UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane
Abstract
1. Introduction
2. Materials and Methods
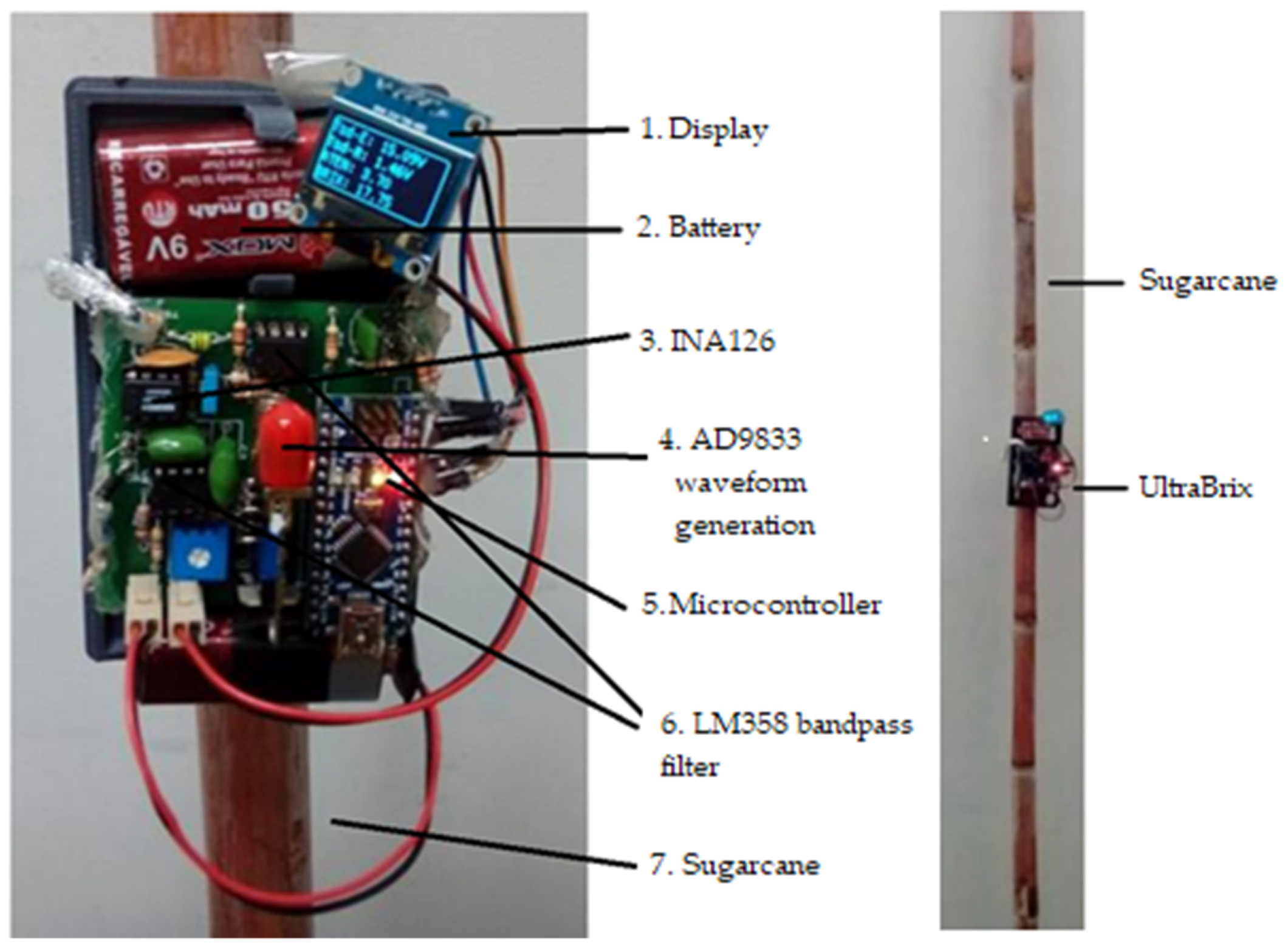
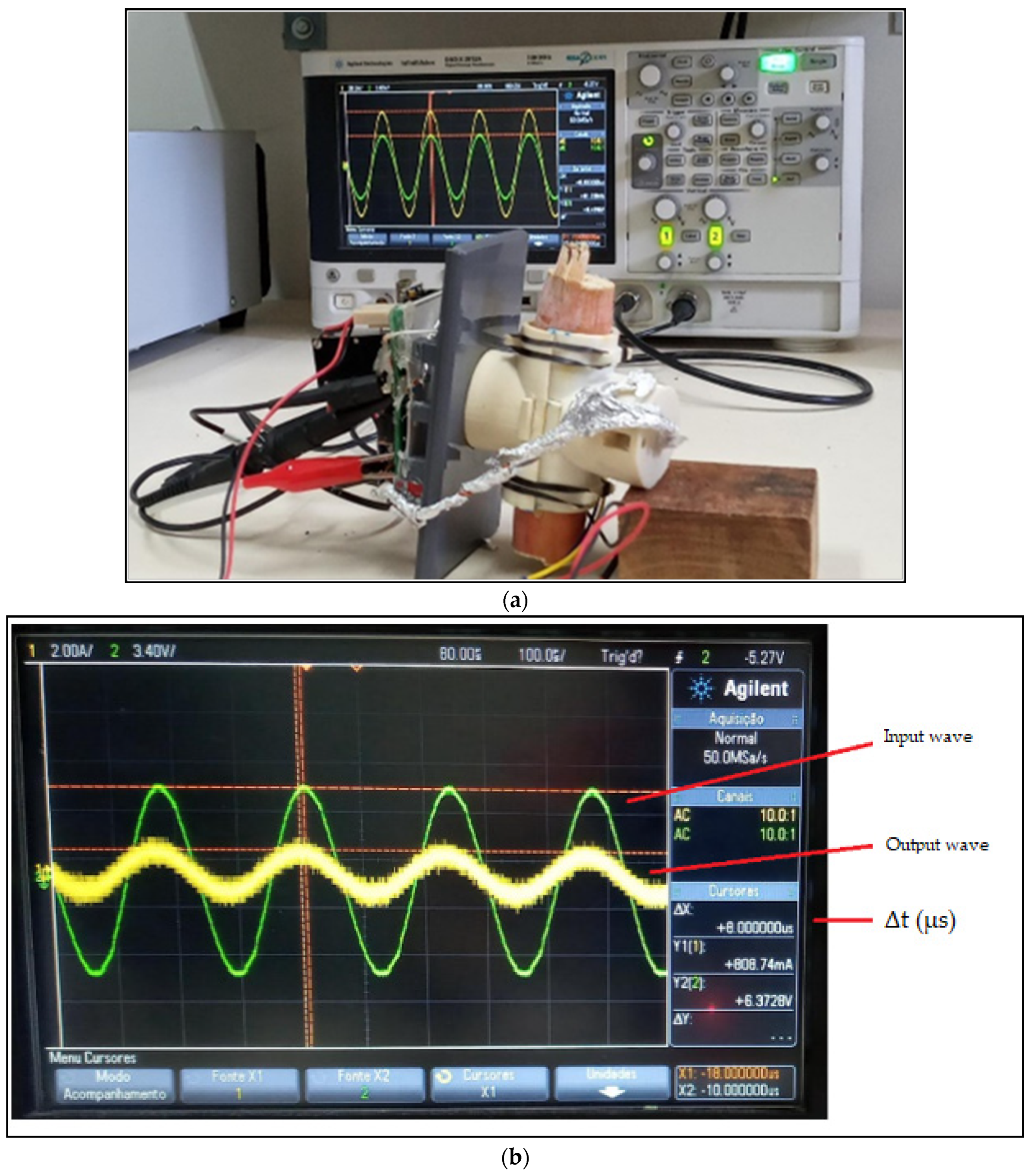
2.1. UltraBrix Device
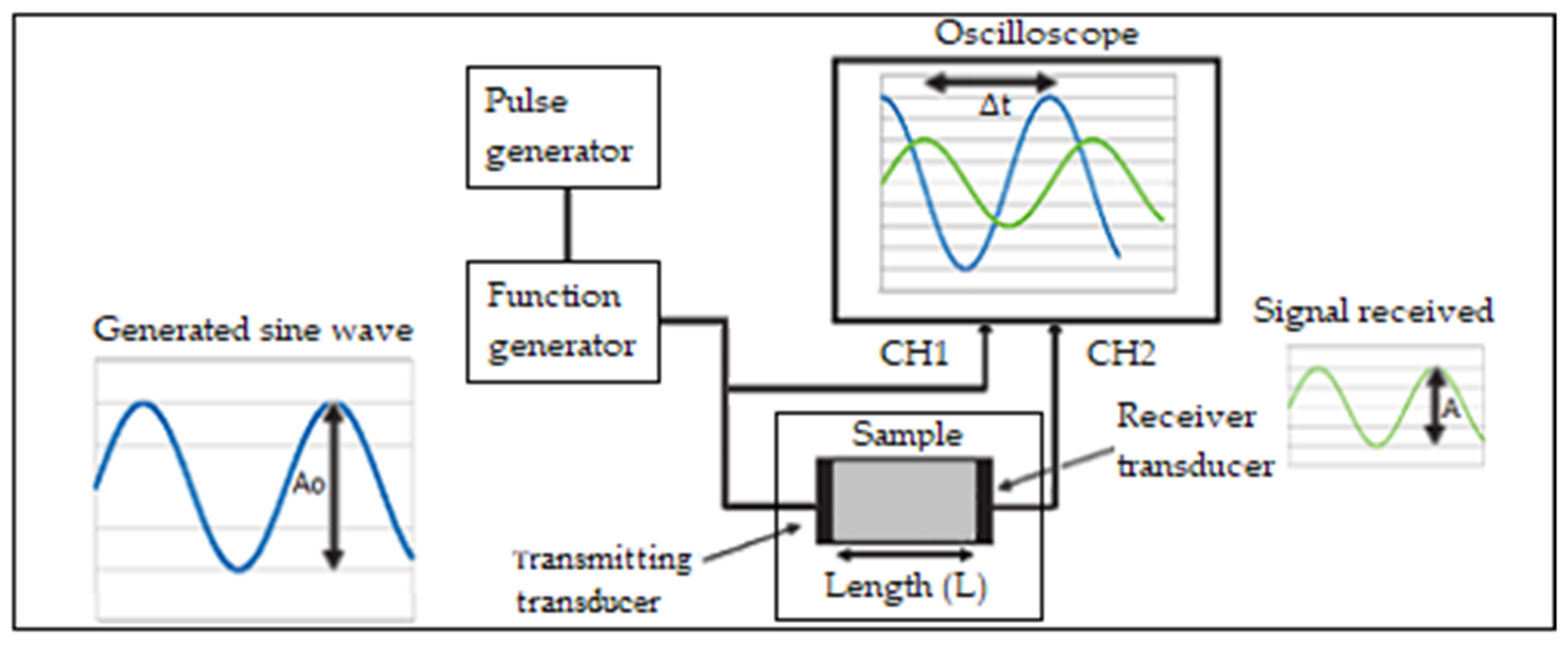
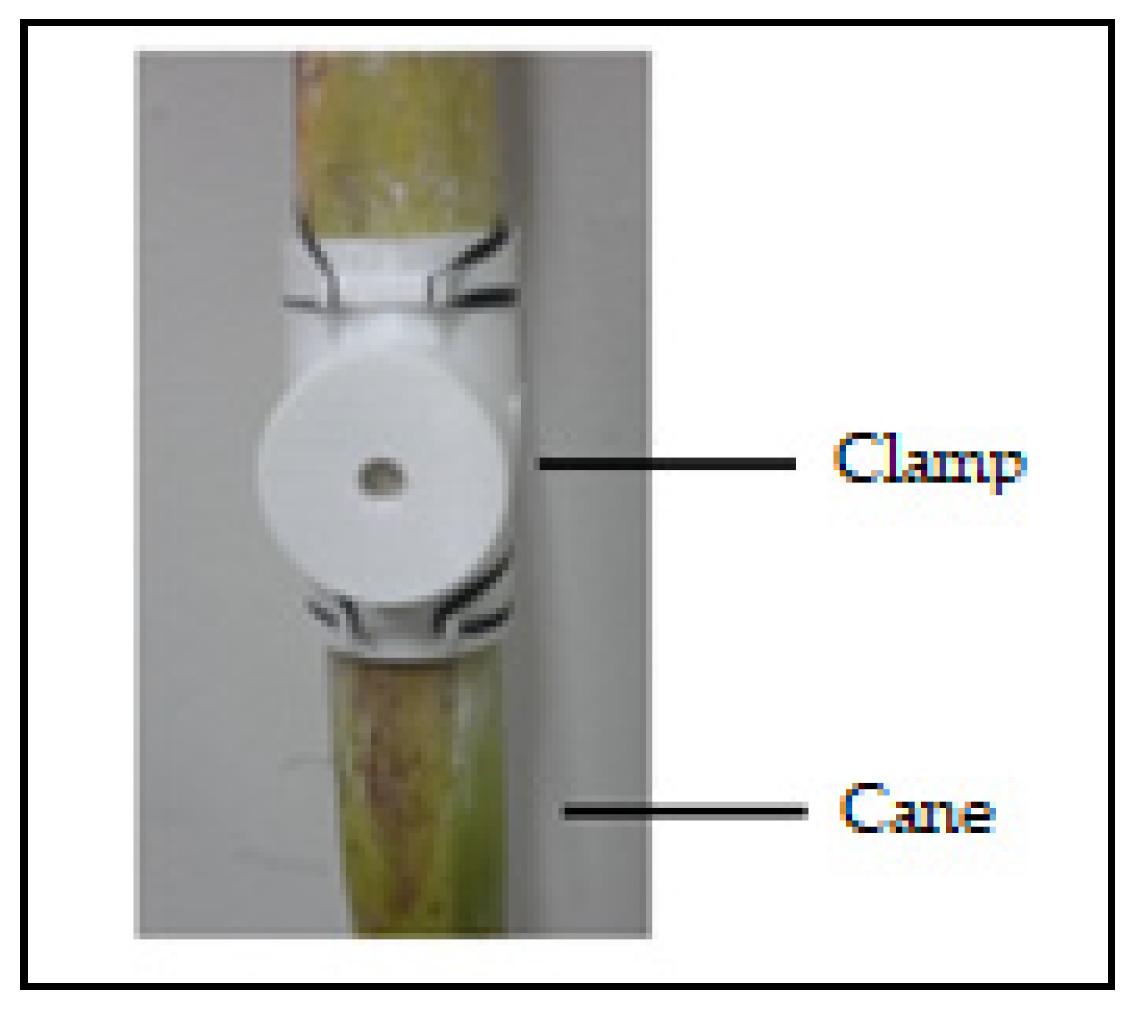
2.2. Transducer
- Display that shows the voltage measurements on the emitting transducer (Tsd-E) and receiver (Tsd-R), the attenuation value and the °Brix;
- Battery used to power the electronic circuit (UltraBrix operation);
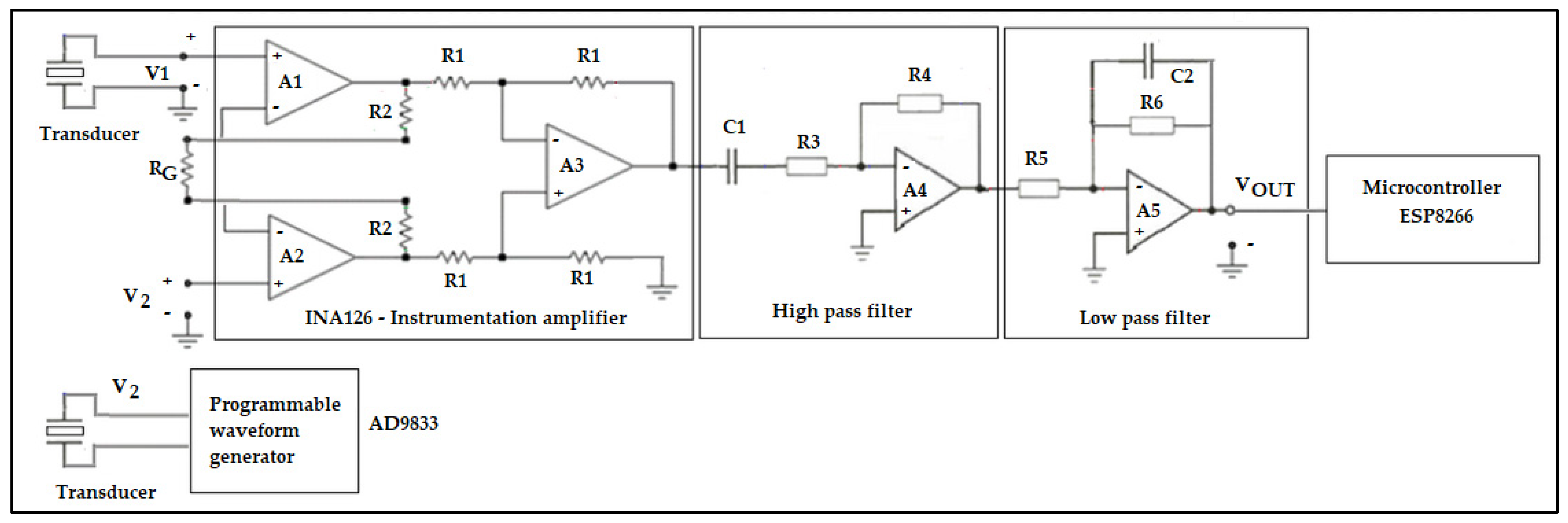
- Integrated circuit instrumentation amplifier (INA126) whose function is to increase the signal received at the receiving transducer;
- 4.3 kHz sine wave signal generator (AD9833 module) for the emitting transducer;
- Arduino One ATMEGA328 microcontroller board—configures AD9833 module for 4.3 kHz sine wave generation, receives the signal from receiver transducer, measures start and end amplitudes of the sine signal, calculates signal attenuation and estimates °Brix;
- LM358 integrated circuit used to allow only the sine signal to pass near the 4.3 kHz frequency (bandpass filter) to the Arduino, thus removing noise and miscellaneous signals.
2.3. Instrumentation Amplifier with Bandpass Filter
3. Results
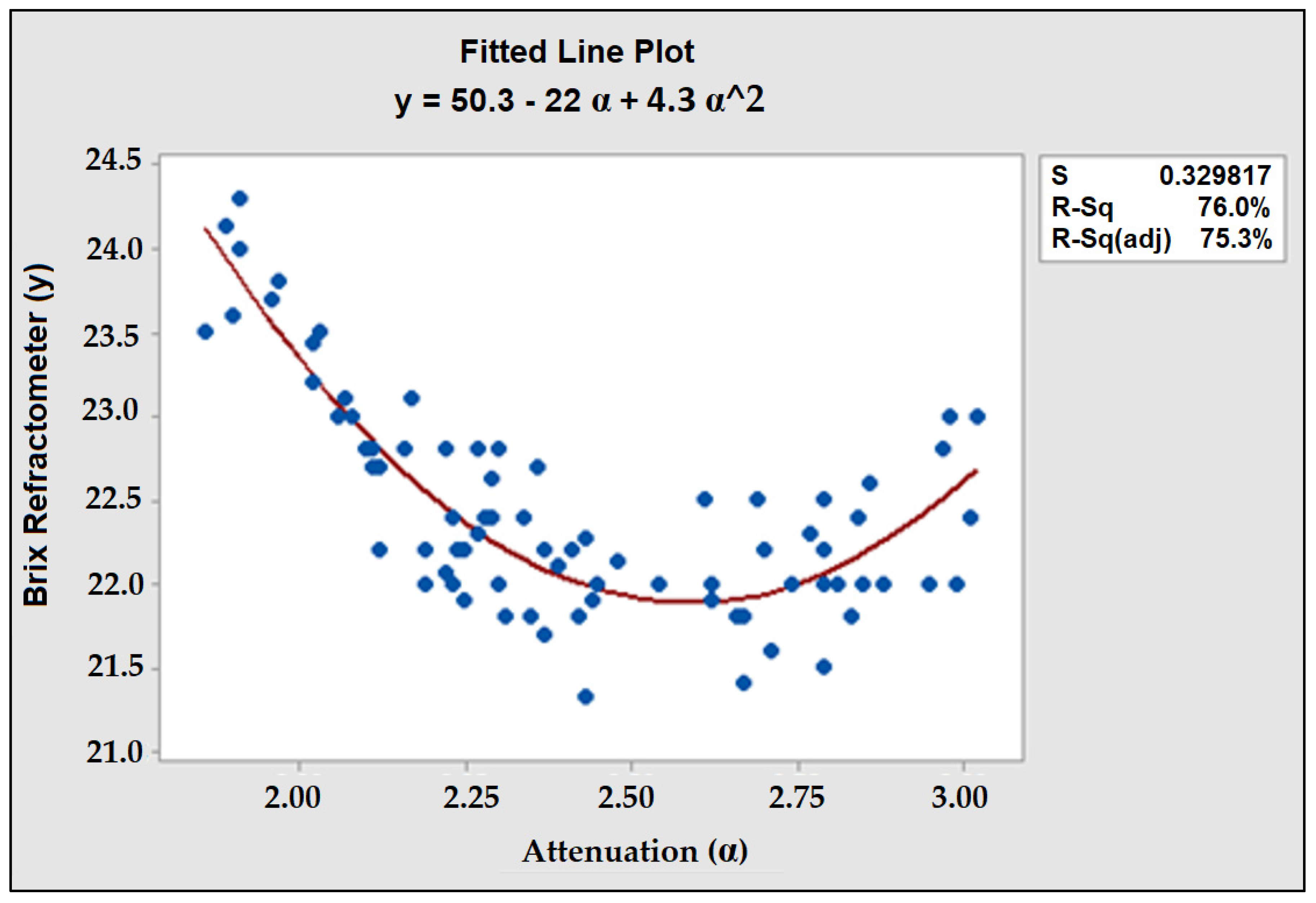
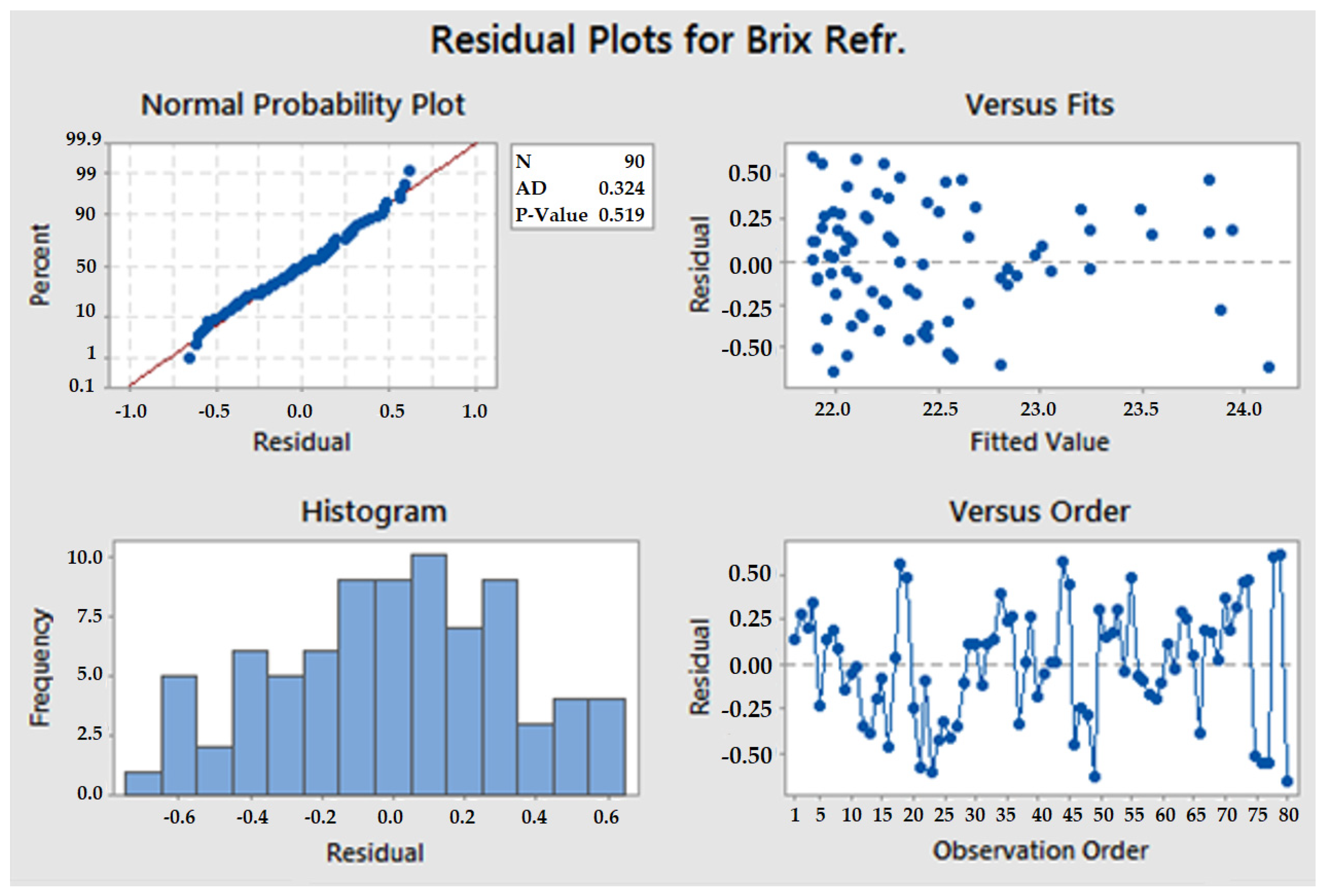
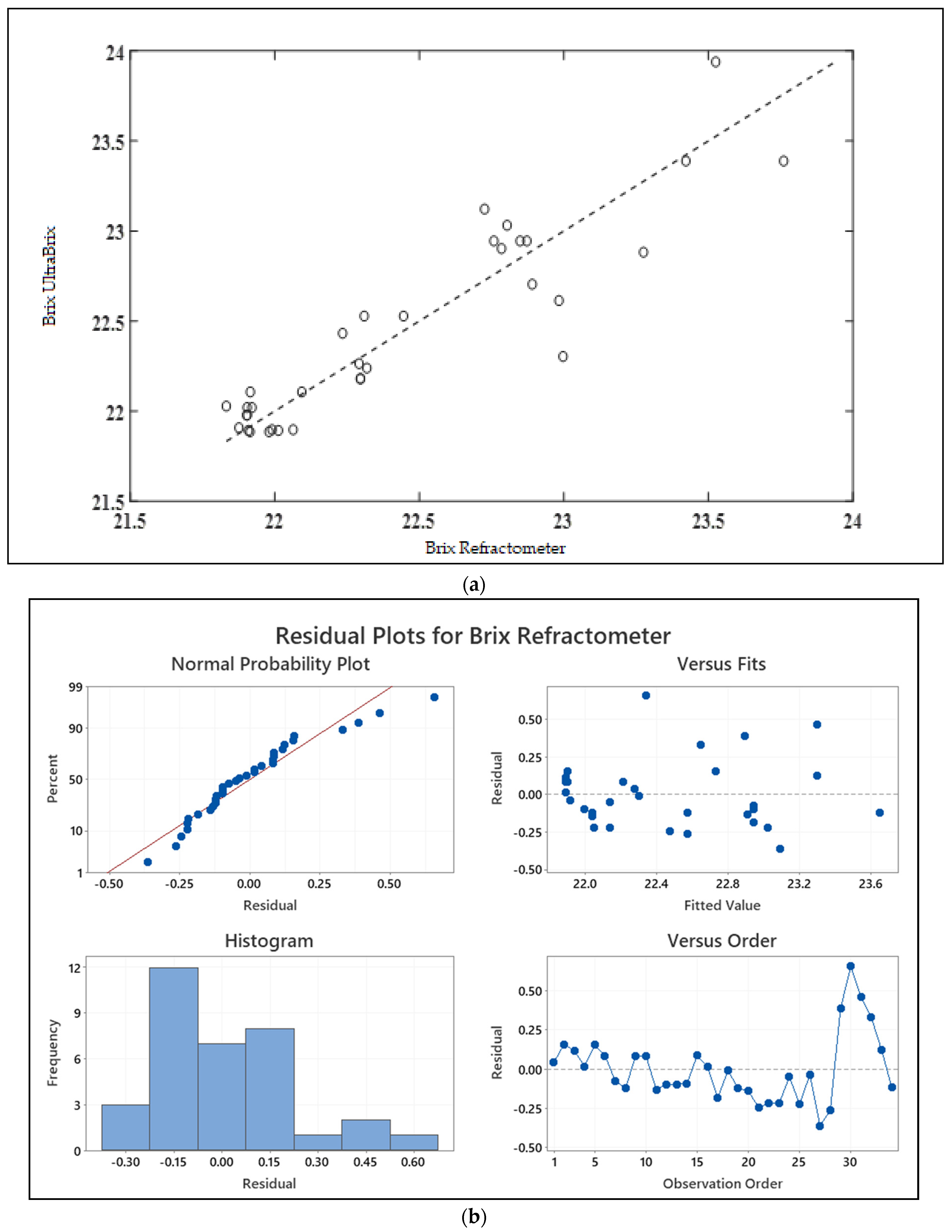
3.1. Test Sample and Validation
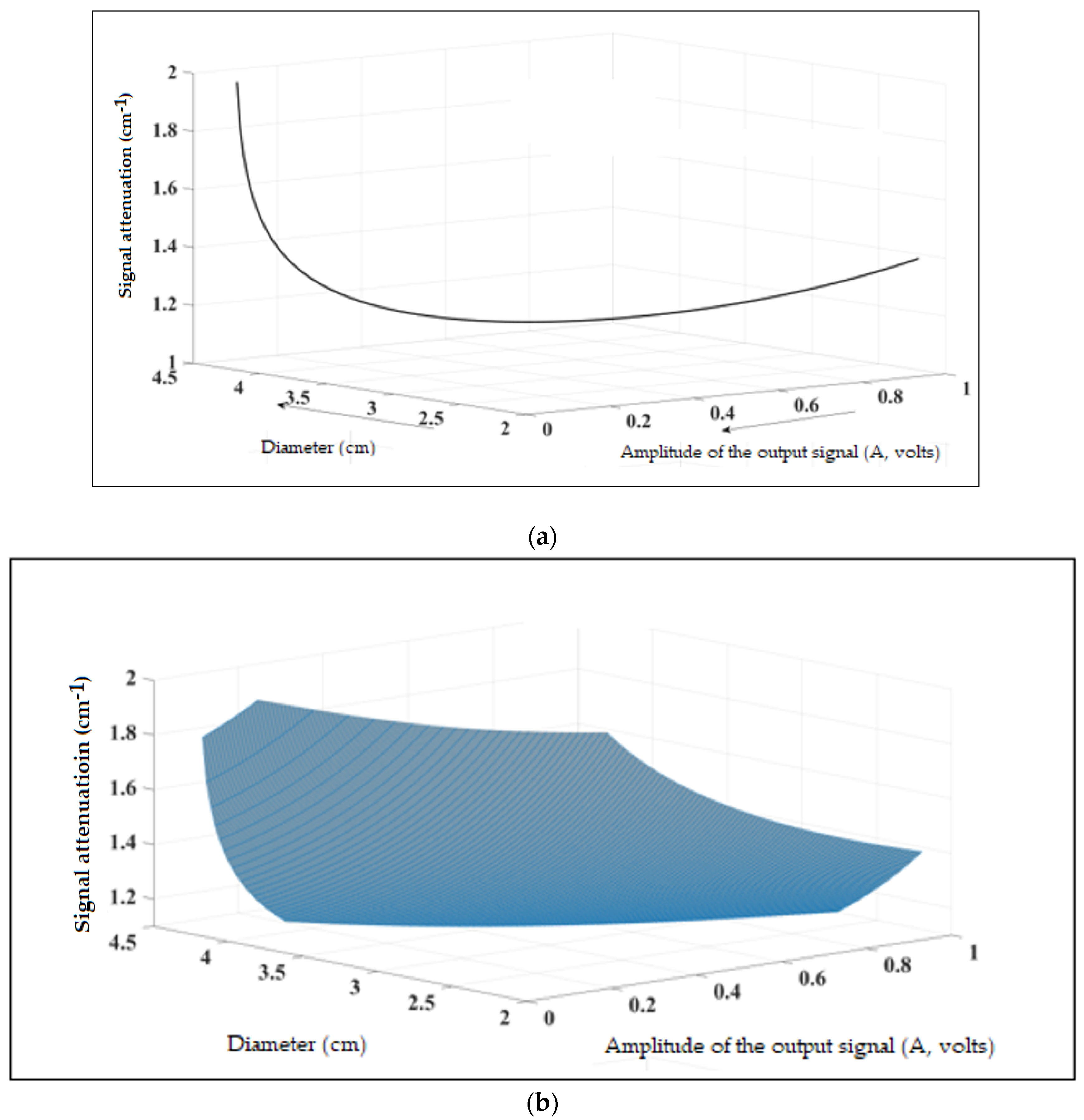
3.2. Sound Propagation around the Specimen and Application in the Field
3.3. Evaluation of Benefits for Sugarcane Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Esp | Stem a | Stem Diameter (cm) | Attenuation | °Brix Refr | Estimated Brix | Error |
|---|---|---|---|---|---|---|
| 1 | 1 | 3.20 | 2.16 | 22.80 | 22.64 | 0.16 |
| 2 | 7 | 3.00 | 2.43 | 22.27 | 21.95 | 0.31 |
| 3 | 8 | 2.80 | 2.48 | 22.13 | 21.89 | 0.24 |
| 4 | 3 | 3.00 | 2.22 | 22.80 | 22.42 | 0.38 |
| 5 | 5 | 3.00 | 2.30 | 22.00 | 22.20 | 0.20 |
| 6 | 7 | 3.00 | 2.29 | 22.40 | 22.21 | 0.19 |
| 7 | 11 | 2.90 | 2.41 | 22.20 | 21.98 | 0.22 |
| 8 | 5 | 3.42 | 2.07 | 23.10 | 23.00 | 0.10 |
