Pineapple Residue Ash Reduces Carbon Dioxide and Nitrous Oxide Emissions in Pineapple Cultivation on Tropical Peat Soils at Saratok, Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Peat Soil Physical and Chemical Characteristics
2.3. Preparation and Characterization of Pineapple Residue Ash
2.4. Field Experimental Design and Treatments
2.5. Gas Flux Measurements
2.6. Laboratory Experiment
2.7. Statistical Analysis
3. Results
3.1. Peat Soil Physicochemical Properties
3.2. Characteristics of Pineapple Residue ash
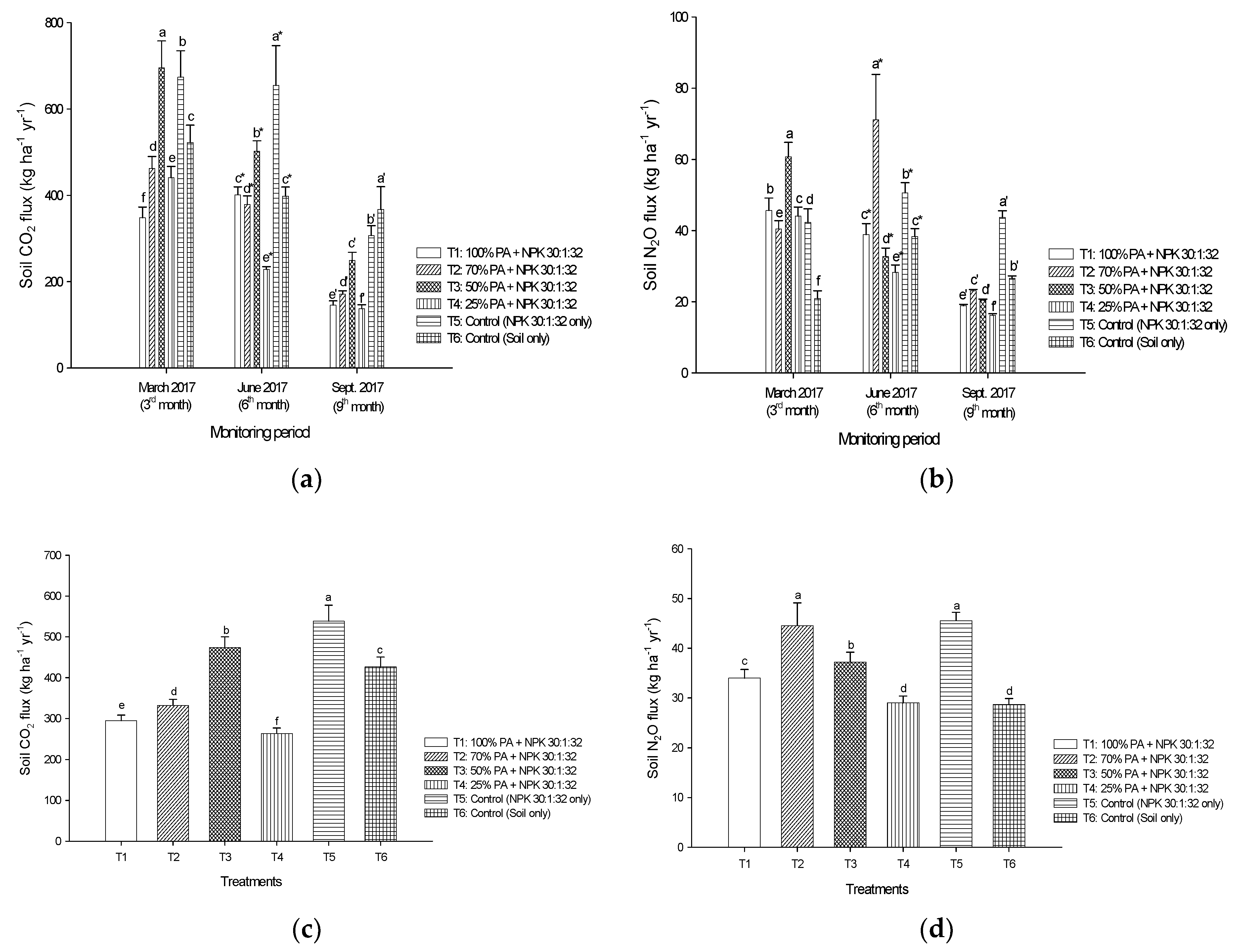
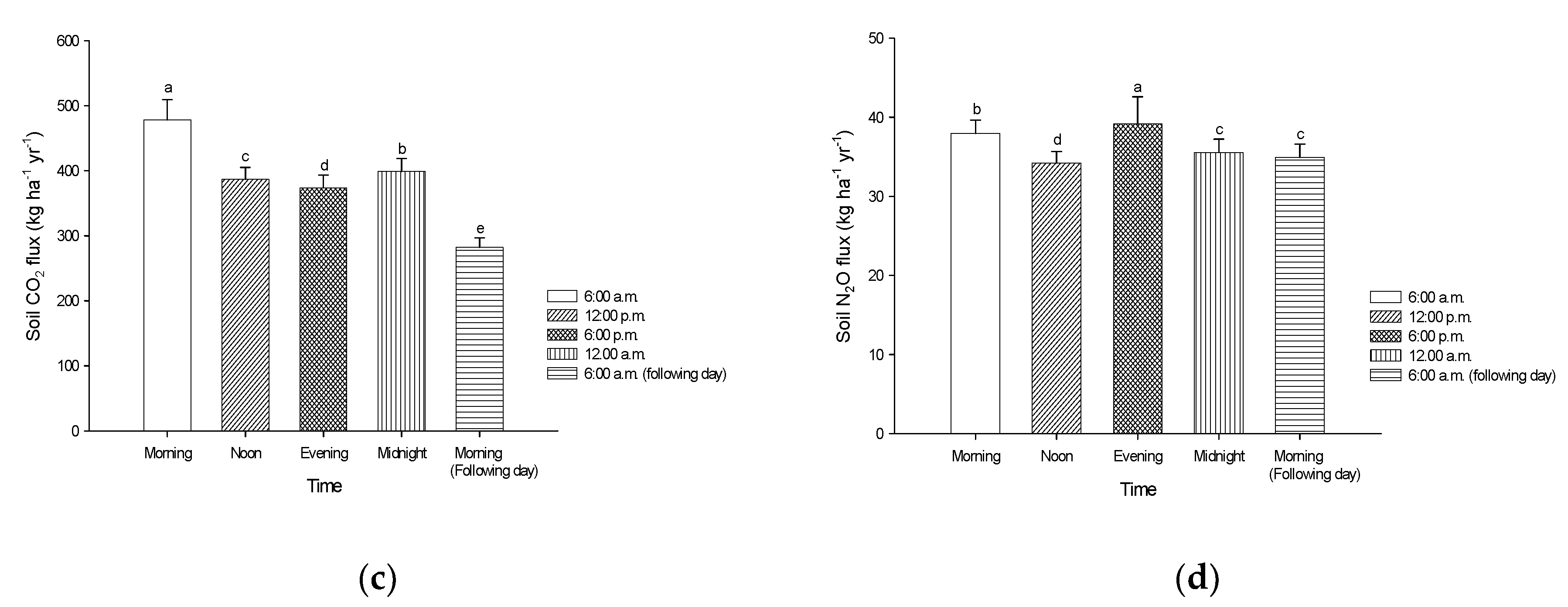
3.3. Soil CO2 from Peat Soils Grown with Pineapples
3.4. Soil N2O from Peat Soils Grown with Pineapples
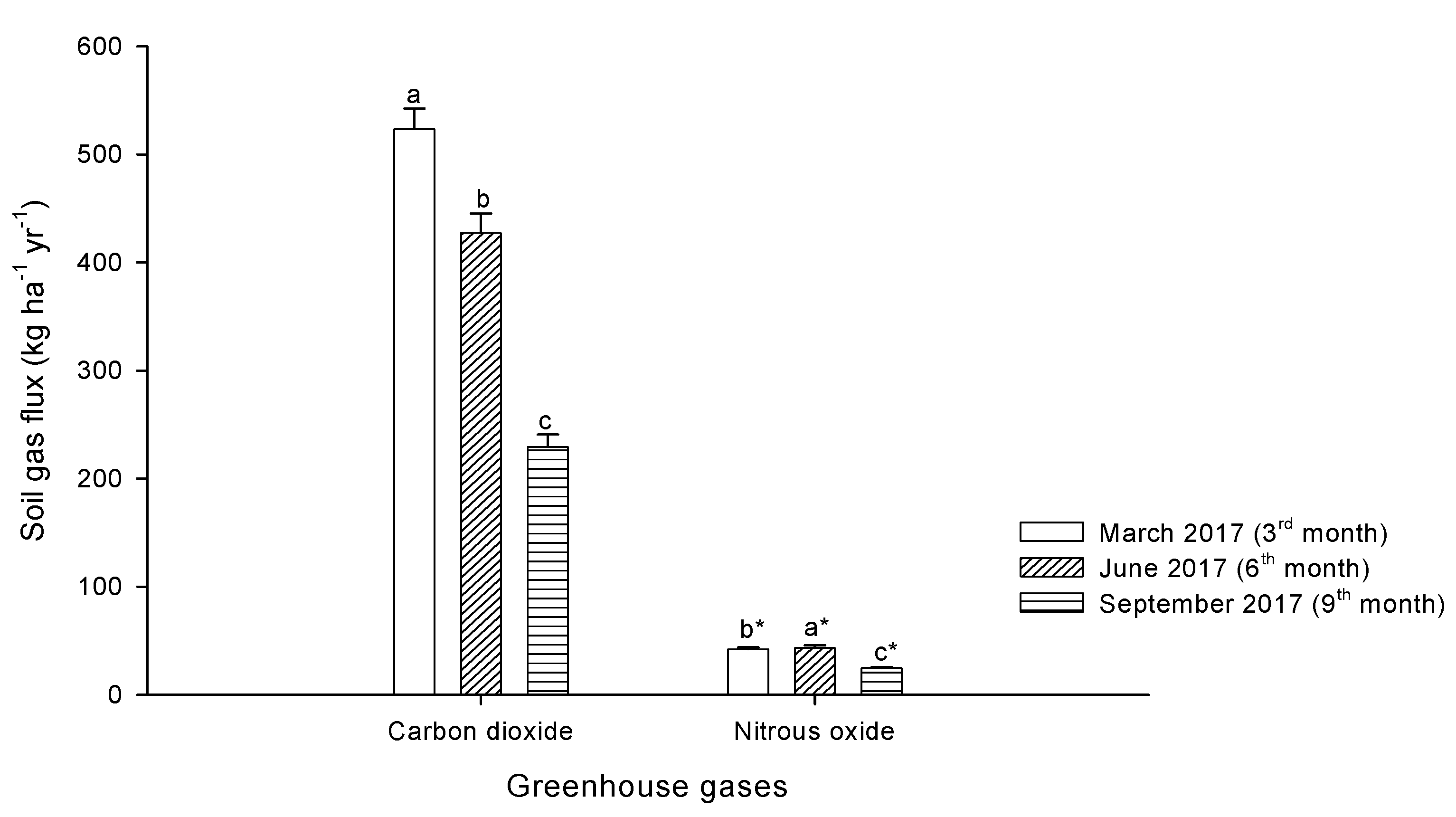
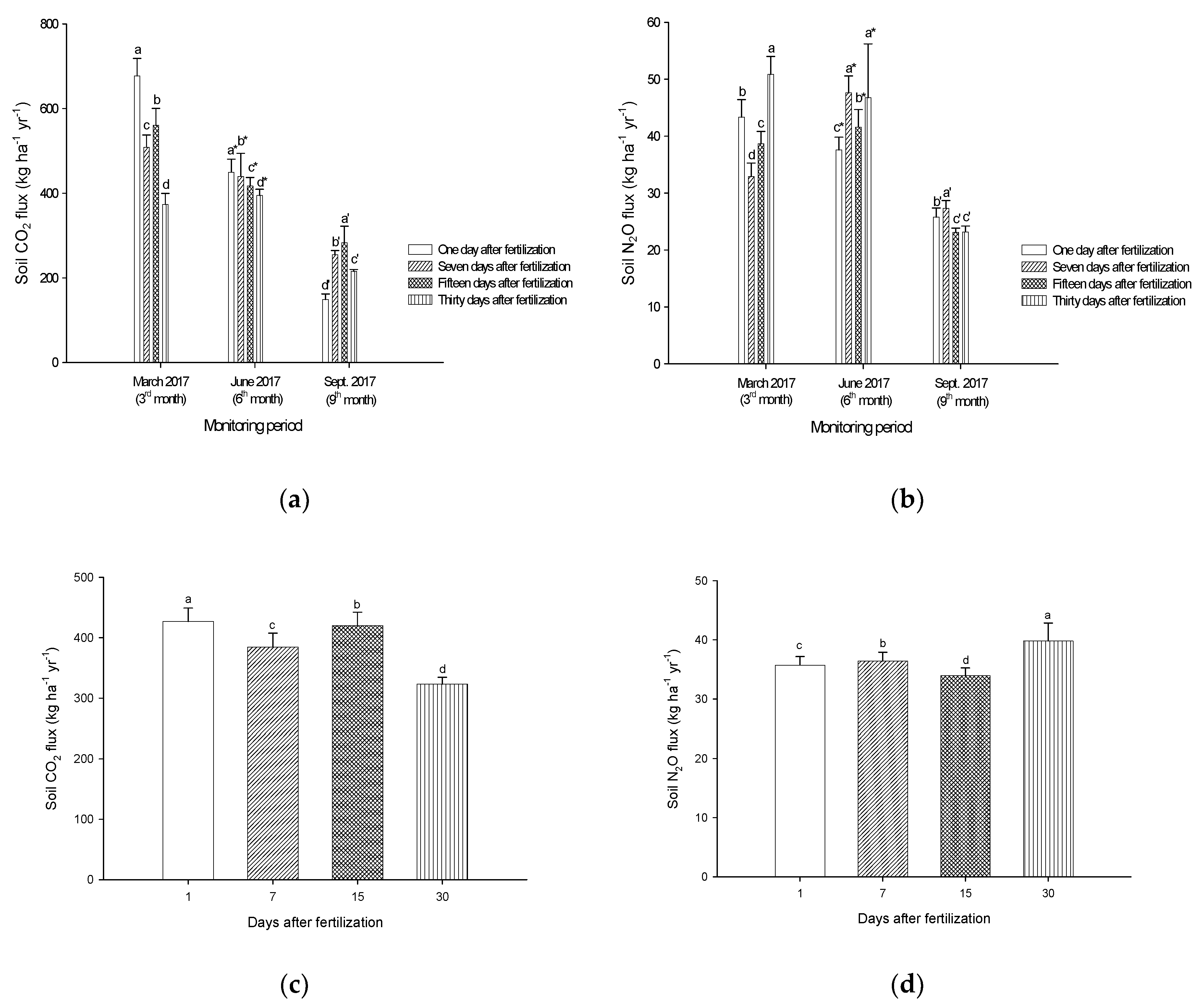
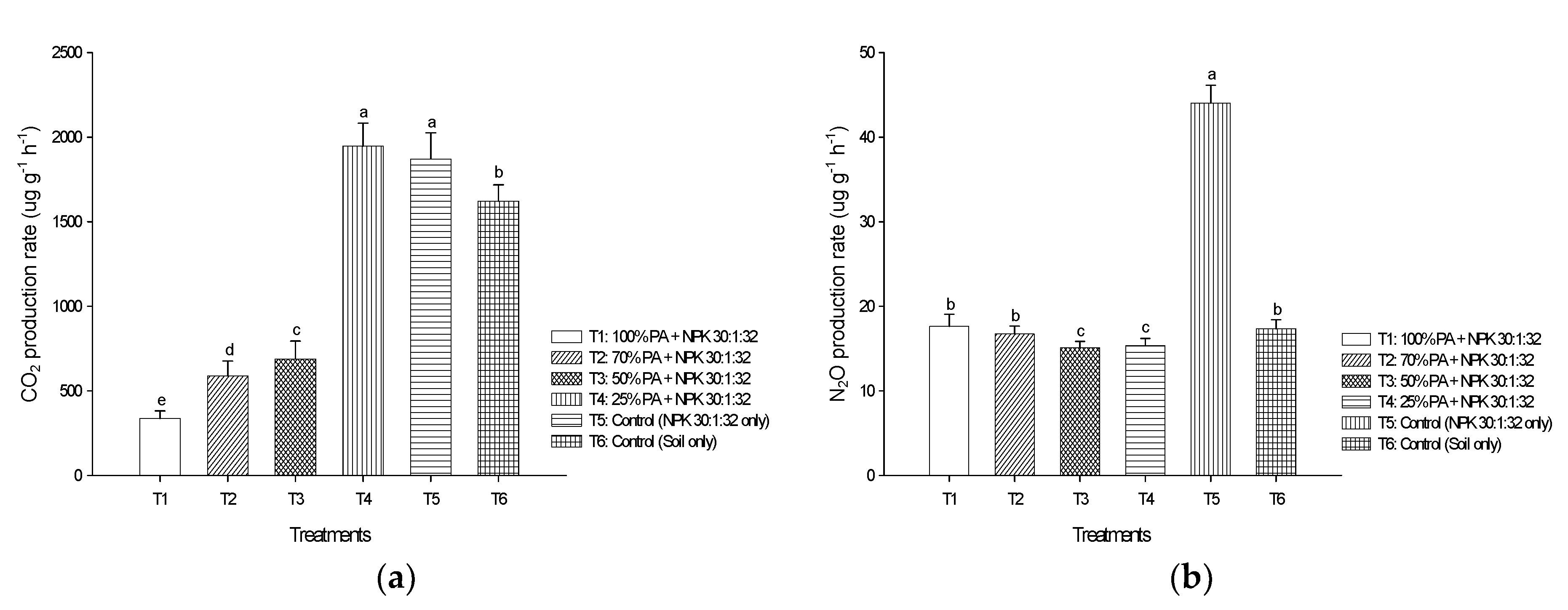
3.5. Soil CO2 and N2O Emissions from Laboratory Incubation Experiment
4. Discussion
4.1. Peat Soil Physicochemical Properties
4.2. Pineapple Residue Ash Application on CO2 and N2O Emissions in Peat Soils Cultivated with Pineapples
4.3. Pineapple Residue Ash on CO2 and N2O Emissions in Peat Soils—A Laboratory Incubation Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, O.H.; Ahmad Husni, M.H.; Rahim, A.A.; Mohd Hanafi, M. Sustainable Production of Pineapples on Tropical Peat Soils; Universiti Putra Malaysia Press: Serdang, Malaysia, 2013; pp. 1–141. [Google Scholar]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.M.; Rahman, A.A. Status and impact of pineapple technology on mineral soil. Econ. Technol. Manag. Rev. 2010, 5, 11–19. [Google Scholar]
- Marlier, M.E.; Liu, T.; Yu, K.; Buonocore, J.J.; Koplitz, S.N.; DeFries, R.S.; Mickley, L.J.; Jacob, D.J.; Schwartz, J.; Wardhana, B.S. Fires, smoke exposure, and public health: An integrative framework to maximize health benefits from peatland restoration. GeoHealth 2019, 3, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Page, S.E.; Hooijer, A. In the line of fire: The peatlands of Southeast Asia. Philos. Trans. R. Soc. B 2016, 371, 20150176. [Google Scholar] [CrossRef] [PubMed]
- Uda, S.K.; Hein, L.; Atmoko, D. Assessing the health impacts of peatland fires: A case study for Central Kalimantan, Indonesia. Environ. Sci. Pollut. Res. 2019, 26, 31315–31327. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Watanabe, C.; Yoshikawa, M.; Yamaguchi, C.; Yoshimura, N. Chemical compounds in gas emitted from tropical peat soil burning: With and without oxygen. In Proceedings of the International Symposium of Tropical Peatlands, Bogor, Indonesia, 22–23 November 1999; pp. 27–32. [Google Scholar]
