Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Bacterial Cultures
2.2. Fungal Metabolites Preparations
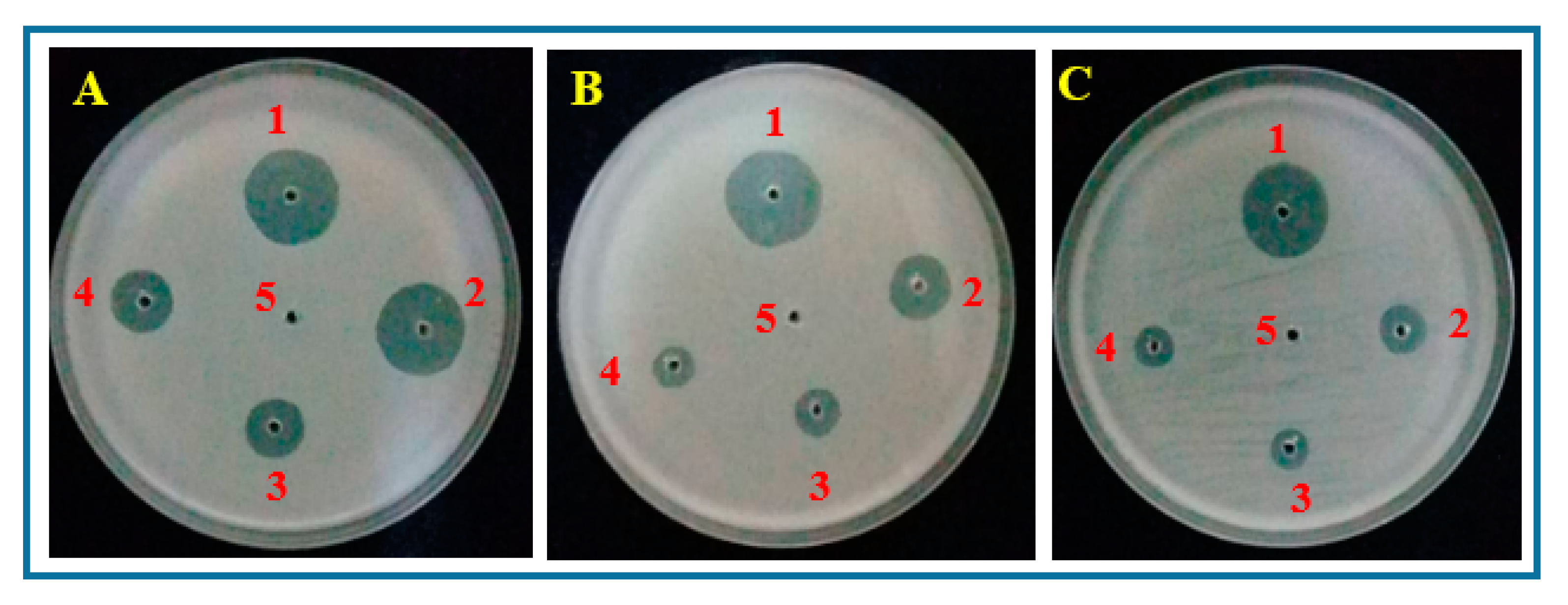
2.3. In Vitro Test
2.4. Morphological Observation of the Bacterial Cells
2.5. In Vivo Test
2.6. Data Parameters
2.7. Bacterial Population in Soil
2.8. Area under Disease Progressive Curve (AUDPC)
2.9. Statistical Analysis
3. Results
3.1. In Vitro Test
3.2. Bacterial Cell Morphology
3.3. Plant Growth
3.4. Soil Bacterial Population
3.5. AUDPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 15 April 2019).
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers (Basel) 2016, 8, E58. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Zhang, Y.; Martin, C. Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep. 2018, 37, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health promoting effects of tomato fruit for biofortified food. Med. Inflamm. 2014. [Google Scholar] [CrossRef]
- Liu, Z.; Alseekh, S.; Brotman, Y.; Zheng, Y.; Fei, Z.; Tieman, D.M. Identification of a Solanum pennellii Chromosome 4 Fruit Flavor and Nutritional Quality-Associated Metabolite QTL. Front. Plant Sci. 2016, 7, 1671. [Google Scholar] [CrossRef][Green Version]
- Huo, J.Y. The current status of tomato industry in China and its safety precautions. Vegetables 2016, 6, 1–4. [Google Scholar]
- Ma, Z.H. The change trend of tomato varieties in China based on the demand of production market. China Veg. 2017, 3, 1–5. [Google Scholar]
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=6123393 (accessed on 5 February 1991). [CrossRef]
- Artal, R.B.; Gopalakrishnan, C.; Thippeswamy, B. An efficient in- oculation method to screen tomato, brinjal and chilli entries for bacterial wilt resistance. Pest Manag. Hortic. Ecosyst. 2012, 18, 70–73. [Google Scholar]
- Singh, S.; Gautam, R.K.; Singh, D.R.; Sharma, T.V.R.S.; Sakthivel, K.; Dam Roy, S. Genetic approaches for mitigating losses caused by bacterial wilt of tomato in tropical islands. Eur. J. Plant Pathol. 2015, 143, 205–222. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.R.; Kumar, K.; Birah, A. Eco-friendly manage- ment modules for bacterial wilt (Ralstonia solanacearum) of tomato for protected cultivation in a tropical island ecosystem. Biol. Agric. Hortic. 2014, 30, 219–227. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial wilt in China: History, current status, and future perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Yuan, S.Q.; Xiong, Z.K.; Lin, M.B. The general situation of related studies on tomato bacterial spot in Guangdong Province. Guangdong Agric. Sci. 2003, 3, 32–34. [Google Scholar]
- Wei, Z.; Yang, X.M.; Yin, S.X.; Shen, Q.R.; Ran, W.; Xu, Y.C. Efficacy of Bacillus -fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl. Soil Ecol. 2011, 48, 152–159. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, J.F.; Hu, J.; Gu, Y.A.; Yang, C.L.; Mei, X.L.; Shen, Q.R.; Xu, Y.C.; Friman, V.P. Altering transplantation time to avoid periods of high temperature can efficiently reduce bacterial wilt disease incidence with tomato. PLoS ONE 2015, 10, e0139313. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Lowe-Power, T.M.; Hendrich, C.G.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Khokhani, D.; Allen, C. How Ralstonia solanacearum exploits and thrives in the flowing plant xylem environment. Trends Microbiol. 2018, 26, 929–942. [Google Scholar] [CrossRef]