| 9 | 8 | 3.32 | 2.11 | 22.70 | 22.83 | 0.13 |
| 10 | 9 | 3.30 | 2.11 | 22.80 | 22.83 | 0.03 |
| 11 | 10 | 3.10 | 2.27 | 22.30 | 22.26 | 0.04 |
| 12 | 11 | 3.20 | 2.19 | 22.20 | 22.53 | 0.33 |
| 13 | 14 | 3.00 | 2.37 | 21.70 | 22.05 | 0.35 |
| 14 | 15 | 2.93 | 2.42 | 21.80 | 21.96 | 0.16 |
| 15 | 16 | 2.90 | 2.44 | 21.90 | 21.93 | 0.03 |
| 16 | 1 | 3.15 | 2.25 | 21.90 | 22.34 | 0.44 |
| 17 | 4 | 2.88 | 2.45 | 22 | 21.92 | 0.08 |
| 18 | 5 | 2.95 | 2.30 | 22.8 | 22.20 | 0.60 |
| 19 | 6 | 2.93 | 2.27 | 22.8 | 22.28 | 0.52 |
| 20 | 12 | 2.52 | 2.88 | 22 | 22.19 | 0.19 |
| 21 | 13 | 2.40 | 2.99 | 22 | 22.52 | 0.52 |
| 22 | 14 | 2.48 | 2.81 | 22 | 22.06 | 0.06 |
| 23 | 1 | 3.17 | 2.12 | 22.2 | 22.77 | 0.57 |
| 24 | 2 | 3.22 | 2.23 | 22 | 22.40 | 0.40 |
| 25 | 3 | 3.03 | 2.35 | 21.8 | 22.09 | 0.29 |
| 26 | 4 | 2.97 | 2.31 | 21.8 | 22.18 | 0.38 |
| 27 | 6 | 2.70 | 2.71 | 21.6 | 21.90 | 0.30 |
| 28 | 7 | 2.67 | 2.66 | 21.8 | 21.86 | 0.06 |
| 29 | 8 | 2.80 | 2.54 | 22 | 21.85 | 0.15 |
| 30 | 9 | 2.83 | 2.37 | 22.2 | 22.05 | 0.15 |
| 31 | 10 | 2.66 | 2.67 | 21.8 | 21.87 | 0.07 |
| 32 | 11 | 2.62 | 2.62 | 22 | 21.84 | 0.16 |
| 33 | 16 | 2.53 | 2.79 | 22.2 | 22.01 | 0.19 |
| 34 | 4 | 2.64 | 2.86 | 22.6 | 22.16 | 0.44 |
| 35 | 5 | 2.66 | 2.84 | 22.4 | 22.10 | 0.30 |
| 36 | 6 | 2.63 | 2.77 | 22.3 | 21.98 | 0.32 |
| 37 | 7 | 2.65 | 2.83 | 21.8 | 22.09 | 0.29 |
| 38 | 8 | 2.65 | 2.62 | 21.9 | 21.85 | 0.05 |
| 39 | 9 | 2.58 | 2.70 | 22.2 | 21.89 | 0.31 |
| 40 | 10 | 2.60 | 2.85 | 22 | 22.14 | 0.14 |
| 41 | 11 | 2.66 | 2.79 | 22 | 22.02 | 0.02 |
| 42 | 16 | 2.74 | 2.74 | 22 | 21.93 | 0.07 |
| 43 | 17 | 2.78 | 2.74 | 22 | 21.94 | 0.06 |
| 44 | 21 | 2.68 | 2.69 | 22.5 | 21.88 | 0.62 |
| 45 | 24 | 2.68 | 2.79 | 22.5 | 22.02 | 0.48 |
| 46 | 27 | 2.48 | 2.95 | 22 | 22.39 | 0.39 |
| 47 | 30 | 2.50 | 3.01 | 22.4 | 22.60 | 0.20 |
| 48 | 3 | 3.44 | 1.90 | 23.6 | 23.86 | 0.26 |
| 49 | 4 | 3.26 | 1.86 | 23.5 | 24.09 | 0.59 |
| 50 | 5 | 3.43 | 1.97 | 23.8 | 23.47 | 0.33 |
| 51 | 6 | 3.32 | 1.96 | 23.7 | 23.51 | 0.19 |
| 52 | 8 | 3.22 | 1.91 | 24 | 23.82 | 0.18 |
| 53 | 10 | 3.20 | 2.03 | 23.5 | 23.16 | 0.34 |