- Ahmed, O.H.; Husni, M.H.A.; Syed Omar, R.S.; Hanafi, M.M.; Koh, S.K. The effect of residue management practices on phosphorus and potassium uptake in pineapple. Malays. J. Soil Sci. 1999, 3, 29–37. [Google Scholar]
- Klemedtsson, L.; Ernfors, M.; Björk, R.G.; Weslien, P.; Rütting, T.; Crill, P. Reduction of greenhouse gas emissions by wood application to a Picea abis (L.) Karst. Forest on a drained organic soil. Eur. J. Soil Sci. 2010, 61, 734–744. [Google Scholar] [CrossRef]
- Liimatainen, M.; Martikainen, P.J.; Maljanen, M. Why granulated wood ash decreases N2O production in boreal acidic peat soil? Soil Biol. Biochem. 2014, 79, 140–148. [Google Scholar] [CrossRef]
- Couwenberg, J.; Hoojier, A. Towards robust subsidence-based soil carbon emission factors for peat soils in South-East Asia, with special reference to oil palm plantations. Mires Peat 2013, 12, 1–13. Available online: http://www.mires-and-peat.net/pages/volumes/map12/map1201.php (accessed on 31 August 2014).
- Melling, L.; Henson, I.E. Greenhouse gas exchange of tropical peatlands—A review. J. Oil Palm Res. 2011, 23, 1087–1095. [Google Scholar]
- Page, S.E.; Morrison, R.; Malins, C.; Hoojier, A.; Rieley, J.O.; Jauhiainen, J. Review of Peat Surface Greenhouse Gas Emissions from Oil Palm Plantations in Southeast Asia; (ICCT White Paper 15); International Council on Clean Transportation: Washington, DC, USA, 2011; pp. 40–45. [Google Scholar]
- Choo, L.N.L.K.; Ahmed, O.H. Partitioning carbon dioxide emission and assessing dissolved organic carbon leaching of a drained peatland cultivated with pineapple at Saratok, Malaysia. Sci. World J. 2014, 906021. [Google Scholar] [CrossRef]
- Choo, L.N.L.K.; Ahmed, O.H. Nitrous oxide emission of a tropical peat soil grown with pineapple at Saratok, Malaysia. Sustain. Agric. Res. 2017, 6, 75–84. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Choo, L.N.L.K. Greenhouse Gas Emission and Carbon Leaching in Pineapple Cultivation on Tropical Peat Soil; Universiti Putra Malaysia Press: Serdang, Malaysia, 2015; pp. 1–145. [Google Scholar]
