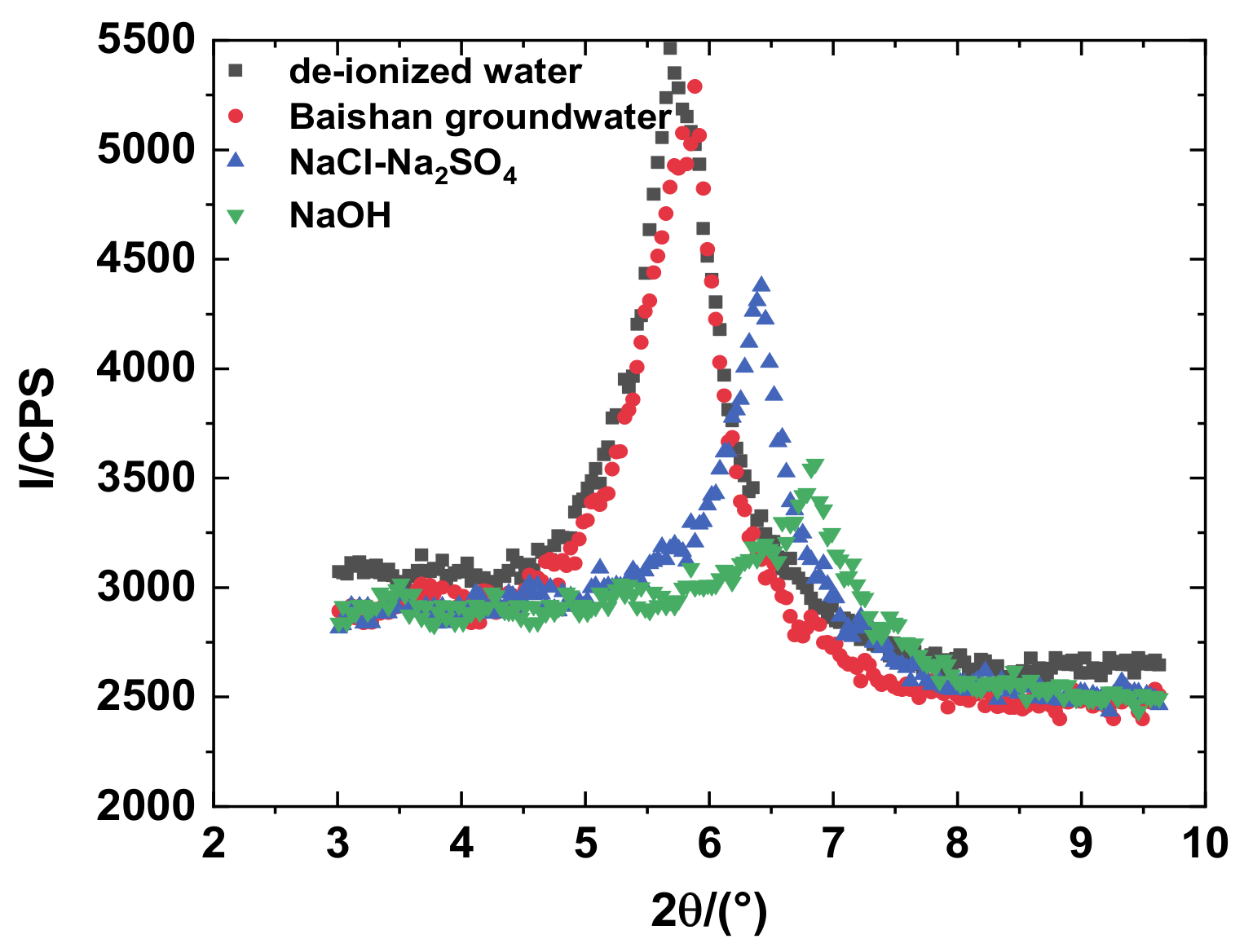
3.2. Effects of pH Value on Mineralogy
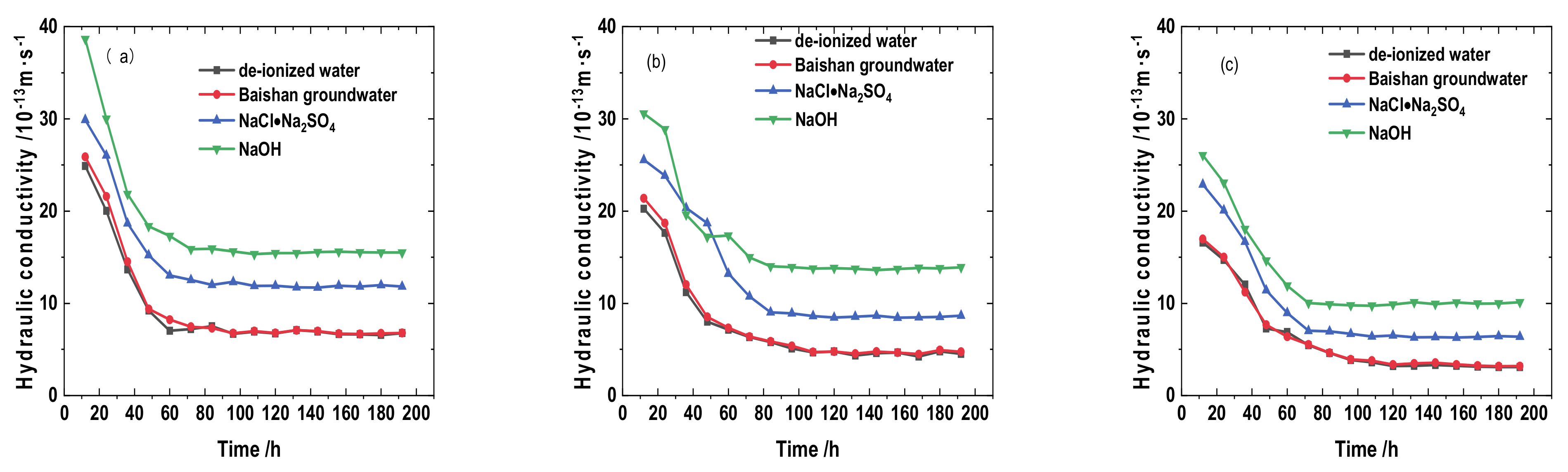
3.3. Solution Infiltration Influences on Saturated Permeability Coefficient
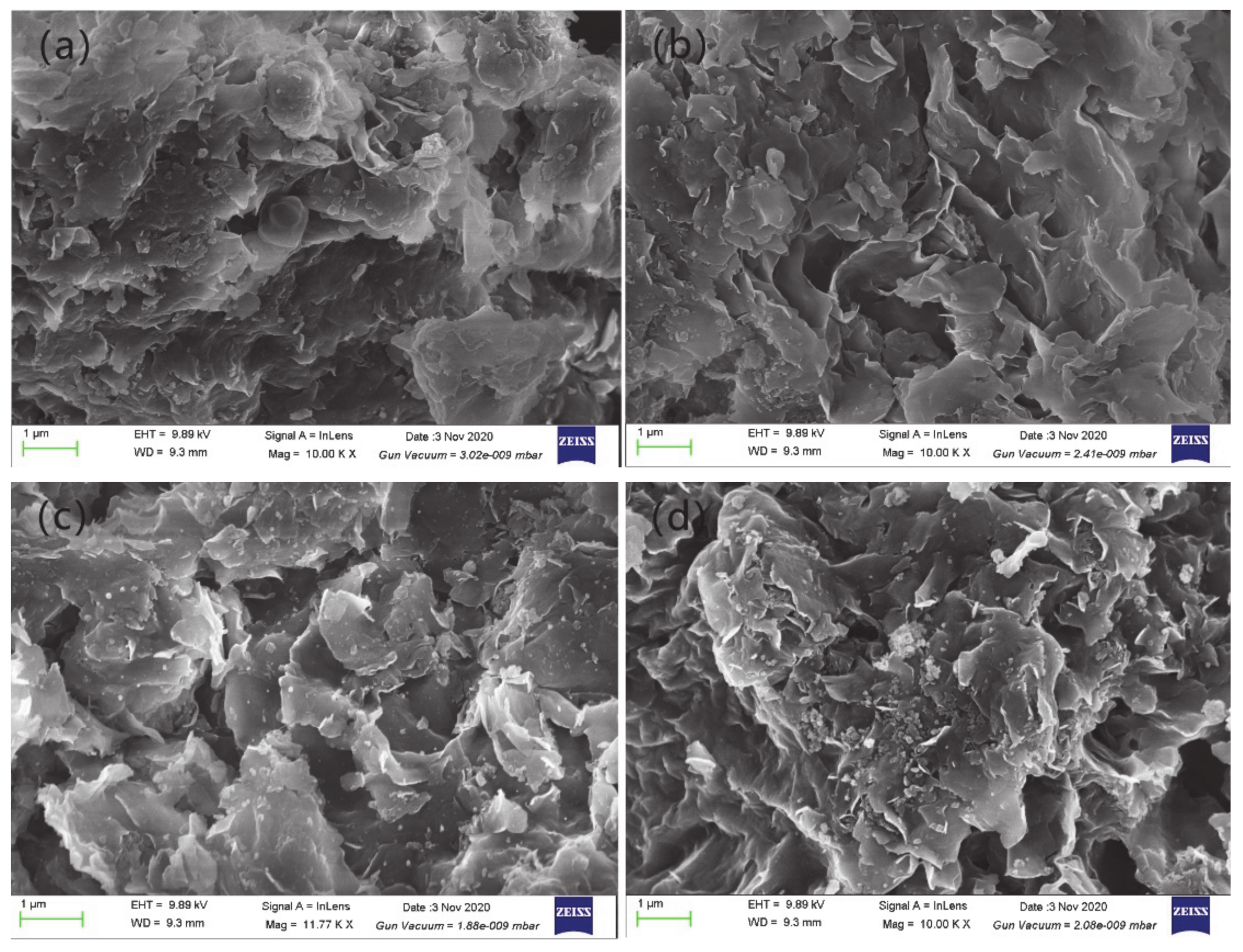
3.4. Characterization of Microstructure
4. Conclusions
- (1)
- The swelling and hydraulic tests were carried out on GMZ bentonite-sand mixtures with different dry densities (1.7, 1.8, and 1.9 g/cm3) and infiltrating solutions: DI water, simulated Baishan groundwater, NaCl-Na2SO4 solution, and NaOH solution. Each solution had various chemical reactions with the mixtures, causing various swelling pressure (SWP) and mineralogy. The value of SWP was the maximum when infiltrated with DI water (>2.5 MPa), decreased slightly in contact with simulated Baishan groundwater (>2 MPa), and was the smallest when infiltrated with NaOH solutions (<1 MPa). The SWP of the mixture increased with time in various solution media, and the history curve of SWP experienced three