| 54 | 12 | 3.28 | 2.02 | 23.2 | 23.20 | 0.00 |
| 55 | 14 | 3.08 | 2.17 | 23.1 | 22.59 | 0.51 |
| 56 | 15 | 3.28 | 2.06 | 23 | 23.04 | 0.04 |
| 57 | 16 | 3.33 | 2.10 | 22.8 | 22.87 | 0.07 |
| 58 | 19 | 3.16 | 2.25 | 22.2 | 22.33 | 0.13 |
| 59 | 20 | 3.10 | 2.24 | 22.2 | 22.37 | 0.17 |
| 60 | 21 | 3.12 | 2.12 | 22.7 | 22.79 | 0.09 |
| 61 | 22 | 3.02 | 2.28 | 22.4 | 22.26 | 0.14 |
| 62 | 27 | 3.17 | 2.23 | 22.4 | 22.40 | 0.00 |
| 63 | 1 | 2.54 | 2.97 | 22.8 | 22.47 | 0.33 |
| 64 | 9 | 2.93 | 2.34 | 22.40 | 22.10 | 0.30 |
| 65 | 10 | 2.90 | 2.39 | 22.10 | 22.02 | 0.08 |
| 66 | 11 | 3.00 | 2.22 | 22.07 | 22.44 | 0.37 |
| 67 | 2 | 3.67 | 1.89 | 24.13 | 23.91 | 0.35 |
| 68 | 5 | 3.36 | 2.02 | 23.43 | 23.24 | 0.23 |
| 69 | 6 | 3.20 | 2.08 | 23.00 | 22.96 | 0.05 |
| 70 | 7 | 3.07 | 2.29 | 22.63 | 22.21 | 0.35 |
| 71 | 2 | 3.67 | 1.89 | 24.13 | 23.91 | 0.23 |
| 72 | 26 | 2.50 | 3.02 | 23 | 22.62 | 0.38 |
| 73 | 29 | 2.53 | 2.98 | 23 | 22.48 | 0.52 |
| 74 | 1 | 3.47 | 1.91 | 24.3 | 23.84 | 0.46 |
| 75 | 8 | 2.65 | 2.67 | 21.4 | 21.87 | 0.47 |
| 76 | 18 | 3.22 | 2.19 | 22 | 22.53 | 0.53 |
| 77 | 12 | 2.64 | 2.79 | 21.5 | 22.01 | 0.51 |
| 78 | 17 | 2.88 | 2.36 | 22.70 | 22.07 | 0.63 |
| 79 | 13 | 2.82 | 2.61 | 22.5 | 21.84 | 0.66 |
| 80 | 20 | 2.82 | 2.48 | 21.33 | 21.90 | 0.56 |
| Esp | Stem a | Stem Diameter (cm) | Attenuation | °Brix Refr | Estimated Brix | Error |
|---|---|---|---|---|---|---|
| 1 | 2 | 3.15 | 2.30 | 22.32 | 22.24 | 0.08 |
| 2 | 23 | 3.08 | 2.15 | 22.89 | 22.70 | 0.19 |
| 3 | 15 | 2.63 | 2.63 | 22.01 | 21.89 | 0.12 |
| 4 | 15 | 2.63 | 2.63 | 21.91 | 21.89 | 0.02 |
| 5 | 7 | 2.72 | 2.64 | 22.06 | 21.90 | 0.17 |
| 6 | 7 | 2.72 | 2.64 | 21.99 | 21.90 | 0.09 |
| 7 | 12 | 3.32 | 2.09 | 22.87 | 22.94 | 0.07 |
| 8 | 13 | 3.10 | 2.20 | 22.45 | 22.53 | 0.08 |
| 9 | 15 | 2.42 | 2.85 | 22.30 | 22.18 | 0.12 |
| 10 | 15 | 2.42 | 2.85 | 22.30 | 22.18 | 0.12 |
| 11 | 1 | 3.10 | 2.10 | 22.78 | 22.90 | 0.12 |
| 12 | 3 | 3.10 | 2.44 | 21.90 | 21.98 | 0.07 |
| 13 | 3 | 3.10 | 2.44 | 21.90 | 21.98 | 0.07 |
| 14 | 17 | 3.20 | 2.09 | 22.85 | 22.94 | 0.10 |
| 15 | 5 | 2.87 | 2.60 | 21.98 | 21.89 | 0.09 |
