Humidification-Dehumidification Desalination System Powered by Simultaneous Air-Water Solar Heater
Abstract
:1. Introduction
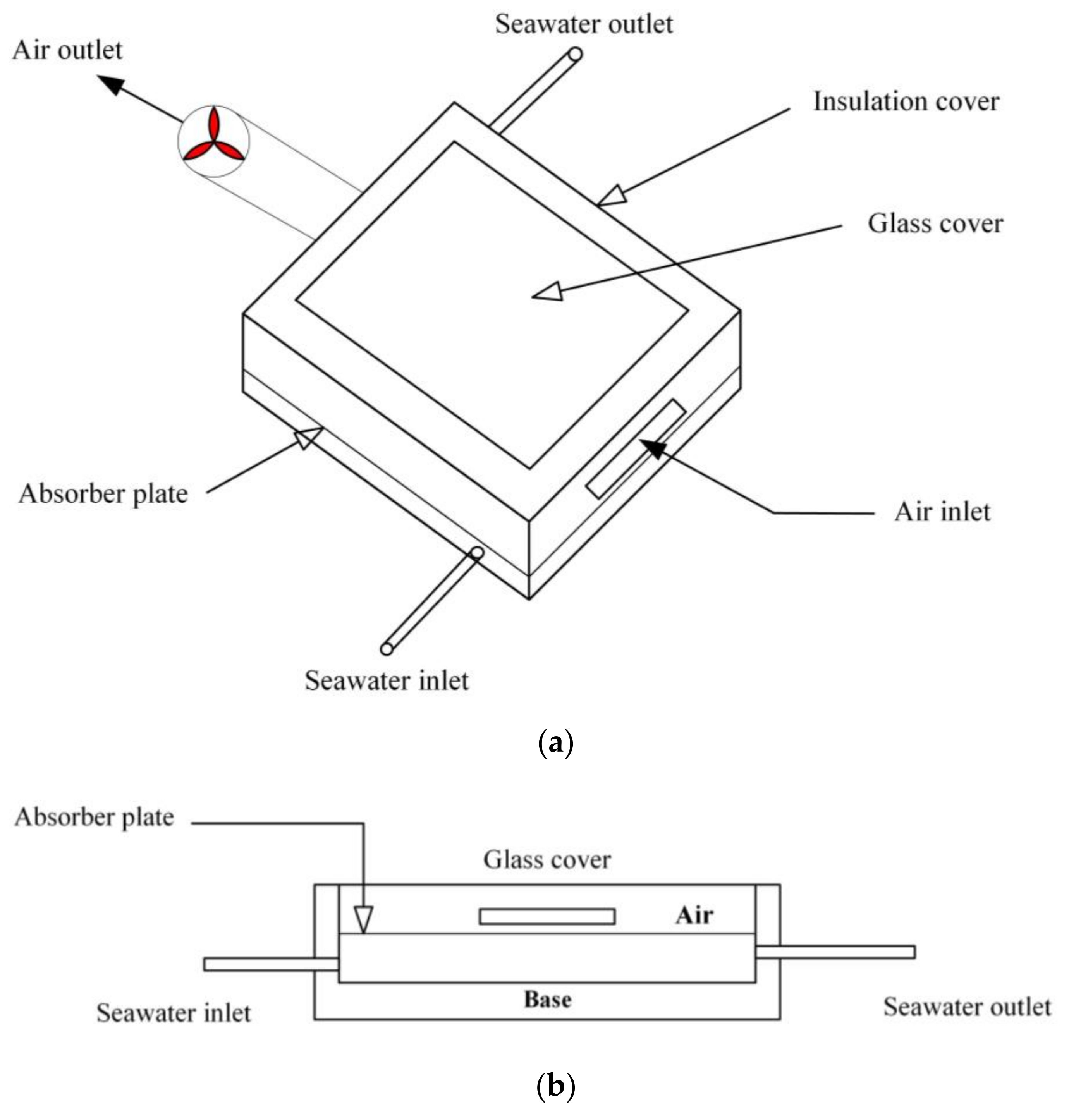
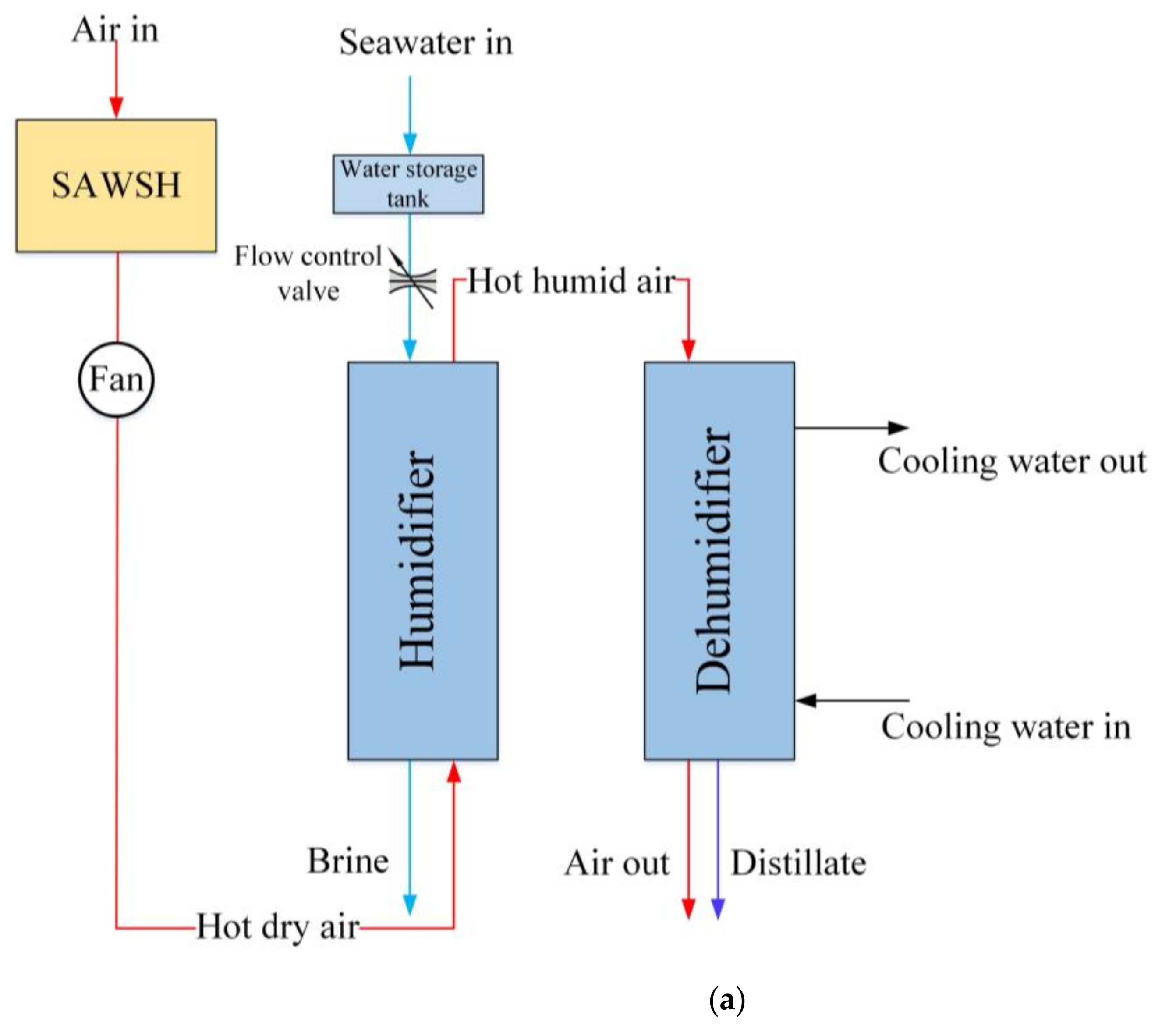
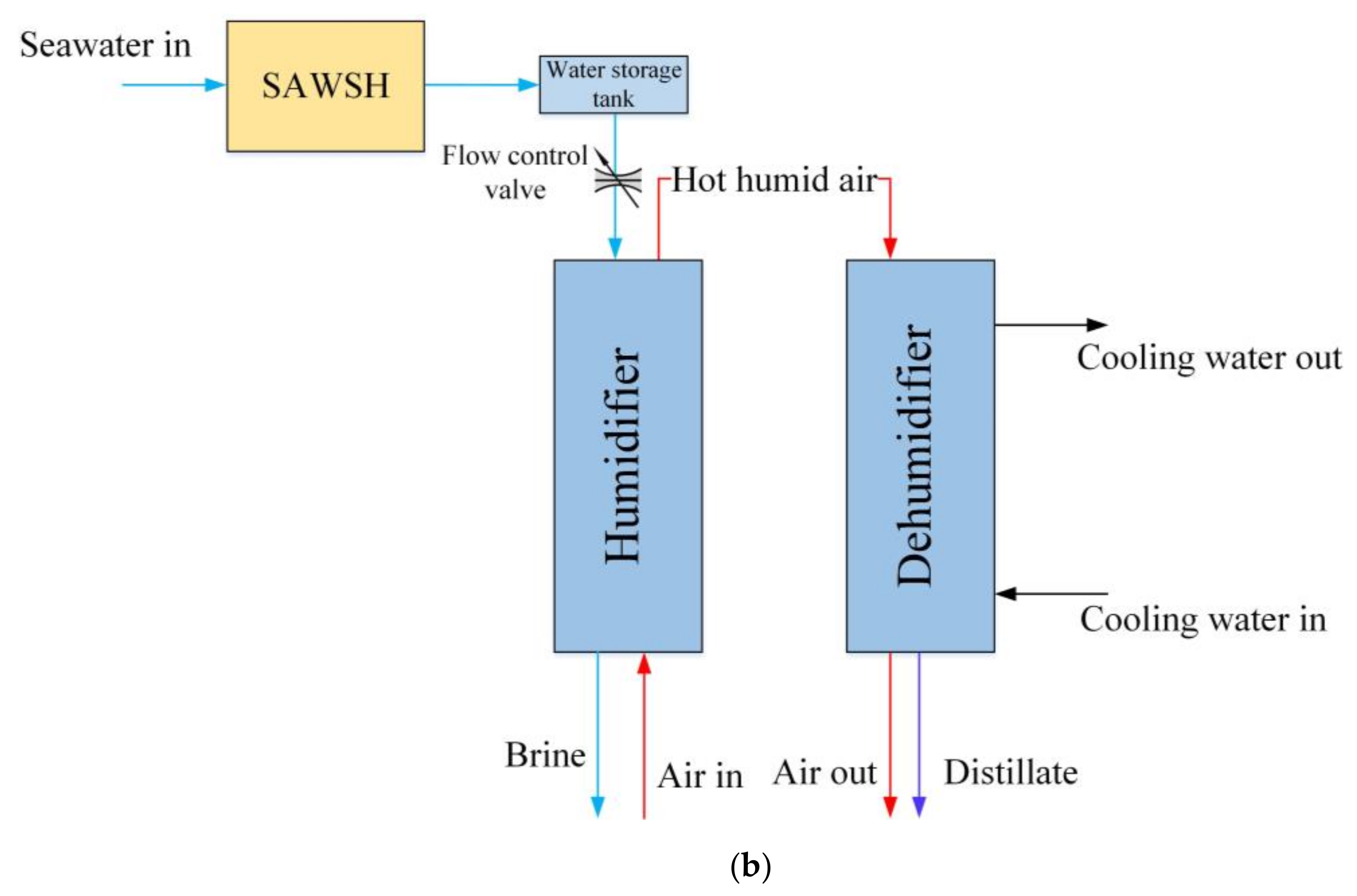
2. System Description
3. Mathematical Modeling
- The system operates under a steady-state condition.