stages. The influence of NaOH solution on the SWP of the mixtures primarily happened in the second stage. The evolution of the solution on the hydraulic conductivity of samples first decreased and then tended to be stable, and the permeability coefficient decreased with dry density rise. The concentration of ionic solution had a significant effect on the permeability coefficient of mixed soil, which was greater than DI water.
- (2)
- XRD test results indicated that after the samples were permeated by ionic solution, and the characteristic peak layer spacing of montmorillonite changed in different degrees. The crest value of diffraction intensity of montmorillonite diminished with the increasing solution concentration, which indicates that montmorillonite dissolved with high salt and high alkali.
- (3)
- The results of FESEM showed the denudation and breakage of montmorillonite aggregates and the formation of cracks. After hydration with deionized water and simulated Baishan groundwater, it was found that the layered montmorillonite structure was basically intact and smooth and had smooth wing-like montmorillonite aggregates with good shape characteristics. The wing-like montmorillonite aggregates at the surface might have blocked the pores. After hydration with hyper-alkaline and hyper-salinity solutions, lamellar montmorillonite had obvious lamellar dissolution traces, and the dissolution and denudation degree of hyper-alkaline was more serious than that of hyper-salinity. The integrity of the montmorillonite structural was seriously damaged. The increase in macropores was more obvious, which widened the permeability path of the solution and improved the permeability of bentonite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency IAEA. Geological Disposal Facilities for Radioactive Waste-Specific Safety Guide No. SSG-14. 2011. Available online: https://www.iaea.org/publications/8535/geological-disposal-facilities-for-radioactive-waste (accessed on 15 July 2020).