| 16 | 5 | 2.87 | 2.60 | 21.91 | 21.89 | 0.03 |
| 17 | 2 | 3.10 | 2.09 | 22.76 | 22.94 | 0.19 |
| 18 | 2 | 3.00 | 2.29 | 22.29 | 22.26 | 0.03 |
| 19 | 5 | 2.97 | 2.41 | 21.92 | 22.02 | 0.10 |
| 20 | 5 | 2.97 | 2.41 | 21.90 | 22.02 | 0.12 |
| 21 | 3 | 3.07 | 2.23 | 22.23 | 22.43 | 0.20 |
| 22 | 24 | 3.27 | 2.07 | 22.80 | 23.03 | 0.23 |
| 23 | 13 | 3.00 | 2.36 | 21.92 | 22.11 | 0.19 |
| 24 | 13 | 3.00 | 2.36 | 22.09 | 22.11 | 0.01 |
| 25 | 7 | 2.77 | 2.77 | 21.83 | 22.03 | 0.20 |
| 26 | 14 | 2.67 | 2.66 | 21.88 | 21.91 | 0.03 |
| 27 | 26 | 3.32 | 2.05 | 22.73 | 23.12 | 0.40 |
| 28 | 12 | 2.97 | 2.20 | 22.31 | 22.53 | 0.22 |
| 29 | 6 | 2.53 | 3.07 | 23.28 | 22.88 | 0.39 |
| 30 | 7 | 2.52 | 2.90 | 23.00 | 22.30 | 0.69 |
| 31 | 5 | 2.52 | 3.18 | 23.76 | 23.39 | 0.37 |
| 32 | 13 | 2.40 | 3.00 | 22.98 | 22.61 | 0.37 |
| 33 | 11 | 2.45 | 3.18 | 23.42 | 23.39 | 0.03 |
| 34 | 3 | 2.55 | 3.28 | 23.53 | 23.94 | 0.41 |
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 16 December 2018).
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for Bioenergy Production: An Assessment of Yield and Regulation of Sucrose Content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef]
- Schneider, F. Sugar Analysis: Official and Tentative Methods Recommended by the International Commission for Uniform Methods of Sugar Analysis (ICUMSA); Hyperion Books: New York, NY, USA, 1979. [Google Scholar]
- Froese, R.E.; Waterstraut, J.R.; Johnson, D.M.; Shonnard, D.R.; Whitmarsh, J.H.; Miller, C.A. Lignocellulosic Ethanol: Is It Economically and Financially Viable as a Fuel Source? Environ. Qual. Manag. 2008, 18, 23–45. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological Trends, Global Market, and Challenges of Bio-Ethanol Production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Smogrovicova, D.; Obulam, V.S.R.; Ko, S. Very High Gravity (VHG) Ethanolic Brewing and Fermentation: A Research Update. J. Ind. Microbiol. Biotechnol. 2011, 38, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Americano da Costa, M.V.; Pasamontes, M.; Normey-Rico, J.E.; Guzmán, J.L.; Berenguel, M. Advanced Control Strategy Combined with Solar Cooling for Improving Ethanol Production in Fermentation Units. Ind. Eng. Chem. Res. 2014, 53, 11384–11392. [Google Scholar] [CrossRef]