- Ahmed, O.H.; Jeffary, A.V.; Luta, W.; Choo, L.N.L.K. Tropical Peat Soil and Transportation of Greenhouse Gases; Universiti Putra Malaysia Press: Serdang, Malaysia, 2018; pp. 1–120. [Google Scholar]
- Jeffary, A.V.; Ahmed, O.H.; Heng, R.K.J.; Choo, L.N.L.K.; Omar, L. Horizontal and vertical emissions of carbon dioxide and methane from a tropical peat soil cultivated with pineapple (Ananas comosus (L.) Merr.). Sustain. Agric. Res. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Luta, W.; Ahmed, O.H.; Heng, R.K.J.; Choo, L.N.L.K. Water table fluctuation and carbon dioxide emission from a tropical peat soil cultivated with pineapples (Ananas comosus L. Merr.). Int. J. Biosci. 2017, 10, 172–178. [Google Scholar] [CrossRef]
- Kløve, B.; Sveistrup, T.E.; Hauge, A. Leaching of nutrients and emission of greenhouse gases from peatland cultivation at Bodin, Northern Norway. Geoderma 2010, 154, 219–232. [Google Scholar] [CrossRef]
- Maljanen, M.; Komulainen, V.-M.; Hytönen, J.; Martikainen, P.J.; Laine, J. Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics. Soil Biol. Biochem. 2004, 36, 1801–1808. [Google Scholar] [CrossRef]
- Kasimir-Klemedtsson, Å.; Klemedtsson, L.; Berglund, K.; Martikainen, P.; Silvola, J.; Oenema, O. Greenhouse gas emissions from farmed organic soils: A review. Soil Use Manag. 1997, 13, 245–250. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Veloo, R.; van Ranst, E.; Selliah, P. Peat characteristics and its impact on oil palm yield. NJAS Wagening. J. Life Sci. 2015, 72–73, 33–40. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Silvennoinen, H.; Hämäläinen, R.; Limin, S.; Raison, R.J.; Vasander, H. Nitrous oxide fluxes from tropical peat with different disturbance history and management. Biogeosciences 2012, 9, 1337–1350. [Google Scholar] [CrossRef]
- Maljanen, M.; Martikkala, M.; Koponen, H.T.; Virkajärvi, P.; Martikainen, P.J. Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil. Soil Biol. Biochem. 2007, 39, 914–920. [Google Scholar] [CrossRef]
- Hatala, J.A.; Detto, M.; Sonnentag, O.; Deverel, S.J.; Verfaille, J.; Baldocchi, D.D. Greenhouse gas (CO2, CH4, H2O) fluxes from drained and flooded agricultural peatlands in the Sacramento-San Joaquin Delta. Agr. Ecosyst. Environ. 2012, 150, 1–18. [Google Scholar] [CrossRef]