- Pressure and heat losses from the HDH system components are neglected.
3.1. Modeling of SAWSH Unit
3.1.1. Glass Cover
3.1.2. Air Stream
3.1.3. Absorber Plate
3.1.4. Base
3.1.5. Water
3.2. Modeling of HDH System
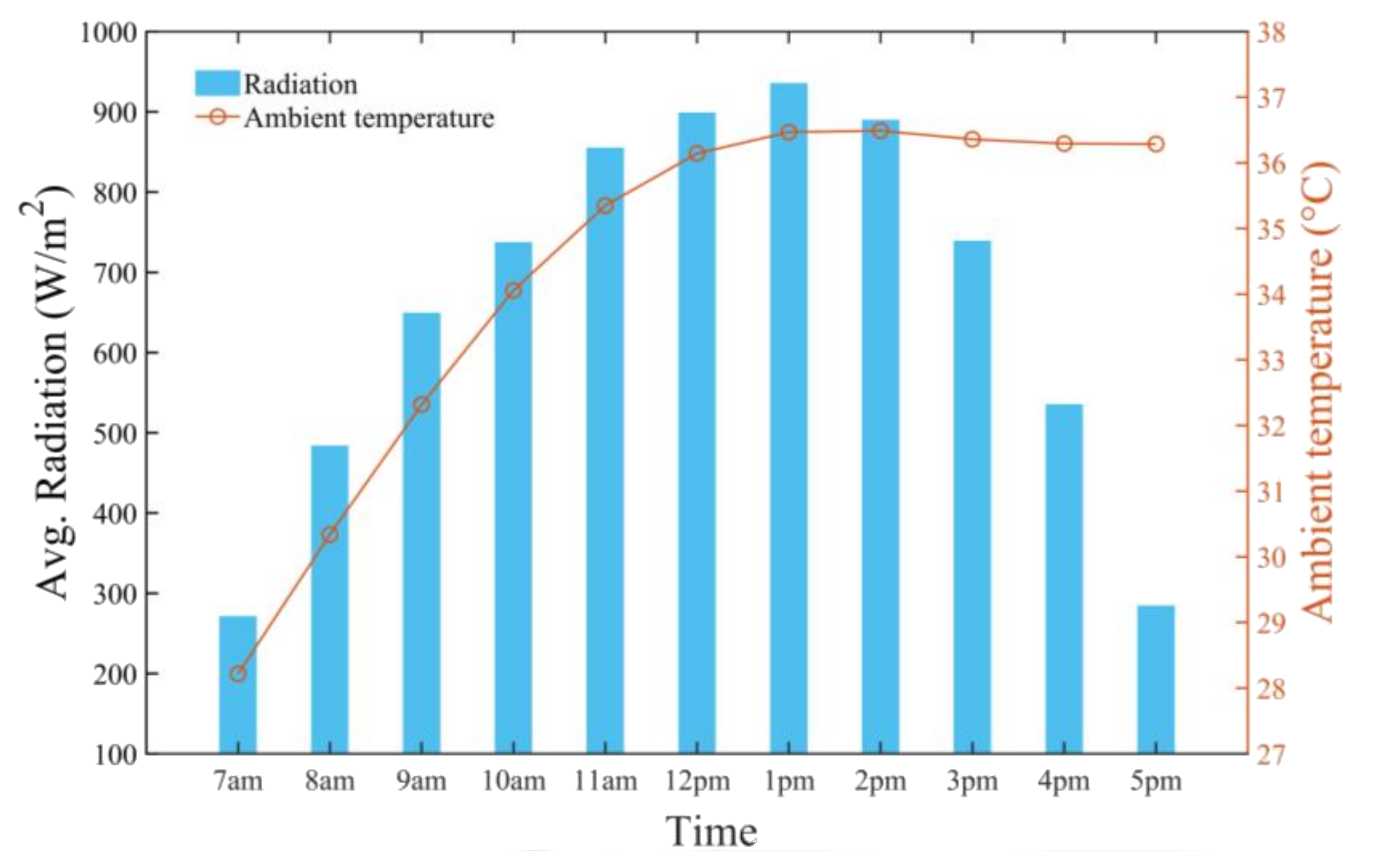
4. Results and Discussion
5. Conclusions
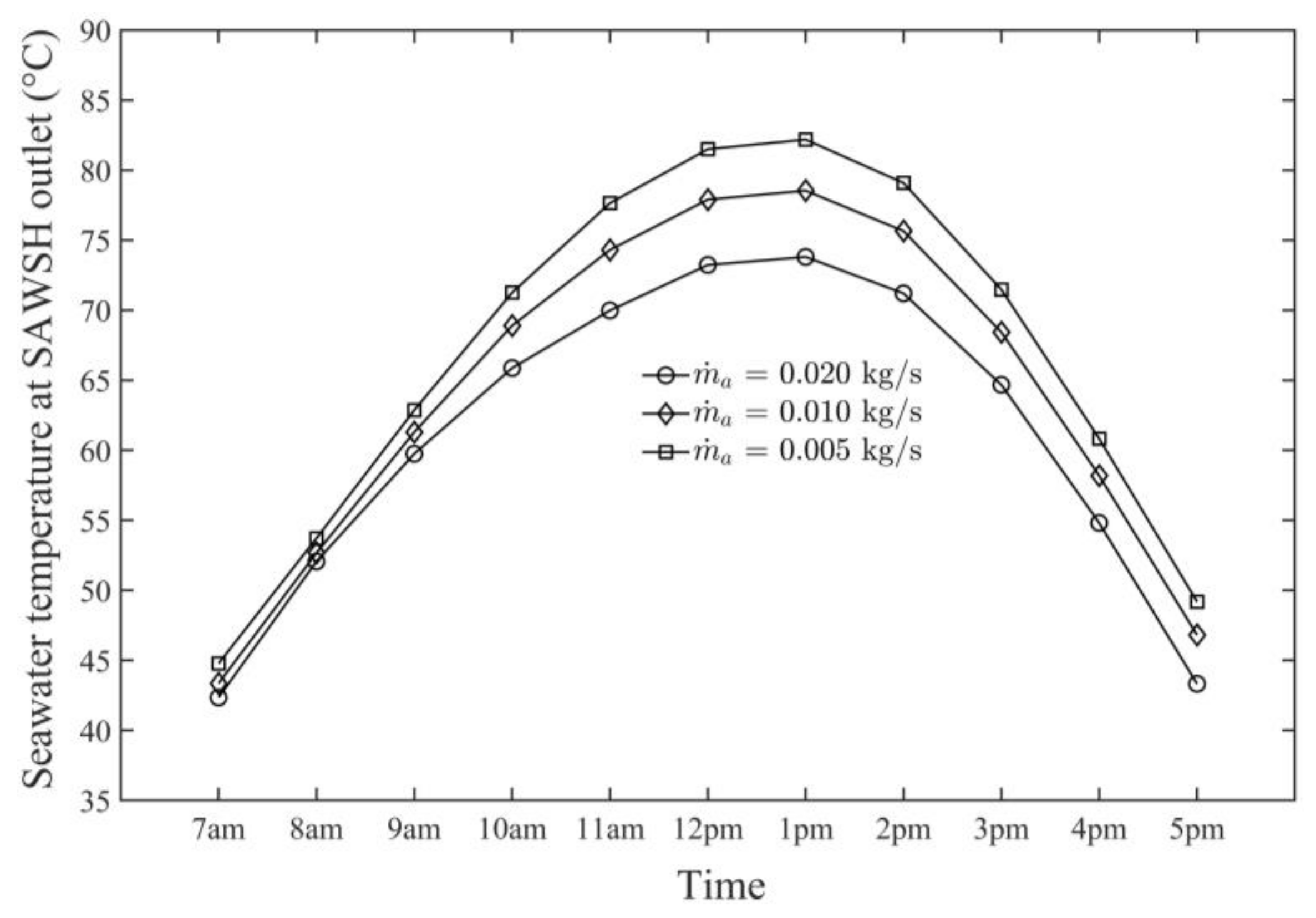
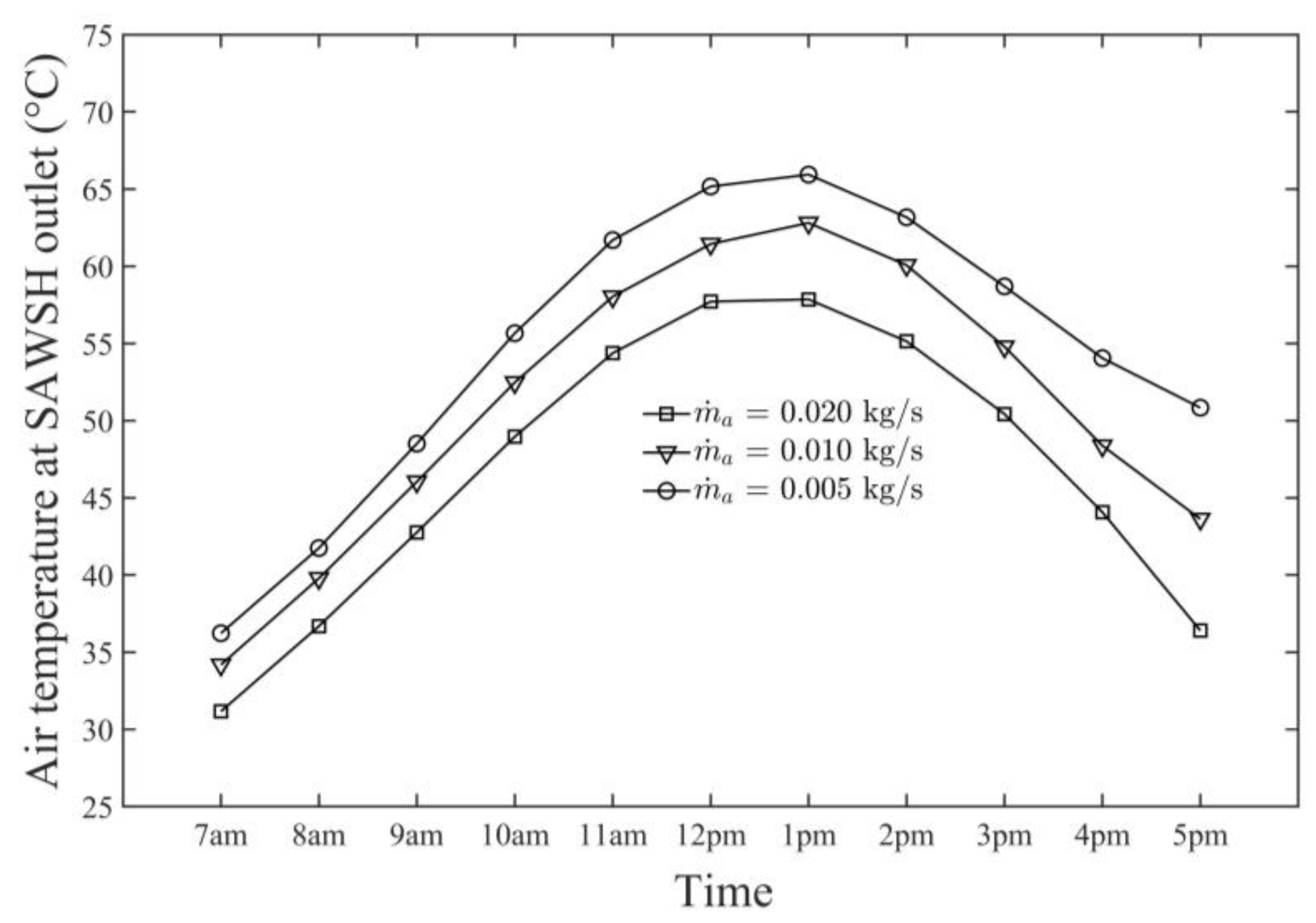
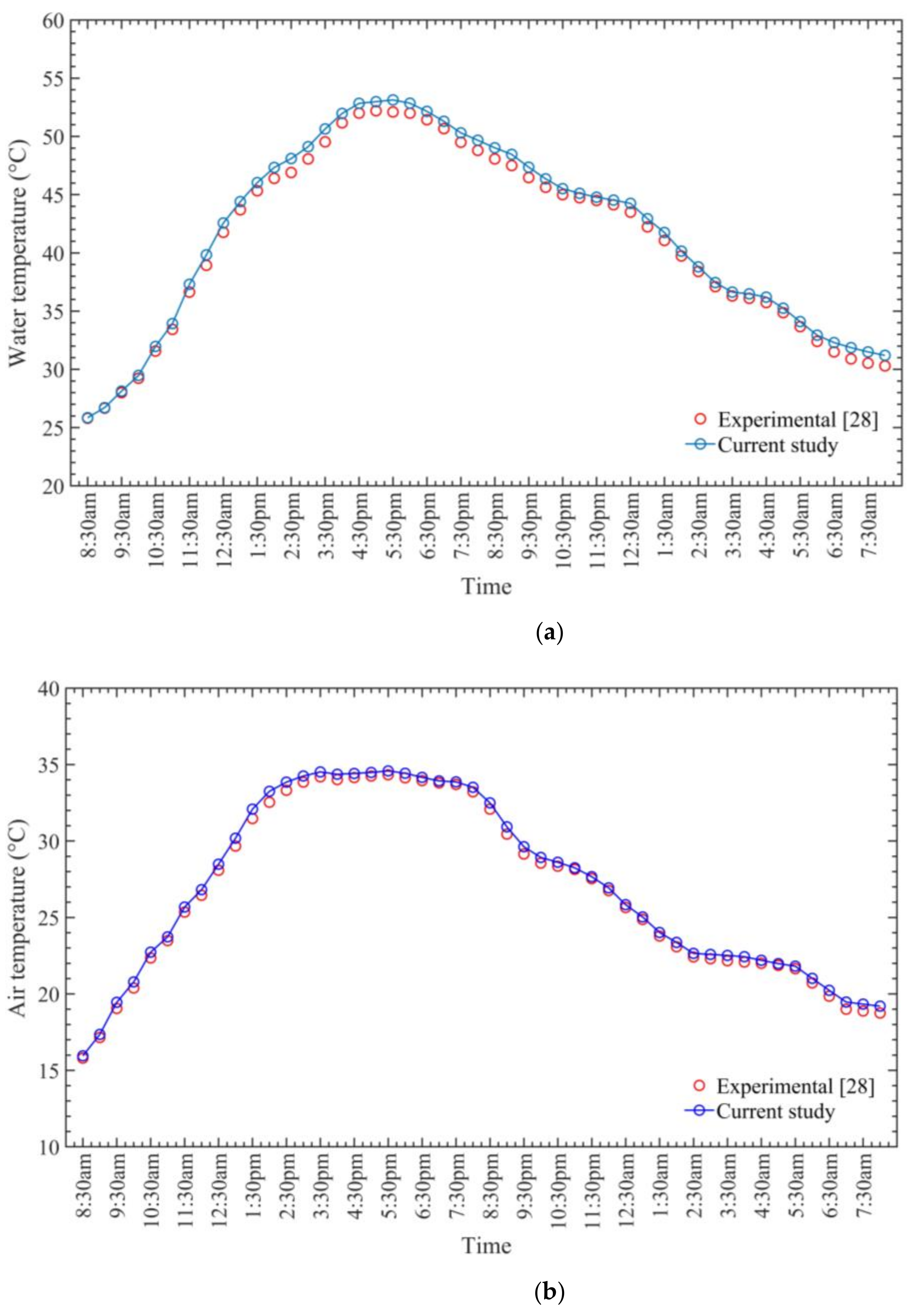