- Nawi, N.M.; Chen, G.; Jensen, T.; Mehdizadeh, S.A. Prediction and Classification of Sugar Content of Sugarcane Based on Skin Scanning Using Visible and Shortwave near Infrared. Biosyst. Eng. 2013, 115, 154–161. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Real-Time Monitoring of Sucrose, Sorbitol, d-Glucose and d-Fructose Concentration by Electromagnetic Sensing. Food Chem. 2017, 232, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Mishima, M.; Kajiwara, I.; Maeda, S. Non-Destructive Firmness Assessment of Apples Using a Non-Contact Laser Excitation System Based on a Laser-Induced Plasma Shock Wave. Postharvest Biol. Technol. 2017, 128, 11–17. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.-W. Hyperspectral Imaging Technique for Evaluating Food Quality and Safety during Various Processes: A Review of Recent Applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Tesfay, S.Z. A Review of Destructive and Non-Destructive Methods for Determining Avocado Fruit Maturity. Food Bioprocess Technol. 2015, 8, 1995–2011. [Google Scholar] [CrossRef]
- Mizrach, A. Ultrasonic Technology for Quality Evaluation of Fresh Fruit and Vegetables in Pre- and Postharvest Processes. Postharvest Biol. Technol. 2008, 48, 315–330. [Google Scholar] [CrossRef]
- Mizrach, A. Nondestructive Ultrasonic Monitoring of Tomato Quality during Shelf-Life Storage. Postharvest Biol. Technol. 2007, 46, 271–274. [Google Scholar] [CrossRef]
- Mizrach, A.; Flitsanov, U. Nondestructive Ultrasonic Determination of Avocado Softening Process. J. Food Eng. 1999, 40, 139–144. [Google Scholar] [CrossRef]
- Morrison, D.S.; Abeyratne, U.R. Ultrasonic Technique for Non-Destructive Quality Evaluation of Oranges. J. Food Eng. 2014, 141, 107–112. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic Innovations in the Food Industry: From the Laboratory to Commercial Production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Rady, A.M.; Guyer, D.E. Rapid and/or Nondestructive Quality Evaluation Methods for Potatoes: A Review. Comput. Electron. Agric. 2015, 117, 31–48. [Google Scholar] [CrossRef]