- Kechavarzi, C.; Dawson, Q.; Bartlett, M.; Leeds-Harrison, P.B. The role of soil moisture, temperature and nutrient amendment on CO2 efflux from agricultural peat soil microcosms. Geoderma 2010, 154, 203–210. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Kerojoki, O.; Silvennoinen, H.; Limin, S.; Vasander, H. Heterotrophic respiration in drained tropical peat is greatly affected by temperature—A passive ecosystem cooling experiment. Environ. Res. Lett. 2014, 9, 105013. [Google Scholar] [CrossRef]
- Mäkiranta, P.; Minkkinen, K.; Hytönen, J.; Laine, J. Factors causing temporal and spatial variation in heterotrophic and rhizospheric components of soil respiration in afforested organic soil croplands in Finland. Soil Biol. Biochem. 2008, 40, 1592–1600. [Google Scholar] [CrossRef]
- Murayama, S.; Bakar, Z.A. Decomposition of tropical peat soils 2. Estimation of in situ decomposition by measurement of CO2 flux. Jpn. Agric. Res. Q. 1996, 30, 153–158. [Google Scholar]
- Oktarita, S.; Hergoualc’h, K.; Anwar, S.; Verchot, V. Substantial N2O emissions from peat decomposition and N fertilization in an oil palm plantation exacerbated by hotspots. Environ. Res. Lett. 2017, 12, 104007. [Google Scholar] [CrossRef]
- Van Beek, C.L.; Pleijter, M.; Kuikman, P.J. Nitrous oxide emissions from fertilized and unfertilized grasslands on peat soil. Nutr. Cycl. Agroecosys. 2011, 89, 453–461. [Google Scholar] [CrossRef]
- Mohammed Selamat, M.; Abdul Rahman, H. Amalan kultur. In Penanaman Nanas—Nanas Makan Segar dan Nanas Kaleng; Mohammed Selamat, M., Ed.; MARDI: Serdang, Malaysia, 1996; pp. 22–34. [Google Scholar]
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Curr. Sci. 2002, 82, 510–517. [Google Scholar]
- Aerts, R.; Toet, S. Nutritional controls on carbon dioxide and methane emission from carex-dominated peat soils. Soil Biol. Biochem. 1997, 29, 1683–1690. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Chen, J.; Kim, H.; Yoo, G. Effects of biochar addition on CO2 and N2O emissions following fertilizer application to a cultivated grassland soil. PLoS ONE 2015, 10, e1026841. [Google Scholar] [CrossRef] [PubMed]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar properties influencing greenhouse gas emissions in tropical soils differing in texture and mineralogy. J. Environ. Qual. 2016, 45, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- NRE. Malaysia Biennial Update Report to the UNFCCC; Ministry of Natural Resources and Environment Malaysia: Putrajaya, Malaysia, 2015; pp. 31–44.