- Graham, J.; Jones, D.A.; Lloyd, A.B. Survival of Pseudomonas solanacearum race-3 in plant debris and in latently infected potato-tubers. Phytopathology 1979, 69, 1100–1103. [Google Scholar] [CrossRef]
- Grey, B.E.; Steck, T.R. The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection. Appl. Environ. Microbiol. 2001, 67, 3866–3872. [Google Scholar] [CrossRef]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, e173. [Google Scholar]
- Meyer, V. Genetic engineering of filamentous fungi-Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species–opportunistic avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C. Tanshinone II A and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Monte, E. Understanding Trichoderma: Between agricultural biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [PubMed]
- Vizcaino, J.A.; Sanz, L.; Cardoza, R.E.; Monte, E.; Gutierrez, S. Detection of putative peptide synthetase genes in Trichoderma species. Application of this method to the cloning of a gene from T. harzianum CECT 2413. FEMS Microbiol. Let. 2005, 244, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2008, 8, 127–139. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Ghisalberti, E.L. Secondary metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium. Basic Biology, Taxonomy and Genetics; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis: London, UK, 1998; Volume 1, pp. 139–191. [Google Scholar]
- Luckner, M. Secondary Metabolism in Microorganisms, Plants and Animals, 3rd ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- Mohamed, B.F.F.; Sallam, N.M.A.; Alamri, S.A.M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt J. Biol. Pest Control 2020, 30, 61. [Google Scholar] [CrossRef]
- Kariuki, C.K.; Mutitu, E.W.; Muiru, W.M. Effect of Bacillus and Trichoderma species in the management of the bacterial wilt of tomato (Lycopersicum esculentum) in the field. Egypt. J. Biol. Pest Control 2020, 30, 109. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef]
- Tong, X.; Shen, X.Y.; Hou, C.L. Antimicrobial activity of fungal endophytes from Vaccinium dunalianum var. urophyllum. Sains. Malaysiana 2018, 47, 1685–1692. [Google Scholar] [CrossRef]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial activity of Aqularia crassna leaf extract against Staphylococcus epidermis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Ahmad, M.; Khan, R.A.A.; Naz, I.; Ali, A.; Alam, S.S. Management of bacterial wilt in tomato using dried powder of Withania coagulan (L) Dunal. Austral. Plant Pathol. 2019, 48, 183–192. [Google Scholar] [CrossRef]