- Sorol, N.; Arancibia, E.; Bortolato, S.A.; Olivieri, A.C. Visible/near Infrared-Partial Least-Squares Analysis of Brix in Sugar Cane Juice: A Test Field for Variable Selection Methods. Chemom. Intell. Lab. Syst. 2010, 102, 100–109. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Non-Destructive Sensing Methods for Quality Assessment of on-Tree Fruits: A Review. J. Food Meas. Charact. 2018, 12, 497–526. [Google Scholar] [CrossRef]
- Naderi-Boldaji, M.; Fazeliyan-Dehkordi, M.; Mireei, S.A.; Ghasemi-Varnamkhasti, M. Dielectric Power Spectroscopy as a Potential Technique for the Non-Destructive Measurement of Sugar Concentration in Sugarcane. Biosyst. Eng. 2015, 140, 1–10. [Google Scholar] [CrossRef]
- Jackson, B.; Jayanthy, T. Determination of Sucrose in Raw Sugarcane Juice by Microwave Method. Indian J. Sci. Technol. 2014, 7, 5. [Google Scholar] [CrossRef]
- Ramasami, P.; Jhaumeer-Laulloo, S.; Rondeau, P.; Cadet, F.; Seepujak, H.; Seeruttun, A. Quantification of Sugars in Soft Drinks and Fruit Juices by Density, Refractometry, Infrared Spectroscopy and Statistical Methods. S. Afr. J. Chem. 2004, 57, 24–27. [Google Scholar]
- Abdel-Rahman, E.M.; Ahmed, F.B.; van den Berg, M. Estimation of Sugarcane Leaf Nitrogen Concentration Using in Situ Spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, S52–S57. [Google Scholar] [CrossRef]
- Purcell, D.E.; Leonard, G.J.; O’Shea, M.G.; Kokot, S. A Chemometrics Investigation of Sugarcane Plant Properties Based on the Molecular Composition of Epicuticular Wax. Chemom. Intell. Lab. Syst. 2005, 76, 135–147. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.-U. Applications and Potential of Ultrasonics in Food Processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Mizrach, A. Assessing Plum Fruit Quality Attributes with an Ultrasonic Method. Food Res. Int. 2004, 37, 627–631. [Google Scholar] [CrossRef]
- Buckin, V.; Kudryashov, E.; Driscoll, B.O. High-Resolution Ultrasonic Spectroscopy for Material Analysis. Int. Lab. 2003, 33, 10–13. [Google Scholar]