- Yoon, S.; Jeon, J.S.; Kim, G.Y.; Seong, J.H.; Baik, M.H. Specific heat capacity model for compacted bentonite buffer materials. Ann. Nucl. Energy 2019, 125, 18–25. [Google Scholar] [CrossRef]
- Bossart, P.; Bernier, F.; Birkholzer, J.; Bruggeman, C.; Connolly, P.; Dewonck, S.; Fukaya, M.; Herfort, M.; Jensen, M.; Matray, J.M.; et al. Mont Terri rock laboratory, 20 years of research: Introduction, site characteristics and overview of experiments. Swiss J. Geosci. 2017, 110, 3–22. [Google Scholar] [CrossRef]
- Takeuchi, M.R.H.; Hasegawa, T.; McKinley, L.; Marquez, G.P.; Ishihara, K.N. What is suitable leadership for high-level radioactive waste (HLW) management. Sustainability 2020, 12, 8691. [Google Scholar] [CrossRef]
- Komine, H.; Ogata, N. Experimental study on swelling characteristics of sand-bentonite mixture for nuclear waste disposal. Soils Found. 1999, 39, 83–97. [Google Scholar] [CrossRef]
- Villar, M.V.; Lloret, A. Influence of temperature on the hydromechanical behaviour of a compacted bentonite. Appl. Clay Sci. 2004, 26, 337–350. [Google Scholar] [CrossRef]
- Siddiqua, S.; Blatz, J.; Siemens, G. Evaluation of the impact of pore fluid chemistry on the hydromechanical behaviour of clay-based sealing materials. Can. Geotech. J. 2011, 48, 199–213. [Google Scholar] [CrossRef]
- Rao, S.M.; Ravi, K. Influence of initial degree of saturation on swell pressures of compacted Barmer bentonite specimens. Ann. Nucl. Energy 2015, 80, 303–311. [Google Scholar] [CrossRef]
- Jia, L.Y.; Chen, Y.G.; Ye, W.M.; Cui, Y.J. Effects of a simulated gap on anisotropic swelling pressure of compacted GMZ bentonite. Eng. Geol. 2019, 248, 155–163. [Google Scholar] [CrossRef]
- Zhu, C.M.; Ye, W.M.; Chen, Y.G.; Chen, B.; Cui, Y.J. Influence of salt solutions on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Eng. Geol. 2013, 166, 74–80. [Google Scholar] [CrossRef]
- Chen, B.; Guo, J.X.; Zhang, H.X. Alteration of compacted GMZ bentonite by infiltration of alkaline solution. Clay Miner. 2016, 51, 237–247. [Google Scholar]
- Ye, W.M.; Zheng, Z.J.; Chen, B.; Chen, Y.G.; Cui, Y.J.; Wang, J. Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl. Clay Sci. 2014, 101, 192–198. [Google Scholar] [CrossRef]
- Studds, P.G.; Stewart, D.I.; Cousens, T.W. The effect of ion valance on the swelling behaviour of sodium bentonite. In Polluted and Marginal Land-96, Proceeding of the 4th International Conference on Re-Use of Contaminated Land and Landfills, London, UK, 2–4 July 1996; Engineering Technics Press: Edinburgh, Scotland, 2–4 July 1996; pp. 139–142. [Google Scholar]
- Karnland, O.; Olsson, S.; Nilsson, U.; Sellin, P. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys. Chem. Earth 2007, 32, 275–286. [Google Scholar] [CrossRef]
- Castellanos, E.; Villar, M.V.; Romero, E.; Lloret, A.; Gens, A. Chemical impact on the hydro-mechanical behaviour of high-density FEBEX bentonite. Phys. Chem. Earth Parts A/B/C 2008, 33, S516–S526. [Google Scholar] [CrossRef]
- Lee, J.O.; Lim, J.G.; Kang, I.M.; Kwon, S. Swelling pressures of compacted Ca-bentonite. Eng. Geol. 2012, 129, 20–26. [Google Scholar] [CrossRef]