- The average daily maximum air and seawater temperature values obtained by the SAWSH were 66 and 83 °C, respectively, on 21 June at 1 p.m.
- The SAWSH-HDH system performed well at lower air mass flow rates. At mass flow rates of 0.005, 0.010, and 0.020 kg/s, the maximum air temperature values were 66, 62, and 57 °C, respectively, whereas the maximum seawater temperature values were 83, 78, and 73 °C, respectively.
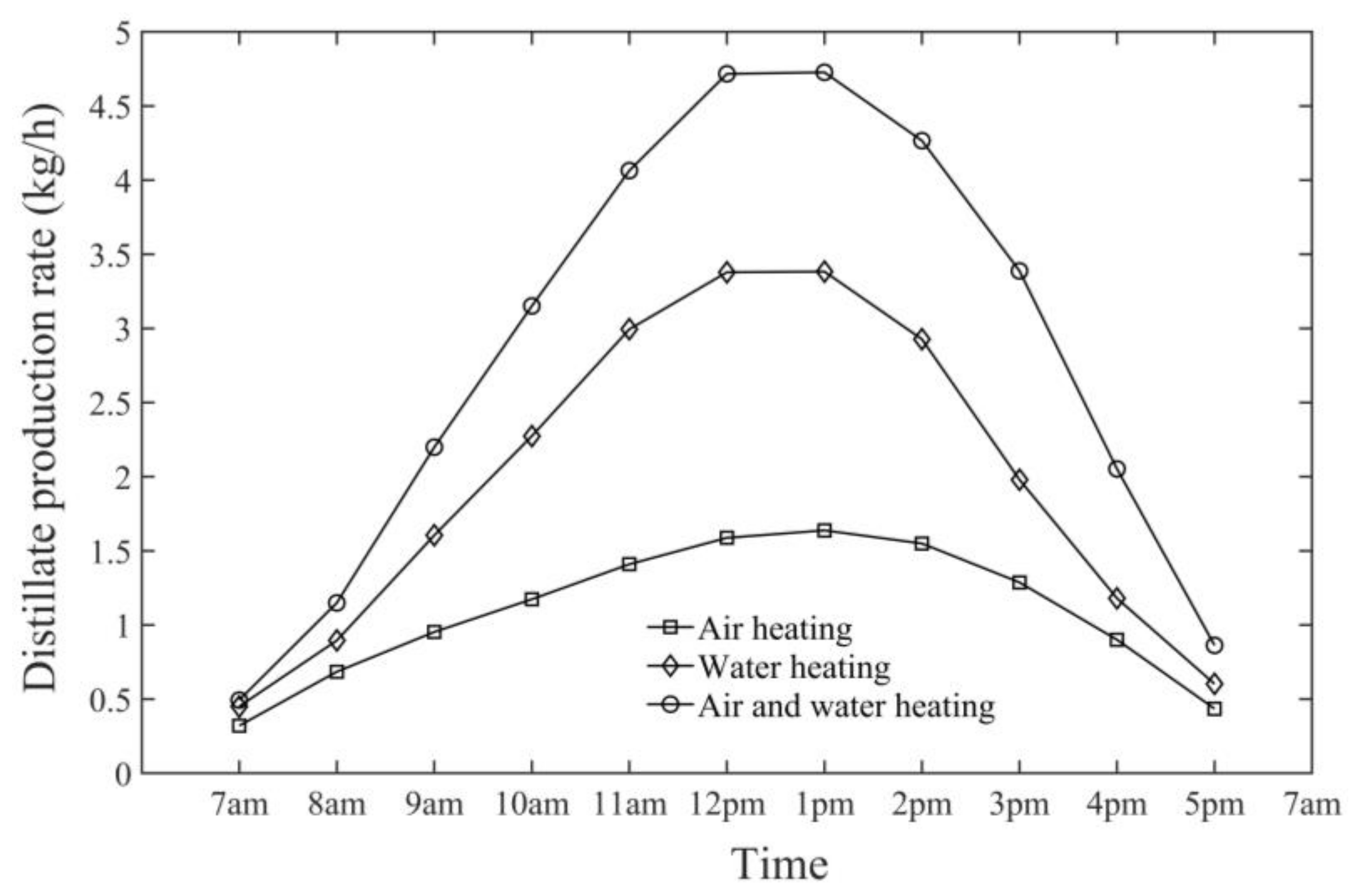
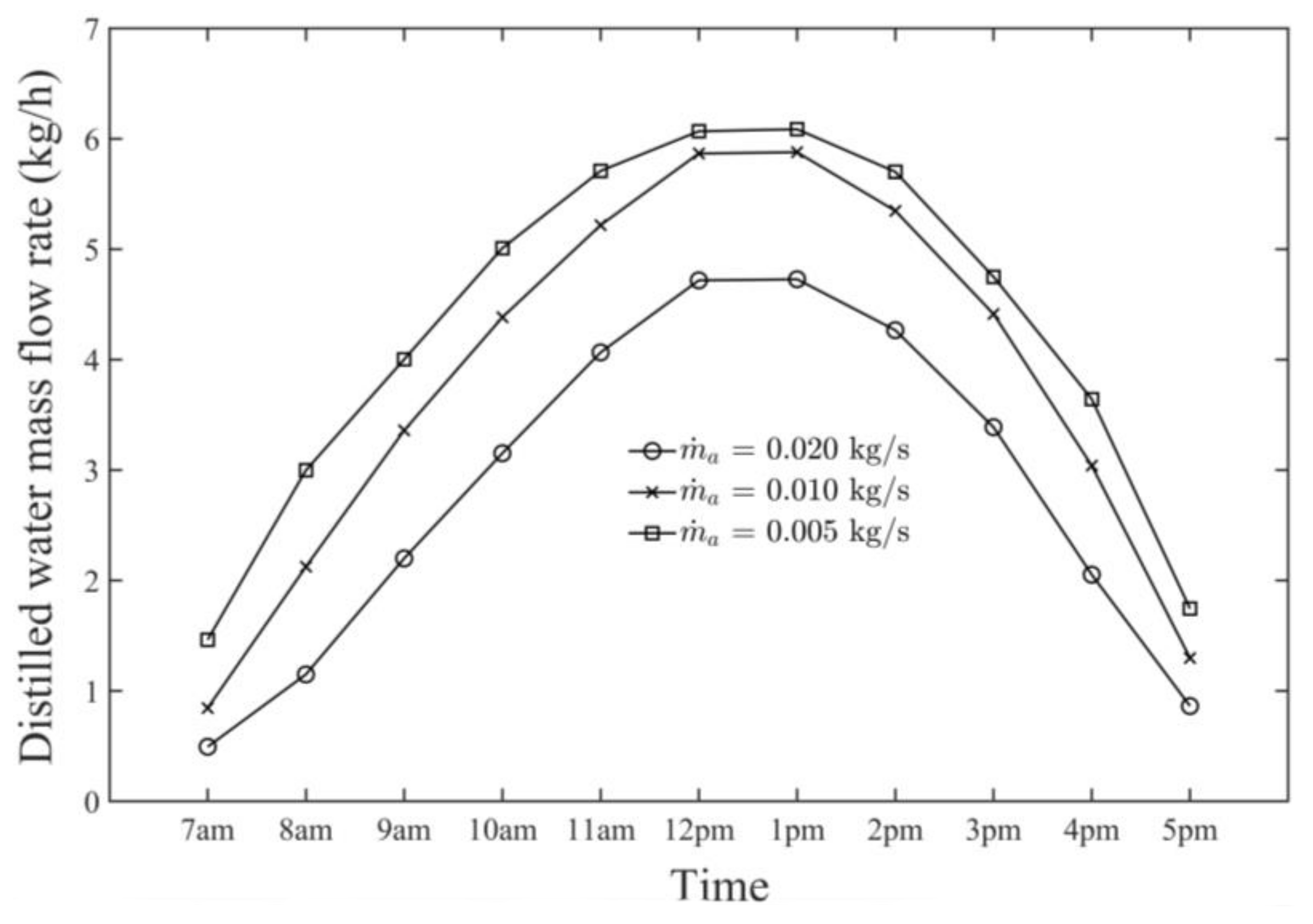
- The observed hourly maximum productivity (i.e., distillate water production rate) from the HDH system was 6.20 kg/h at 1 p.m. on 21 June at an air mass flow rate of 0.005 kg/s.
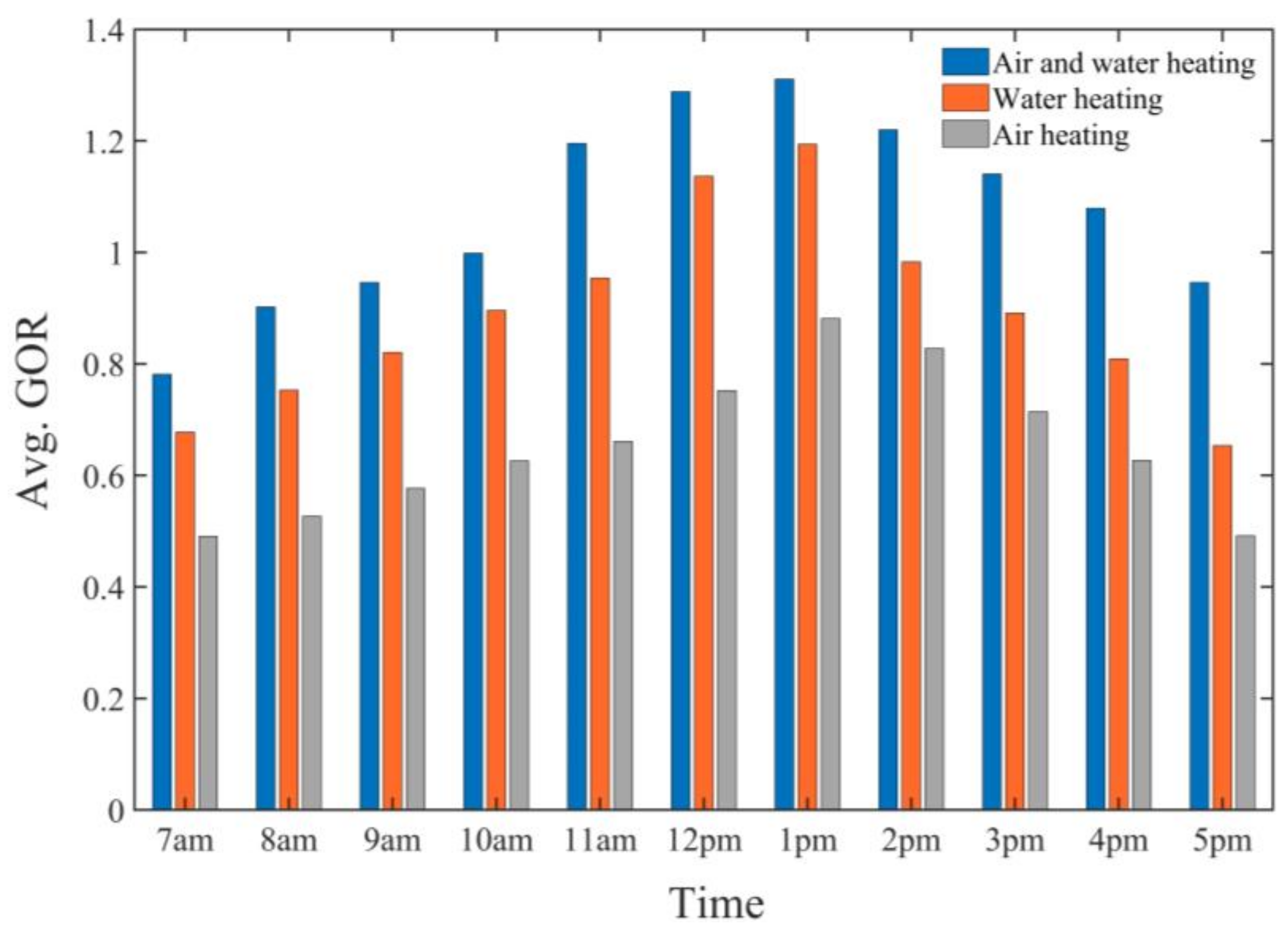
- The comparison between air-heating and water-heating systems and the dual-fluid heating system demonstrated that the air-preheating system had lower performance in terms of productivity and GOR, the water-preheating system had higher productivity and GOR than the air-preheating system, and the dual-fluid heating system was more efficient than both systems in terms of productivity and GOR.