- Moraes Júnior, E.; Fontes, C.H.O.; Americano da Costa, M.V. Eletronic Dendrometer, Patent: Privilege of Innovation. National Institute of Industrial Property. Record number: BR10201701535. 2020. [Google Scholar]
- Anderson, T.W.; Darling, D.A. Asymptotic Theory of Certain “Goodness of Fit” Criteria Based on Stochastic Processes. Ann Math Stat. 1952, 23, 193–212. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of Ultrasound in Analysis, Processing and Quality Control of Food: A Review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Casella, G.; Fienberg, S.; Olkin, I. An Introduction to Statistical Learning with Application in R; Springer Texts un Statistics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Brooks, G.; Barcikowski, R. The PEAR Method for Sample Sizes in Multiple Linear Regression. Mult. Linear Regres. Viewp. 2012, 38, 1–16. [Google Scholar]
- Berding, N.; Brotherton, G.A.; le Brocq, D.G.; Skinner, J.C. Near Infrared Reflectance Spectroscopy for Analysis of Sugarcane from Clonal Evaluation Trials: I. Fibrated Cane. Crop Sci. 1991, 31. [Google Scholar] [CrossRef]
- Madsen, L.R.; White, B.E.; Rein, P.W.; Madsen, L.R.; White, B.E.; Rein, P.W. Evaluation of a near Infrared Spectrometer for the Direct Analysis of Sugar Cane. J. Am. Soc. Sugarcane Technol. 2003, 23, 80–92. [Google Scholar]
- Zgouz, A.; Héran, D.; Barthès, B.; Bastianelli, D.; Bonnal, L.; Baeten, V.; Lurol, S.; Bonin, M.; Roger, J.-M.; Bendoula, R.; et al. Dataset of Visible-near Infrared Handheld and Micro-Spectrometers—Comparison of the Prediction Accuracy of Sugarcane Properties. Data Brief 2020, 31, 106013. [Google Scholar] [CrossRef] [PubMed]



| Method | Equipment Cost (USD) | Sampling Time (min) a |
|---|---|---|
| Refractometry | 500 | 20 |
| Polarimeter | 11,300 | 20 |
| Chromatography | 9200–23,400 | 30 |
| Biosensor | 4250 | 5 |
| °Brix hydrometer | 30 | 15–20 |
| Spectroscopy | 7000–128,000 71,000–99,300 | 0.2–1 |
| Validation/Calibration Sample (N = 80) | |||||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Minimum | Median | Max | |
| Attenuation (cm−1) | 2.41 | 0.33 | 1.86 | 2.35 | 3.02 |