- Murdiyarso, D.; Hergoualc’h, K.; Verchot, L.V. Opportunities for reducing greenhouse gas emissions in tropical peatlands. Proc. Natl. Acad. Sci. USA 2010, 107, 19655–19660. [Google Scholar] [CrossRef] [PubMed]
- Reukeith, G.O. State to up Production of MD2 Variety Pineapples-Jabu. Available online: https://www.theborneopost.com/2015/12/01/state-to-up-production-of-md2-variety-pineapples-jabu/ (accessed on 30 November 2015).
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash management reviews—applications of biomass bottom ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Quirantes, M.; Calvo, F.; Romero, E.; Nogales, R. Soil nutrient availability affected by different biomass-ash applications. J. Soil Sci. Plant Nutr. 2016, 16, 159–163. [Google Scholar] [CrossRef]
- Salvo, M.; Rizzo, S.; Caldirola, M.; Novajra, G.; Canonico, F.; Bianchi, M.; Ferraris, M. Biomass ash as supplementary cementitious material (SCM). Adv. Appl. Ceram. 2015, 114, S3–S10. [Google Scholar] [CrossRef]
- Kalus, K.; Koziel, J.A.; Opaliński, S. A review of biochar properties and their utilization in crop agriculture and livestock production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A sustainable approach for improving plant growth and soil properties. In Biochar—An Imperative Amendment for Soil and the Environment; Abrol, V., Sharma, P., Eds.; IntechOpen: London, UK, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and biomass ash as a soil ameliorant: The effect on selected soil properties and yield of giant Miscanthus (Mischanthus x giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Tan, R.R. Data challenges in optimizing biochar-based carbon sequestration. Renew. Sustain. Energy Rev. 2019, 174–177. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołebiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Tomczzyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Jaji, K.; Man, N.; Nawi, N.M. Factors affecting pineapple market supply in Johor, Malaysia. Int. Food Res. J. 2018, 25, 366–375. [Google Scholar]
- DOA. Crop Statistics Booklet (Food Crop Sub-Sector); Department of Agriculture Malaysia: Putrajaya, Malaysia, 2018; p. 49. [Google Scholar]
- Othman, A.F. Pineapples New Source of Wealth for Malaysia. Available online: https://www.nst.com.my/news/nation/2017/12/313347/pineapples-new-source-wealth-malaysia (accessed on 11 December 2017).