- Sun, D.A.; Zhang, L. Swelling characteristics of Gaomiaozi bentonite saturated by salt solution and their prediction. Rock Soil Mech. 2013, 34, 2790–2795. (In Chinese) [Google Scholar]
- Karnland, O.; Nilsson, U.; Weber, H.; Wersin, P. Sealing ability of Wyoming bentonite pellets foreseen as buffer material-Laboratory results. Phys. Chem. Earth Parts A/B/C 2008, 33, S472–S475. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Cui, S.L.; Zhang, M.; Jia, L.Y. Swelling behaviors of GMZ bentonite-sand mixtures inundated in NaCl-Na2SO4 solutions. Nucl. Eng. Des. 2012, 242, 115–123. [Google Scholar] [CrossRef]
- Nakayama, S.; Sakamoto, Y.; Yamaguchi, T.; Akai, M.; Tanaka, T.; Sato, T.; Iida, Y. Dissolution of montmorillonite in compacted bentonite by highly alkaline aqueous solutions and diffusivity of hydroxide ions. Appl. Clay Sci. 2004, 27, 53–65. [Google Scholar] [CrossRef]
- Alawaji, H.A. Swell and compressibility characteristics of sand-bentonite mixtures inundated with liquids. Appl. Clay Sci. 1999, 15, 411–430. [Google Scholar] [CrossRef]
- Cui, Y.J.; Tang, A.M.; Qian, L.X.; Ye, W.M.; Chen, B. Thermal-mechanical behaviour of compacted GMZ bentonite. Soils Found. 2011, 51, 1065–1074. [Google Scholar] [CrossRef]
- Bag, R.; Rabbani, A. Effect of temperature on swelling pressure and compressibility characteristics of soil. Appl. Clay Sci. 2017, 136, 1–7. [Google Scholar] [CrossRef]
- Neaman, A.; Pelletier, M.; Villieras, F. The effects of exchanged cation, compression, heating and hydration on textural properties of bulk bentonite and its corresponding purified montmorillonite. Appl. Clay Sci. 2003, 22, 153–168. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakamoto, Y.; Akai, M.; Takazawa, M.; Iida, Y.; Tanaka, T.; Nakayama, S. Experimental and modeling study on long-term alteration of compacted bentonite with alkaline groundwater. Phys. Chem. Earth Parts A/B/C 2007, 32, 298–310. [Google Scholar] [CrossRef]
- Alfatlawi, T.J.M.; Alkafaji, S.S. Investigation of groundwater pollution minimization using compacted bentonite barriers. KSCE J. Civ. Eng. 2019, 23, 507–512. [Google Scholar] [CrossRef]
- Balagosa, J.; Yoon, S.; Choo, Y.W. Experimental investigation on small-strain dynamic properties and unconfined compressive strength of gyeongju compacted bentonite for nuclear waste repository. KSCE J. Civ. Eng. 2020, 24, 2657–2668. [Google Scholar] [CrossRef]
- Wen, Z.J. Physical property of China’s buffer material for high-level radioactive waster repositories. Chin. J. Rock Mech. Eng. 2006, 25, 794–800. (In Chinese) [Google Scholar]
- Liu, L.N.; Chen, Y.G.; Ye, W.M.; Cui, Y.J.; Wu, D.B. Effects of hyperalkaline solutions on the swelling pressure of compacted Gaomiaozi (GMZ) bentonite from the viewpoint of Na+ cations and OH− anions. Appl. Clay Sci. 2018, 161, 334–342. [Google Scholar] [CrossRef]
- Herbert, H.J.; Kasbohm, J.; Sprenger, H.; Fernandez, A.M.; Reichelt, C. Swelling pressures of MX-80 bentonite in solutions of different ionic strength. Phys. Chem. Earth Parts A/B/C 2008, 33, S327–S342. [Google Scholar] [CrossRef]
- Ye, W.M.; Zheng, Z.J.; Chen, B.; Chen, Y.G. Progress of research on the influence of alkaline cation and alkaline solution on bentonite properties. World Nucl. Geosci. 2011, 28, 221–227. (In Chinese) [Google Scholar]