- The observed highest monthly average air temperature and seawater temperature were 60 and 69 °C, respectively.
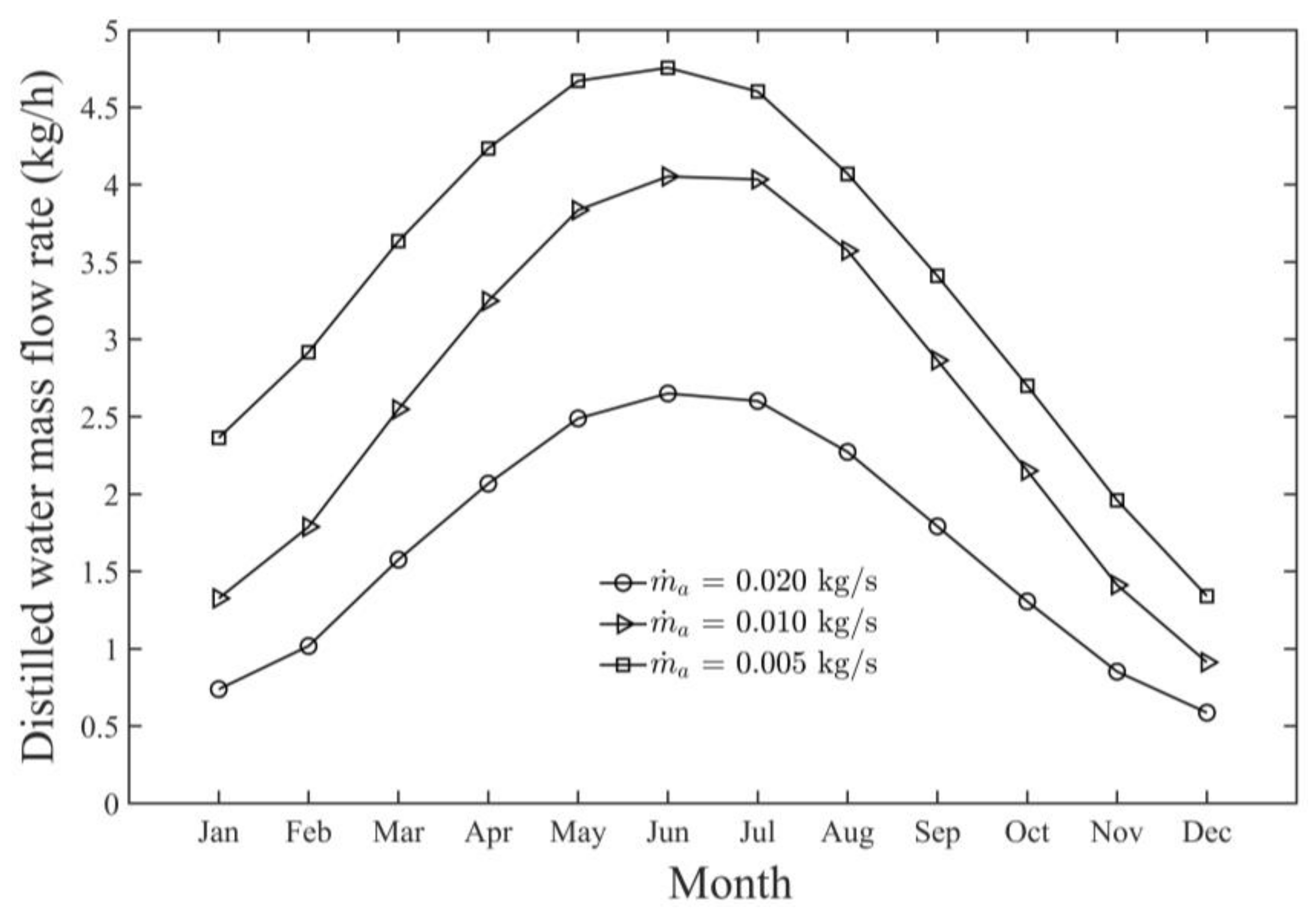
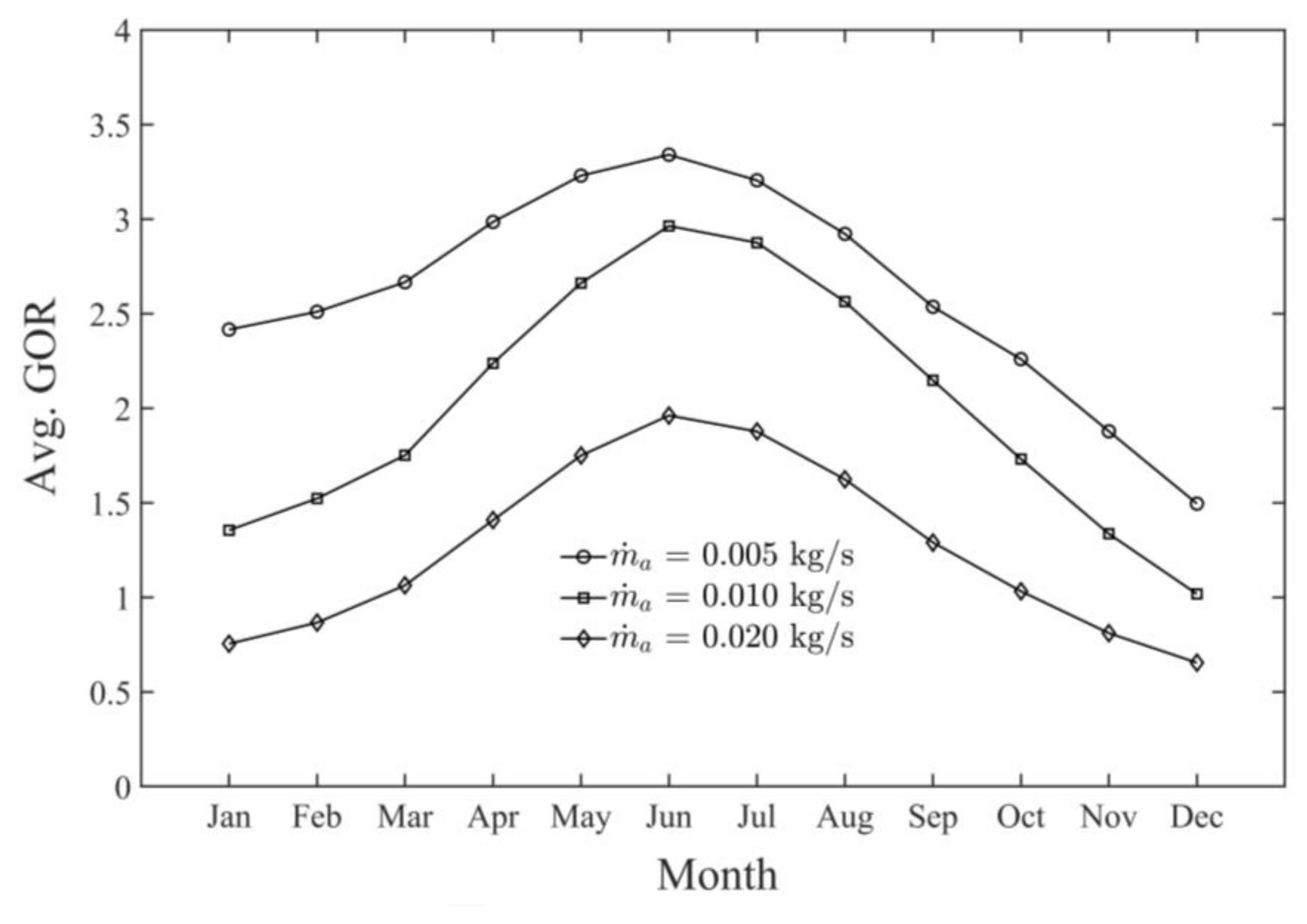
- The highest monthly productivity and GOR of the SAWSH-HDH system for June were found to be 4.60 kg/h and 3.35 kg/h, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ac | Cross-sectional area of upper compartment (air-heating channel) (m2) |
| ap | Perimeter of the upper compartment (m2) |
| Ap | Area of the absorber plate (m2) |
| Acol | Collector area (m2) |
| cair | Specific heat capacity of air (1.009 kJ/kg °C) |
| cw | Specific heat capacity of seawater (4.193 kJ/kg °C) |
| cv | Specific heat capacity of water vapor (1.88 kJ/kg °C) |
| De | Equivalent diameter (m) |
| Ha | Enthalpy of air (kJ/kg) |
| Hw | Enthalpy of seawater (kJ/kg) |
| haap | Convective heat transfer coefficient between air and plate (W/m2 °C) |
| hagc | Convective heat transfer coefficient between air and glass cover (W/m2 °C) |
| hba | Convective heat transfer coefficient between bottom and air (W/m2 °C) |
| hgca | Convective heat transfer coefficient between glass cover and ambient air |
| (W/m2 °C) | |
| hpw | Convective heat transfer coefficient between plate and water (W/m2 °C) |
| hfg | Latent heat of evaporation and condensation (kJ/kg) |
| hram | Radiation heat transfer coefficient between glass cover and ambient air |
| (W/m2 °C) | |
| hrpgc | Radiation heat transfer coefficient between plate and glass cover |
| (W/m2 °C) | |
| kair | Thermal conductivity of air (W/m °C) |
| Ki | Thermal conductivity of insulation (W/m °C) |
| L | Length of collector (m) |
| W | Width of collector (m) |
| Mass flow rate of air (kg/s) | |
| Mass flow rate of brine (kg/s) | |
| Mass flow rate of cooling water (kg/s) | |
| ṁd | Mass flow rate of distilled water (kg/s) |
| Mass flow rate of seawater (kg/s) | |
| Mw | Mass of seawater in collector (kg) |
| MR | Mass ratio |
| Nu | Nusselt number |
| Patm | Atmospheric pressure (kPa) |
| Ps | Saturation pressure (kPa) |
| Re | Reynolds number |
| S | Solar radiation intensity (W/m2) |
| s | Salinity of seawater (kgsalt/kgseawater) |
| ti | Thickness of insulation (m) |
| T1 | Temperature of cooling water at inlet of dehumidifier (°C) |
| T2 | Temperature of cooling water at outlet of dehumidifier (°C) |
| T3 | Temperature of seawater at inlet of humidifier (°C) |
| T4 | Temperature of seawater at outlet of humidifier (°C) |
| T5 | Temperature of air at inlet of collector (°C) |
| T6 | Temperature of air at outlet of collector and inlet of humidifier (°C) |
| T7 | Temperature of air at outlet of humidifier and inlet of dehumidifier (°C) |
| T8 | Temperature of air leaving dehumidifier (°C) |
| Tair | Temperature of air flowing through upper compartment (°C) |
| Tam | Ambient temperature (°C) |
| Tap | Temperature of absorber plate (°C) |
| Tb | Temperature of base (°C) |
| Tgc | Temperature of glass cover (°C) |
| Tw | Temperature of seawater in lower compartment (°C) |
| Uo | Overall heat transfer coefficient between glass cover and ambient air |
| (W/m2 °C) | |
| Uba | Overall heat transfer coefficient between base and ambient air (W/m2 °C) |
| vair | Velocity of ambient air (m/s) |
| Greek letters | |
| αγγχ | Absorptance of glass cover |
| ατ | Effective transmittance–absorptance product |
| εγχ | Emittance of glass |
References
- Miletto, M. The United Nations World Water Development Report-Valuing Water; UNESCO: Paris, France, 2021. [Google Scholar]
- Santosh, R.; Arunkumar, T.; Velraj, R.; Kumaresan, G. Technological advancements in solar energy driven humidification–dehumidification desalination systems—A review. J. Clean. Prod. 2019, 207, 826–845. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Solar powered desalination—Technology, energy and future outlook. Desalination 2019, 453, 54–76. [Google Scholar] [CrossRef] [Green Version]
- Giwa, A.; Akther, N.; Al Housani, A.; Haris, S.; Hasan, S.W. Recent advances in humidification dehumidification (HDH) desalination processes: Improved designs and productivity. Renew. Sustain. Energy Rev. 2016, 57, 929–944. [Google Scholar] [CrossRef]
- Alnaimat, F.; Ziauddin, M.; Mathew, B. A review of recent advances in humidification and dehumidification desalination technologies using solar energy. Desalination 2021, 499, 114860. [Google Scholar] [CrossRef]
- Rajaseenivasan, T.; Srithar, K. An investigation into a laboratory scale bubble column humidification dehumidification desalination system powered by biomass energy. Energy Convers. Manag. 2017, 139, 232–244. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Zubair, M.I.; Atif, M.; Gandhidasan, P.; Al-Dini, S.A.; Antar, M.A. Humidification dehumidification desalination system using parabolic trough solar air collector. Appl. Therm. Eng. 2015, 75, 809–816. [Google Scholar] [CrossRef]