- Gruter, D.; Schmid, B.; Brandl, H. Influence of plant diversity and elevated atmospheric carbon dioxide levels on below ground bacterial diversity. BMC Microbiol. 2006, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Goszczynska, T.; Serfontein, J.J.; Serfontein, S. Media and Diagnostic Tests, Introduction to Practical Phytobacteriology, Bacterial Diseases Unit; ARC-Plant Protection Research Institute: Pretoria, South Africa, 2000; pp. 60–73. [Google Scholar]
- Abdel-Monaaim, M.F.; Abo-Elyousr, K.M.; Morsy, K.M. Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinustermis forsik). Crop Prot. 2011, 30, 185–191. [Google Scholar] [CrossRef]
- Madden, L.V.; Hughes, G.; Van den, B. The Study of Plant Disease Epidemics; APS Press: Minnesota, MN, USA, 2007; Available online: https://apsjournals.apsnet.org/doi/pdf/10.1094/9780890545058.fm (accessed on 7 May 2007).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Xiao-yan, S.; Qing-tao, S.; Shu-tao, X.; Xiu-lan, C.; Cai-yan, S.; Yu-zhong, Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125. [Google Scholar] [CrossRef]
- Utkhede, R.; Koch, C. Biological treatments to control bacterial canker of greenhouse tomatoes. Biocontrology 2004, 49, 305–313. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Detection of viridiofungin A and another antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- Tanaka, J.C.A.; da Silva, C.C.; de Oliveira, A.J.B.; Nakamura, C.V.; Dias, B.P. Antibacterial activity of indole alkaloids from Aspidosperma ramiflorum. Braz. J. Med. Biol. Res. 2006, 39, 387–391. [Google Scholar] [CrossRef]
- Tsuchiya, H. Memberane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophy. Res. Commu. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef] [PubMed]
- Benitez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Contreras-Cornejo, H.A.; Macìas-Rodrìguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Pl. Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Cont. 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Cutler, H.G.; Cox, R.H.; Crumley, F.G.; Cole, P.D. 6-Pentyl-α-pyrone from Trichoderma harzianum: Its plant growth inhibitory and antimicrobial properties. Agric. Biol. Chem. 1986, 50, 2943–2945. [Google Scholar] [CrossRef]
- Cutler, H.G.; Himmetsbach, D.S.; Arrendale, R.F.; Cole, P.D.; Cox, R.H. Koninginin A: A novel plant regulator from Trichoderma koningii. Agri. Biol. Chem. 1989, 53, 2605–2611. [Google Scholar] [CrossRef][Green Version]
- Parker, S.R.; Cutler, H.G.; Jacyno, J.M.; Hill, R.A. Biological activity of 6-pentyl-2H-pyran-2-one and its analogs. J. Agri. Food Chem. 1997, 45, 2774–2776. [Google Scholar] [CrossRef]
- Parker, S.R.; Cutler, H.G.; Schreiner, P.R. Koninginin E: Isolation of a biologically active natural product from Trichoderma koningii. Biosc. Biotechnol. Biochem. 1995, 59, 1747–1749. [Google Scholar] [CrossRef][Green Version]