| °Brix | 22.46 | 0.66 | 21.33 | 22.29 | 24.30 |
| Test sample (N = 34) | |||||
| Attenuation (cm−1) | 2.54 | 0.36 | 2.05 | 2.44 | 3.28 |
| °Brix | 22.44 | 0.55 | 21.83 | 22.30 | 23.76 |
| Components | Value |
|---|---|
| RG (Resistor) | 1 kΩ |
| R3 R4 R5 e R6 | 22 kΩ |
| C1 (Capacitor) | 10 nF |
| C2 (Capacitor) | 1 nF |
| Method | Coefficient of Determination (R2) | Author |
|---|---|---|
| Capacitive | 0.98 | Naderi-Boldaji (2015) [21] |
| Electromagnetic sensor | 0.99 | Harnsoongnoen (2017) [9] |
| Microwave | -- | Jackson (2014) [22] |
| NIR | 0.91 | Berding et al. (1991a) [34] |
| MIR | 0.97 | Madsen et al. (2003) [35] |
| VIS/NIR (absorption) | 0.89 | Nawi et al. (2013) [24] |
| VIS/NIR (reflectance) | 0.91 | Nawi et al. (2013) [24] |
| Device and Manufacturer | RMSE | R2 | Cost |
|---|---|---|---|
| DLP NIRscan Nano EVM | 6.2 | 0.864 | $1136.00 |
| F750 (Felix Instrument) | 8.9 | 0.720 | $7500.00–8500.00 |
| LabSpec 4 (ASD) | 2.6 | 0.976 | Not found |
| MicroNIR1700 (Viavi) | 3.8 | 0.949 | Not found |
| MicroNIR2200 (Viavi) | 2.8 | 0.972 | Not found |
| NIRONE 2.2 (Spectral Engines) | 7.7 | 0.791 | $2434.00 |
| Scio (Consumer Physics) | 9.4 | 0.687 | $1999.00 |
| TellSpec (TellSpec) | 9.3 | 0.692 | $1500.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.V.A.d.; Fontes, C.H.; Carvalho, G.; Júnior, E.C.d.M. UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane. Sustainability 2021, 13, 1227. https://doi.org/10.3390/su13031227
Costa MVAd, Fontes CH, Carvalho G, Júnior ECdM. UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane. Sustainability. 2021; 13(3):1227. https://doi.org/10.3390/su13031227
Chicago/Turabian StyleCosta, Marcus V. Americano da, Cristiano Hora Fontes, Gregor Carvalho, and Edison Camilo de Moraes Júnior. 2021. "UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane" Sustainability 13, no. 3: 1227. https://doi.org/10.3390/su13031227
APA StyleCosta, M. V. A. d., Fontes, C. H., Carvalho, G., & Júnior, E. C. d. M. (2021). UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane. Sustainability, 13(3), 1227. https://doi.org/10.3390/su13031227