- Narayan, G.P.; Sharqawy, M.H.; Summers, E.K.; Lienhard, J.H.; Zubair, S.M.; Antar, M.A. The potential of solar-driven humidification-dehumidification desalination for small-scale decentralized water production. Renew. Sustain. Energy Rev. 2010, 14, 1187–1201. [Google Scholar] [CrossRef]
- Rahimi-Ahar, Z.; Hatamipour, M.S.; Ahar, L.R. Air humidification–dehumidification process for desalination: A review. Prog. Energy Combust. Sci. 2020, 80, 100850. [Google Scholar] [CrossRef]
- Mohamed, A.M.I.; Elminshawy, N.A.S. Theoretical investigation of solar humidification–dehumidification desalination system using parabolic trough concentrators. Energy Convers. Manag. 2011, 52, 3112–3119. [Google Scholar] [CrossRef]
- Khalil, A.; El-Agouz, S.A.; El-Samadony, Y.A.F.; Abdo, A. Solar water desalination using an air bubble column humidifier. Desalination 2015, 372, 7–16. [Google Scholar] [CrossRef]
- Yildirim, C.; Solmus, I. A parametric study on a humidification–dehumidification desalination unit powered by solar air and water heaters. Energy Convers. Manag. 2014, 86, 568–575. [Google Scholar] [CrossRef]
- Sharshir, S.W.; Peng, G.; Yang, N.; Eltawil, M.A.; Ali, M.K.A.; Kabeel, A.E. A hybrid desalination system using humidification–dehumidification and solar stills integrated with evacuated solar water heater. Energy Convers. Manag. 2016, 124, 287–296. [Google Scholar] [CrossRef]
- Antar, M.A.; Sharqawy, M.H. Experimental investigations on the performance of an air heated humidification–dehumidification desalination system. Desalin. Water Treat. 2013, 51, 837–843. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wang, R.Z.; Zhang, H.F. Parametric analysis to improve the performance of a solar desalination with humidification and dehumidification. Desalination 2002, 142, 107–118. [Google Scholar] [CrossRef]
- Dai, Y.J.; Zhang, H.F. Experimental investigation of a solar desalination unit with humidification and dehumidification. Desalination 2000, 130, 169–175. [Google Scholar] [CrossRef]
- Houcine, I.; Amara, M.B.; Guizani, A.; Maalej, M. Pilot plant testing of a new solar desalination process by a multiple-effect-humidification technique. Desalination 2006, 196, 105–124. [Google Scholar] [CrossRef]
- Orfi, J.; Galanis, N.; Laplante, M. Air humidification–dehumidification for a water desalination system using solar energy. Desalination 2007, 203, 471–481. [Google Scholar] [CrossRef]
- Zhani, K.; Bacha, H.B. Experimental investigation of a new solar desalination prototype using the humidification dehumidification principle. Renew. Energy 2010, 35, 2610–2617. [Google Scholar] [CrossRef]
- Kabeel, A.E.; El Said, E.M.S. A hybrid solar desalination system of air humidification dehumidification and water flashing evaporation Part 1: A numerical investigation. Desalination 2013, 320, 56–72. [Google Scholar] [CrossRef]
- Yamali, C.; Solmus, I. Theoretical investigation of a humidification dehumidification desalination system configured by a double-pass flat plate solar air heater. Desalination 2007, 205, 163–177. [Google Scholar] [CrossRef]
- Nafey, A.S.; Fath, H.E.S.; El-Helaby, S.O.; Soliman, A.M. Solar desalination using humidification–dehumidification processes. Part I: A numerical investigation. Energy Convers. Manag. 2004, 45, 1243–1261. [Google Scholar] [CrossRef]
- Nafey, A.S.; Fath, H.E.S.; El-Helaby, S.O.; Soliman, A.M. Solar desalination using humidification–dehumidification processes. Part II: An experimental investigation. Energy Convers. Manag. 2004, 45, 1263–1277. [Google Scholar] [CrossRef]
- Rajaseenivasan, T.; Srithar, K. Potential of a dual purpose solar collector on humidification dehumidification desalination system. Desalination 2017, 404, 35–40. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, H.; Ma, X.; Kutlu, C.; Su, Y. Experimental investigation of a multi-stage humidification–dehumidification desalination system heated directly by a cylindrical Fresnel lens solar concentrator. Energy Convers. Manag. 2017, 143, 241–251. [Google Scholar] [CrossRef]
- Xiao, J.; Zheng, H.; Jin, R.; Liang, S.; Wang, G.; Ma, X. Experimental investigation of a bubbling humidification–dehumidification desalination system directly heated by cylindrical Fresnel lens solar concentrator. Sol. Energy 2021, 220, 873–881. [Google Scholar] [CrossRef]
- Santosh, R.; Kumaresan, G.; Kumar, G.K.; Velraj, R. Experimental parametric investigation of waste heat powered humidification dehumidification system for production of freshwater from wastewater. Desalination 2020, 484, 114422. [Google Scholar] [CrossRef]