- Ahmed, O.H.; Husni, A.M.H.; Anuar, R.A.; Hanafi, M.M. Alternative means of recycling pineapple leaf residues. Fruits 2003, 58, 53–60. [Google Scholar] [CrossRef][Green Version]
- Daud, Z.; Hatta, M.Z.M.; Kassim, A.S.M.; Aripin, A.M. Suitability of Malaysia’s pineapple leaf and napier grass as fibre substitution for paper making industry. In Proceedings of the 6th Engineering Conference—Energy and Environment, Kuching, Malaysia, 2–4 July 2013; p. 4. [Google Scholar]
- Liu, C.H.; Liu, Y.; Fan, C.; Kuang, S.Z. The effects of composted pineapple residue return on soil properties and the growth and yield of pineapple. J. Soil Sci. Plant Nutr. 2013, 433–444. [Google Scholar] [CrossRef]
- dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Lim, E.T. Peat Soils of Sarawak and the Analytical Methods; Department of Agriculture Sarawak: Kuching, Malaysia, 1991; pp. 25–28. [Google Scholar]
- Ismail, A.B.; Asing, J.; Zulkefli, M. Residual impact of various land clearing techniques on peat chemical characteristics. In A Case Study at MARDI Peat Research Station, Sessang, Sarawak, Malaysia; Ismail, A.B., Ong, H.K., Mohamad Hanif, M.J., Umi Kalsom, M.S., Eds.; MARDI: Serdang, Malaysia, 2007; pp. 33–61. [Google Scholar]
- Harada, Y.; Inoko, A. The measurement of the cation exchange capacity of composts for the estimation of the degree of maturity. Soil Sci. Plant Nutr. 1980, 26, 127–134. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Bremner, J.M.; Keeney, D.R. Determination and isotope-ratio analysis of different forms of nitrogen in soils: 3. Exchangeable ammonium, nitrate and nitrite by extraction-distillation methods. Soil Sci. Soc. Am. J. 1966, 30, 577–582. [Google Scholar] [CrossRef]
- Leng, L.Y.; Husni, M.H.; Samsuri, A.W. Comparison of the carbon-sequestering abilities of pineapple leaf residue chars produced by controlled combustion and by field burning. Bioresour. Technol. 2011, 102, 10759–10762. [Google Scholar] [CrossRef] [PubMed]
- Wiersum, L.K.; Bakema, K. Competitive adaptation of the cation exchange capacity of roots. Plant Soil 1959, 11, 287–292. [Google Scholar] [CrossRef]
- Abbas, H.; Mahmood, Z.; Maamun, T.M.T.; Ghazali, M.S. Formulation for pineapple in Malaysia. MARDI Tech. Bull. 2015, 8, 7–15. [Google Scholar]
- Choo, L.N.L.K.; Ahmed, O.H.; Nik Majid, N.M.; Ab Aziz, Z.F. Improving nitrogen availability on a tropical peat soil cultivated with Ananas comosus L. Merr. using pineapple residue ash. J. Soil Sci. Plant Nutr. 2020, 20, 657–672. [Google Scholar] [CrossRef]
- Lehmann, J.; Kaiser, K.; Peter, I. Exchange resin cores for the estimation of nutrient fluxes in highly permeable tropical soil. J. Plant Nutr. Soil Sci. 2001, 164, 57–64. [Google Scholar] [CrossRef]
- IAEA. Methane and nitrous oxide flux measurements form soil and plant systems. In Manual on Measurement of Methane and Nitrous Oxide Emissions from Agriculture; IAEA-TECDOC-674; IAEA: Vienna, Austria, 1992; pp. 45–79. [Google Scholar]
- Widén, B.; Lindroth, A. A calibration system for soil carbon dioxide—efflux measurement chambers. Soil Sci. Soc. Am. J. 2003, 67, 327–334. [Google Scholar] [CrossRef]
- Sim, A.K.F.; Ishak, C.F.; Hanif, A.H.M.; Melling, L. Effect of N fertilization on N2O emission from a tropical peat soil: A laboratory incubation study. In Proceedings of the 14th International Peat Congress: Peatlands in Balance, Stockholm, Sweden, 3–8 June 2012; p. 7. [Google Scholar]
- Andriesse, J.P. Nature and Management of Tropical Peat Soils; FAO Soils Bulletin 59; FAO: Rome, Italy, 1988. [Google Scholar]
- Funakawa, S.; Yonebayashi, K.; Shoon, J.F.; Khun, E.C.O. Nutritional environment of tropical peat soils in Sarawak, Malaysia based on soil solution composition. Soil Sci. Plant Nutr. 1996, 42, 833–843. [Google Scholar] [CrossRef]
- Hashim, S.A.; Teh, C.B.S.; Ahmed, O.H. Influence of water table depths, nutrient leaching losses, subsidence of tropical peat soil and oil palm (Elaeis guineensis Jacq.) seedling growth. Malays. J. Soil Sci. 2019, 23, 13–30. [Google Scholar]
- Huat, B.B.K.; Kazemian, S.; Prasad, A.; Barghchi, M. State of an art review of peat: General perspective. Int. J. Phys. Sci. 2011, 6, 1988–1996. [Google Scholar]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat under stabilized land uses. Mires Peat 2015, 16, 8. Available online: http://www.mires-and-peat.net/pages/volumes/map16/map1608.php (accessed on 16 September 2016).
- MARDI. Master Plan for Malaysian Agricultural Research and Development Institute Sessang Peat Research Station; MARDI: Serdang, Malaysia, 1996; pp. 16–22.