- Sanchez, L.; Cuevas, J.; Ramirez, S.; Leon, D.R.D.; Fernandez, R.; Villa, R.V.D.; Leguey, S. Reaction kinetics of FEBEX bentonite in hyperalkaline conditions resembling the cement-bentonite interface. Appl. Clay Sci. 2006, 33, 125–141. [Google Scholar] [CrossRef]
- Bauer, A.; Velde, B. Smectite transformation in high molar KOH solutions. Clay Miner. 1999, 34, 259–273. [Google Scholar] [CrossRef]
- Xiang, G.S.; Xu, Y.F.; Wang, Y.; Fang, Y. Change Law of the Swelling Deformation of GMZ Bentonite Corroded by Alkaline Pore Water. J. Shanghai Jiao Tong Univ. 2018, 52, 141–146. (In Chinese) [Google Scholar]
- Ye, W.M.; Wan, M.; Chen, B.; Chen, Y.G.; Cui, Y.J.; Wang, J. Temperature effects on the unsaturated permeability of the densely compacted GMZ01 bentonite under confined conditions. Eng. Geol. 2012, 126, 1–7. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, H.H.; Wen, Y.N.; Shi, B.; Gao, L. Experimental investigation of consolidation properties of nano-bentonite mixed clayey soil. Sustainability 2020, 12, 459. [Google Scholar] [CrossRef]
- Studds, P.G.; Stewart, D.I.; Cousens, T.W. The effects of salt solutions on the properties of bentonite-sand mixtures. Clay Miner. 1998, 33, 651–660. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ohtsubo, M.; Li, L.Y.; Higashi, T.; Park, J. Effect of salt of various concentrations on liquid limit and hydraulic conductivity of different soil-bentonite mixtures. Environ. Geol. 2009, 57, 1145–1153. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.T.; Yi, F.C.; Niu, S.J.; Ma, P.J. Research on permeability of high compacted sand-bentonite mixtures as buffer material. Sci. Technol. Eng. 2017, 17, 231–235. (In Chinese) [Google Scholar]

| No | Dry Density (g/cm3) | Pore Solution | PH |
|---|---|---|---|
| 1 | 1.70 | DI water | 7 |
| 2 | simulated Baishan groundwater | 8.2 | |
| 3 | NaCl-Na2SO4 solution | 7 | |
| 4 | NaOH solution | 13.8 | |
| 5 | 1.80 | DI water | 7 |
| 6 | simulated Baishan groundwater | 8.2 | |
| 7 | NaCl-Na2SO4 solution | 7 | |
| 8 | NaOH solution | 13.8 | |
| 9 | 1.90 | DI water | 7 |
| 10 | simulated Baishan groundwater | 8.2 | |
| 11 | NaCl-Na2SO4 solution | 7 | |
| 12 | NaOH solution | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-P.; Wang, Z.; Zhao, Y.; Yi, F.-C.; Zhu, B.-L. Swelling Properties and Permeability of GMZ Bentonite-Sand Mixtures during Different Solutions Infiltration. Sustainability 2021, 13, 1622. https://doi.org/10.3390/su13041622
Wang Y-P, Wang Z, Zhao Y, Yi F-C, Zhu B-L. Swelling Properties and Permeability of GMZ Bentonite-Sand Mixtures during Different Solutions Infiltration. Sustainability. 2021; 13(4):1622. https://doi.org/10.3390/su13041622
Chicago/Turabian StyleWang, Yu-Ping, Zhe Wang, Yu Zhao, Fa-Cheng Yi, and Bao-Long Zhu. 2021. "Swelling Properties and Permeability of GMZ Bentonite-Sand Mixtures during Different Solutions Infiltration" Sustainability 13, no. 4: 1622. https://doi.org/10.3390/su13041622
APA StyleWang, Y.-P., Wang, Z., Zhao, Y., Yi, F.-C., & Zhu, B.-L. (2021). Swelling Properties and Permeability of GMZ Bentonite-Sand Mixtures during Different Solutions Infiltration. Sustainability, 13(4), 1622. https://doi.org/10.3390/su13041622