- Macias, F.A.; Varela, R.M.; Simonet, A.M.; Cutler, H.G.; Cutler, S.J.; Eden, M.A.; Hill, R.A. Bioactive carotanes from Trichoderma virens. J. Nat. Prod. 2000, 63, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Cutler, H.G.; Jacyno, J.M.; Phillips, R.S.; von Tersch, R.L.; Cole, P.D.; Montemurro, N. Cyclonerodiol from a novel source, Trichoderma koningii: Plant growth regulatory activity. Agric. Biol. Chem. 1991, 55, 243–244. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Rowland, C.Y. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 1993, 56, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Bilal, A.; Musharaf, A.; Asad, A.; Ishrat, N.; Muhammad, F. Management of Ralstonia solanacearum (Smith) Yabuuchi wilt in tomato (Solanum lycopersicum L.) with dried powder of the medicinal plant Withania somnifera (L.) Dunal. Pak. J. Bot. 2019, 51, 297–306. [Google Scholar] [CrossRef]





| Application Time (DBT) | Trichoderma spp. | |||
|---|---|---|---|---|
| T. harzianum | T. virens | T. koningii | Control | |
| Experiment I | ||||
| 0 | 32.1 ± 3.1 c | 27.3 ± 2.5 de | 25.2 ± 1.7 e | 24.4 ± 1.8 e |
| 4 | 40.3 ± 3.1 b | 33.4 ± 3.2 c | 27.7 ± 2.7 de | 24.2 ± 1.9 e |
| 8 | 51.3 ± 3.6 a | 39.7 ± 2.9 b | 30.4 ± 2.8 cd | 25.6 ± 2.5 e |
| Experiment II | ||||
| 0 | 35.5 ± 2.8 ef | 33.2 ± 3.1 ef | 30.3 ± 2.8 f | 28.5 ± 2.3 f |
| 4 | 46.1 ± 2.9 b | 41.6 ± 3.2 c | 35.4 ± 3.4 ef | 29.3 ± 2.2 f |
| 8 | 59.2 ± 3.8 a | 47.2 ± 3.5 b | 38.6 ± 3.2 de | 27.4 ± 2.2 f |
| Application Time (DBT) | Trichoderma spp. | |||
|---|---|---|---|---|
| T. harzianum | T. virens | T. koningii | Control | |
| Experiment I | ||||
| 0 | 17.4 ± 2.1 c | 14.3 ± 1.4 ef | 10.5 ± 0.9 gh | 9.2 ± 1.1 hi |
| 4 | 20.6 ± 1.6 b | 16.4 ± 1.3 cd | 12.2 ± 1.0 fg | 9.1 ± 0.8 hi |
| 8 | 27.4 ± 2.4 a | 22.3 ± 2.1 b | 15.6 ± 1.6 de | 8.1 ± 0.9 i |
| Experiment II | ||||
| 0 | 19.2 ± 2.3 c | 16.6 ± 2.1 d | 14.3 ± 1.7 de | 12.4 ± 1.4 e |
| 4 | 23.4 ± 2.1 b | 19.7 ± 1.8 c | 16.2 ± 1.4 d | 13.5 ± 1.2 e |
| 8 | 28.7 ± 3.1 a | 23.3 ± 2.4 b | 19.6 ± 1.8 c | 12.8 ± 1.0 e |
| Application Time (DBT) | Trichoderma spp. | |||
|---|---|---|---|---|
| T. harzianum | T. virens | T. koningii | Control | |
| Experiment I | ||||
| 0 | 23.9 ± 2.6 d | 18.3 ± 2.4 e | 16.5 ± 2.1 e | 15.8 ± 1.8 e |
| 4 | 40.6 ± 3.3 b | 33.3 ± 2.4 c | 26.3 ± 1.9 d | 17.2 ± 1.2 e |
| 8 | 46.3 ± 3.3 a | 38.5 ± 3.1 b | 32.3 ± 2.6 c | 15.0 ± 1.5 e |
| Experiment II | ||||
| 0 | 25.6 ± 2.6 d | 23.2 ± 2.5 de | 19.6 ± 3.2 e | 20.3 ± 2.1 e |
| 4 | 39.2 ± 2.8 b | 31.3 ± 2.7 c | 25.5 ± 2.2 d | 19.2 ± 1.3 e |
| 8 | 47.3 ± 3.1 a | 40.6 ± 2.2 b | 32.2 ± 2.5 c | 18.1 ± 2.1 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Fan, Z.; Yi, X.; Zhang, Y.; Khan, R.A.A.; Zhou, Z. Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp. Sustainability 2021, 13, 1491. https://doi.org/10.3390/su13031491
Guo Y, Fan Z, Yi X, Zhang Y, Khan RAA, Zhou Z. Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp. Sustainability. 2021; 13(3):1491. https://doi.org/10.3390/su13031491
Chicago/Turabian StyleGuo, Yancui, Zhenyu Fan, Xiong Yi, Yuhong Zhang, Raja Asad Ali Khan, and Zhiqiang Zhou. 2021. "Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp." Sustainability 13, no. 3: 1491. https://doi.org/10.3390/su13031491
APA StyleGuo, Y., Fan, Z., Yi, X., Zhang, Y., Khan, R. A. A., & Zhou, Z. (2021). Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp. Sustainability, 13(3), 1491. https://doi.org/10.3390/su13031491