- Murtedza, M.; Padmanabhan, E.; Mei, B.L.H.; Siong, W.B. The Peat Soils of Sarawak, STRAPEAT Status Report; UNIMAS: Sarawak, Malaysia, 2002; pp. 1–16. [Google Scholar]
- Othman, H.; Darus, F.M.; Mohammed, A.T. Experiences in peat development for oil palm planting in the MPOB Research Station at Sessang, Saratok. Oil Palm Bull. 2009, 58, 1–13. [Google Scholar]
- Reeza, A.A.; Hussin, A.; Hanif, A.H.M.; Sukari, M.A.M. Effect of liming and fertilizer application in hemic and sapric of tropical peat: Phosphorus mineralization, infra-red spectroscopy and microscopy. Am. J. Agric. Biol. Sci. 2014, 9, 321–333. [Google Scholar] [CrossRef]
- Mutalib, A.A.; Lim, J.S.; Wong, M.H.; Koonvai, L. Characterization, distribution, and utilization of peat in Malaysia. In Proceedings of the International Symposium on Tropical Peatland, Kuching, Malaysia, 6–10 May 1991; pp. 7–16. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Masiello, C.A. Controls on black carbon storage in soils. Glob. Biogeochem. Cycles 2007, 21, GB3005. [Google Scholar] [CrossRef]
- Yangyuoru, M.; Boateng, E.; Adiku, S.G.K.; Acquah, D.; Adjadeh, T.A.; Mawunya, F. Effects of natural and synthetic soil conditioners on soil moisture rentention and maize yield. West Afr. J. Appl. Ecol. 2006, 9, 1–8. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Moradshashi, A.; Vines, H.M.; Black, C.C. CO2 exchange and acidity levels in pineapple, Ananas comosus (L.), Merr., leaves during the day at various temperature, O2 and CO2 concentrations. Plant Physiol. 1977, 59, 274–278. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Bunthawin, S. Photosynthesis in pineapple (Ananas comosus comosus [L.] Merr) measured using PAM (Pulse Amplitude Modulation) Fluorometry. Trop. Plant Biol. 2010, 3, 193–203. [Google Scholar] [CrossRef]
- Mohammed Selamat, M. Biologi tanaman dan keperluan persekitaran. In Penanaman Nanas—Nanas Makan Segar dan Nanas Kaleng; Mohammed Selamat, M., Ed.; MARDI: Serdang, Malaysia, 1996; pp. 7–16. [Google Scholar]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modeling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Perrin, T.S.; Boettinger, J.L.; Drost, D.T.; Norton, J.M. Decreasing nitrogen leaching from sandy soil with ammonium-loaded clinoptilolite zeolite. J. Environ. Qual. 1998, 27, 656–663. [Google Scholar] [CrossRef]
- Mäkiranta, P.; Laiho, R.; Fritze, H.; Hytönen, J.; Laine, J.; Minkkinen, K. Indirect regulation of heterotrophic peat soil respiration by water level via microbial community structure and temperature sensitity. Soil Biol. Biochem. 2009, 41, 695–703. [Google Scholar] [CrossRef]
- Chan, S.L.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. Equilibrium, kinetic and thermodynamic studies of a new potential biosorbent for the removal of basic blue 3 and congo red eyes: Pineapple (Ananas comosus) plant stem. J. Taiwan Inst. Chem. 2016, 61, 306–315. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Ahmad Zaini, M.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeter. Biodegr. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Luey, J.K.; Darab, J.G.; Autrey, T.S.; Vienna, J.D.; Wigent, H.L. Characterization of Offgas Generated during Calcination of Incinerator Ash Surrogates; PNNL-11982-UC-2000; Pacific Northwest National Laboratory: Washington, DC, USA, 1999; pp. 22–34. [Google Scholar]
- Pardo, M.E.S.; Cassellis, M.E.R.; Escobedo, R.M.; Garcia, E.J. Chemical characterization of the industrial residues of the pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K. Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biol. Biochem. 2011, 43, 923–931. [Google Scholar] [CrossRef]
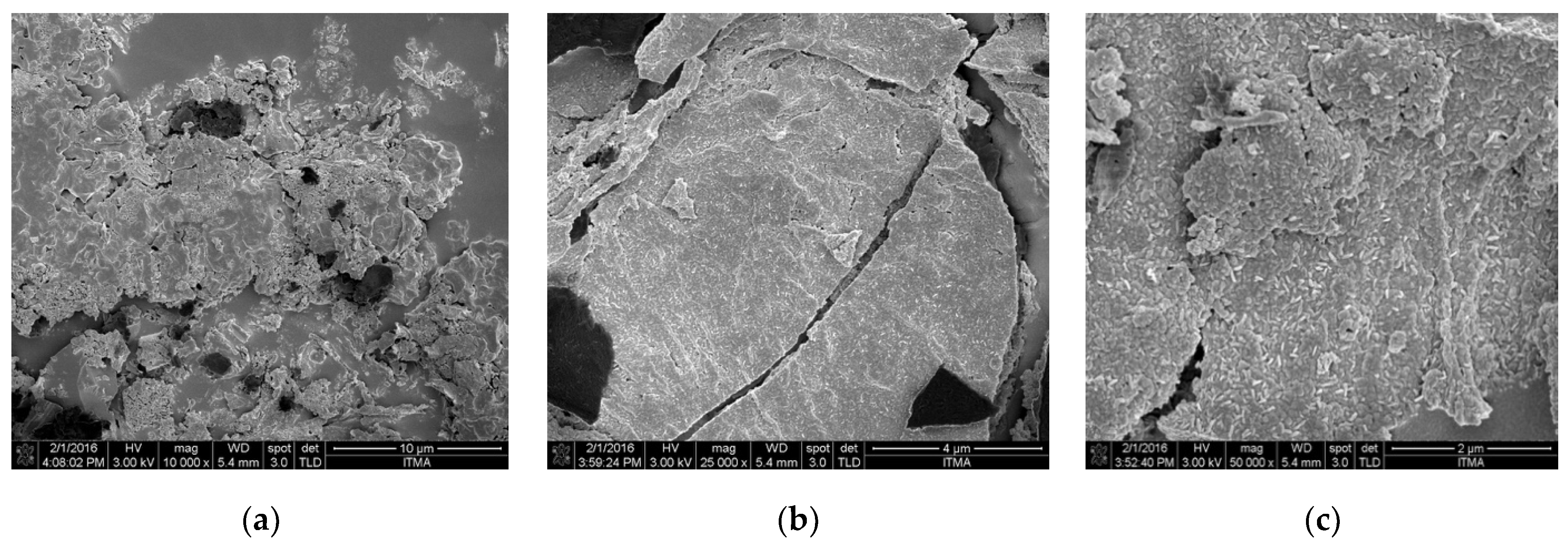

| Fertilization Treatments | Application Rate (per Plant) | |
|---|---|---|
| T1 | 100% PA + compound NPK fertilizer | 20.0 g of PA + 20 g of NPK fertilizer 30:1:32 |
| T2 | 70% PA + compound NPK fertilizer | 14.0 g of PA + 20 g of NPK fertilizer 30:1:32 |
| T3 | 50% PA + compound NPK fertilizer | 10.0 g of PA + 20 g of NPK fertilizer 30:1:32 |
| T4 | 25% PA + compound NPK fertilizer | 5.0 g of PA + 20 g of NPK fertilizer 30:1:32 |
| T5 | Control: Compound NPK fertilizer only | 20 g of NPK fertilizer 30:1:32 |
| T6 | Control: Peat soil alone (without fertilizer) | Nil |
| Variable | Value Obtained per Soil Depth (cm) | Reported Range | ||
|---|---|---|---|---|
| 0 to 20 cm | 20 to 40 cm | 40 to 60 cm | ||
| Bulk density (g cm−3) | 0.14 a ± 0.003 | 0.13 a ± 0.002 | 0.13 a ± 0.002 | 0.1 to 0.2 [80] 0.12–0.20 [81] 0.09–0.16 [84] |
| Moisture (%) | 81.2 c ± 0.5 | 85.6 b ± 0.4 | 89.3a ± 0.3 | 90–95 [83] 75.45 [76] |