- Somwanshi, A.; Sarkar, N. Thermal performance of a dual-purpose collector-cum-storage type air-water heater. Appl. Therm. Eng. 2020, 171, 115094. [Google Scholar] [CrossRef]
- Mamouri, S.J.; Tan, X.; Klausner, J.F.; Yang, R.; Benard, A. Performance of an integrated greenhouse equipped with Light-Splitting material and an HDH desalination unit. Energy Convers. Manag. X 2020, 7, 100045. [Google Scholar] [CrossRef]
- Abbasi, O.A. Water desalination using renewable energy in a humidification-dehumidification cycle for Bahrain climate conditions. KnE Eng. 2018, 213–220. [Google Scholar] [CrossRef]
- Tiwari, G.N. Solar Energy Fundamentals, Design Modeling and Applications; Narosa Publishing House: New Delhi, India, 2002. [Google Scholar]
- Kays, W.M. Convective Heat and Mass Transfer; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Sharqawy, M.H.; Antar, M.A.; Zubair, S.M.; Elbashir, A.M. Optimum thermal design of humidification–dehumidification desalination systems. Desalination 2014, 349, 10–21. [Google Scholar] [CrossRef]
- Chehayeb, K.M.; Narayan, G.P.; Zubair, S.M.; Lienhard, J.H.V. Use of multiple extractions and injections to thermodynamically balance the humidification–dehumidification desalination system. Int. J. Heat Mass Transfer 2014, 68, 422–434. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization (WHO): Valletta, Malta, 2011. [Google Scholar]
- Sharqawy, M.H.; Lienhard, J.H.V.; Zubair, S.M. Thermophysical properties of seawater: A review of existing correlations and date. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Hermosillo, J.J.; Bulnes, C.A.A.; Estrada, C.A. Water desalination by air humidification: Mathematical model and experimental study. Sol. Energy 2012, 86, 1070–1076. [Google Scholar] [CrossRef]
- Hamed, M.H.; Kabeel, A.E.; Omara, Z.M.; Sharshir, S.W. Mathematical and experimental investigation of a solar humidification–dehumidification desalination unit. Desalination 2015, 358, 9–17. [Google Scholar] [CrossRef]
- Ali-Sahali, M.; Ettouney, H.M. Humidification dehumidification desalination process: Design and performance evaluation. Chem. Eng. J. 2008, 143, 257–264. [Google Scholar] [CrossRef]
- Ishida, K.; Ohara, N.; Kavvas, M.L.; Chen, Z.Q.; Anderson, M.L. Impact of air temperature on physically based maximum precipitation estimation through change in moisture holding capacity of air. J. Hydrol. 2018, 556, 1050–1063. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D. Chemical Engineers Handbook, 6th ed.; McGraw-Hill: New York, NY, USA, 1984; pp. 12–13. [Google Scholar]
- Sea Temperature Meteorological Services. Available online: https://seatemperature.net/ (accessed on 20 March 2021).
- The National Renewable Energy Laboratory NREL (USA). Available online: https://www.nrel.gov/grid/solar-resource/saudi-arabia.html (accessed on 22 March 2021).





| Parameter | Value |
|---|---|
| Mass of seawater in collector at any instance (Mw) | 105 kg |
| Area of absorber plate (Ap) | 1.5 m2 |
| Length of collector (L) | 1.5 m |
| Stefan’s constant (σ) | 5.67 × 10−8 W/m2 °C4 |
| Absorptance–transmittance product (ατ) | 0.85 |
| Absorptance of glass cover (αgc) | 0.05 |
| Emissivity of absorber plate (εp) | 0.85 |
| Heat transfer between water and plate (hpw) | 108.6 W/m2 °C |
| Density of air (ρair) | 1.2 kg/m3 |
| Thermal conductivity of insulation (Ki) | 0.004 W/m °C |
| Insulation thickness (ti) | 0.05 m |
| Cross-section of upper compartment (air channel) (C) | 1.5 m2 |
| Air mass flow rate across SAWSH () | 0.005, 0.010, and 0.020 kg/s |
| a1 | −2.348 × 104 | a6 | −44.17 |
| a2 | 3.15 × 105 | a7 | 2.139 × 10−1 |
| a3 | 2.803 × 106 | a8 | −1.997 × 104 |
| a4 | −1.446 × 107 | a9 | 2.778 × 104 |
| a5 | 7.826 × 103 | a10 | 97.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soomro, S.H.; Santosh, R.; Bak, C.-U.; Kim, W.-S.; Kim, Y.-D. Humidification-Dehumidification Desalination System Powered by Simultaneous Air-Water Solar Heater. Sustainability 2021, 13, 13491. https://doi.org/10.3390/su132313491
Soomro SH, Santosh R, Bak C-U, Kim W-S, Kim Y-D. Humidification-Dehumidification Desalination System Powered by Simultaneous Air-Water Solar Heater. Sustainability. 2021; 13(23):13491. https://doi.org/10.3390/su132313491
Chicago/Turabian StyleSoomro, Sadam Hussain, Ravichandran Santosh, Chul-U Bak, Woo-Seung Kim, and Young-Deuk Kim. 2021. "Humidification-Dehumidification Desalination System Powered by Simultaneous Air-Water Solar Heater" Sustainability 13, no. 23: 13491. https://doi.org/10.3390/su132313491
APA StyleSoomro, S. H., Santosh, R., Bak, C.-U., Kim, W.-S., & Kim, Y.-D. (2021). Humidification-Dehumidification Desalination System Powered by Simultaneous Air-Water Solar Heater. Sustainability, 13(23), 13491. https://doi.org/10.3390/su132313491