| pH | 3.9 a ± 0.1 | 3.9 a ± 0.1 | 3.9 a ± 0.1 | 3.0–4.5 [80] 3.59–3.90 [78] |
| Electrical conductivity (μS cm−1) | 177.4 a ± 2.3 | 176.1 a ± 1.5 | 173.2 a ± 1.7 | 159.8–358 [78] <200 [82] |
| Cation exchange capacity (cmol(+) kg−1) | 143.2 a ± 11.1 | 135.5 a ± 10.2 | 139.5 a ± 14.4 | 200 [77] 161.1 [79] 145 [82] |
| Total organic carbon (%) | 41.8 a ± 0.5 | 41.1 a ± 0.3 | 40.7 a ± 0.4 | 12–60 [77] 24.86 [84] 62.2 [85] |
| Total nitrogen (%) | 1.39 a ± 0.02 | 1.13 b ± 0.01 | 1.11 b ± 0.02 | 1.10–1.67 [83] 1.21–2.98 [84] 1.34 [85] |
| Ammonium-nitrogen (mg kg−1) | 1098.3 a ± 15.6 | 1081.4 a ± 14.7 | 738.2 b ± 11.3 | 642.1 [76] |
| Nitrate-nitrogen (mg kg−1) | 549.1 a ± 9.8 | 445.3 b ± 10.6 | 322.9 c ± 14.1 | 174.42 [76] |
| Properties | Values |
|---|---|
| pH | 12.34 (±0.02) |
| Cation exchange capacity (cmol+ kg−1) | 23.0 (±0.15) |
| Chemical composition (weight %) | O2: 67.5 |
| Mg: 20.6 | |
| Ca: 6.8 | |
| P: 4.0 | |
| K: 1.1 | |
| Functional groups (cm−1) | OH: 3696.17 |
| CH2: 1415.87 | |
| C-O: 1038.83 | |
| Surface area (m2 g−1) | 365.2 |
| Treatments | Pineapple Plant Age (Soil pH) | Exchangeable Ammonium (mg kg−1) | Available Nitrate (mg kg−1) | ||
|---|---|---|---|---|---|
| 3 Months | 6 Months | 9 Months | |||
| T1 | 5.84 b ± 0.06 | 5.99 b ± 0.06 | 6.31 c ± 0.03 | 688.76 d ± 5.67 | 279.68 c ± 2.44 |
| T2 | 6.13 a ± 0.07 | 6.18 a ± 0.05 | 6.36 b ± 0.07 | 553.42 e ± 4.39 | 231.85 e ± 3.06 |
| T3 | 5.87 b ± 0.12 | 6.02 b ± 0.08 | 6.43 a ± 0.05 | 1438.68 b ± 10.37 | 246.62 d ± 4.27 |
| T4 | 5.66 c ± 0.09 | 5.96 c ± 0.02 | 6.05 d ± 0.07 | 1465.52 a ± 8.05 | 577.13 a ± 2.85 |
| T5 | 4.40 d ± 0.03 | 4.22 d ± 0.02 | 4.19 e ± 0.02 | 704.25 c ± 3.76 | 324.93 b ± 7.46 |
| T6 | 3.92 e ± 0.07 | 4.14 e ± 0.02 | 4.05 f ± 0.01 | 1460.40 a ± 7.15 | 237.61 e ± 5.74 |
| Treatments | Total Soluble Solids (°Brix) | Fresh Fruit Weight (kg) |
|---|---|---|
| T1 | 13.62 a ± 0.02 | 2.10 a ± 0.05 |
| T2 | 13.48 ab ± 0.06 | 2.00 ab ± 0.003 |
| T3 | 13.51 ab ± 0.03 | 1.92 b ± 0.02 |
| T4 | 13.29 b ± 0.05 | 1.80 c ± 0.01 |
| T5 | 12.82 c ± 0.05 | 1.72 cd ± 0.04 |
| T6 | 12.65 c ± 0.09 | 1.61 d ± 0.01 |
| Variable | Pineapple Growth Period (Soil Temperature) | ||
|---|---|---|---|
| March 2017 (3 Months Old) | June 2017 (6 Months Old) | September 2017 (9 Months Old) | |
| Soil CO2 emission | r = 0.07204 p = 0.2089 | r = −0.09884 p = 0.0756 | r = 0.04833 p = 0.3729 |
| Soil N2O emission | r = −0.21878 p = 0.0001 | r = 0.00602 p = 0.9140 | r = −0.08808 p = 0.1039 |
| Soil temperature (°C) | |||
| Morning | 26.6 d | 27.5 b | 25.9 d |
| Noon | 29.1 b | 29.9 a | 29.6 a |
| Evening | 30.4 a | 30.3 a | 29.6 a |
| Midnight | 27.7 c | 27.7 b | 27.2 b |
| Morning-following day | 26.1 d | 26.7 c | 26.5 c |
| Temperature (°C) | |||
| Mean day-time temperature | 29.2 | 29.5 | 28.7 |
| Mean night-time temperature | 24.3 | 25.0 | 24.4 |
| Mean day- and night-time temperature differences | 4.9 | 4.5 | 4.3 |
| Treatments | pH | Exchangeable Ammonium (mg kg−1) | Available Nitrate (mg kg−1) |
|---|---|---|---|
| T1 | 8.18 a ± 0.02 | 898.01 d ± 2.19 | 31.68 a ± 0.22 |
| T2 | 8.08 a ± 0.02 | 1218.61 b ± 3.48 | 23.70 c ± 0.92 |
| T3 | 7.99 b ± 0.01 | 847.03 d ± 5.27 | 21.97 d ± 0.91 |
| T4 | 7.49 b ± 0.03 | 1067.88 c ± 3.62 | 23.21 c ± 0.49 |
| T5 | 4.71 c ± 0.02 | 1318.93 a ± 3.29 | 21.38 d ± 0.93 |
| T6 | 4.10 c ± 0.02 | 110.49 e ± 1.97 | 25.47 b ± 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, L.N.L.K.; Ahmed, O.H.; Majid, N.M.N.; Aziz, Z.F.A. Pineapple Residue Ash Reduces Carbon Dioxide and Nitrous Oxide Emissions in Pineapple Cultivation on Tropical Peat Soils at Saratok, Malaysia. Sustainability 2021, 13, 1014. https://doi.org/10.3390/su13031014
Choo LNLK, Ahmed OH, Majid NMN, Aziz ZFA. Pineapple Residue Ash Reduces Carbon Dioxide and Nitrous Oxide Emissions in Pineapple Cultivation on Tropical Peat Soils at Saratok, Malaysia. Sustainability. 2021; 13(3):1014. https://doi.org/10.3390/su13031014
Chicago/Turabian StyleChoo, Liza Nuriati Lim Kim, Osumanu Haruna Ahmed, Nik Muhamad Nik Majid, and Zakry Fitri Abd Aziz. 2021. "Pineapple Residue Ash Reduces Carbon Dioxide and Nitrous Oxide Emissions in Pineapple Cultivation on Tropical Peat Soils at Saratok, Malaysia" Sustainability 13, no. 3: 1014. https://doi.org/10.3390/su13031014
APA StyleChoo, L. N. L. K., Ahmed, O. H., Majid, N. M. N., & Aziz, Z. F. A. (2021). Pineapple Residue Ash Reduces Carbon Dioxide and Nitrous Oxide Emissions in Pineapple Cultivation on Tropical Peat Soils at Saratok, Malaysia. Sustainability, 13(3), 1014. https://doi.org/10.3390/su13031014






