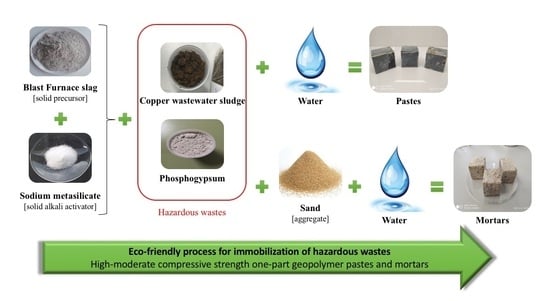
Immobilization of Hazardous Wastes on One-Part Blast Furnace Slag-Based Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymer
- The solid components (precursor, activator, and aggregate, if applied) were mixed and homogenized manually in a plastic bag for about 1–2 min.
- The solids were mechanically mixed while adding the water for 1 min.
- The fresh paste was manually mixed for 1 min.
- The fresh paste was, once more, mechanically mixed for 1.5 min.
- The fresh paste was cast into pieces (40 × 40 × 40 mm), on metallic moulds, and vibrated for 2 min using an electric vibrator.
- The specimens were covered with a plastic film and kept at ambient temperature during the first 24 h of curing.
- All the specimens were unmoulded and introduced into the climatic chamber (20 °C and 65% RH) until the 7th and 28th curing day.
2.3. Materials Characterization
2.4. Geopolymer Characterization Tests
3. Results and Discussion
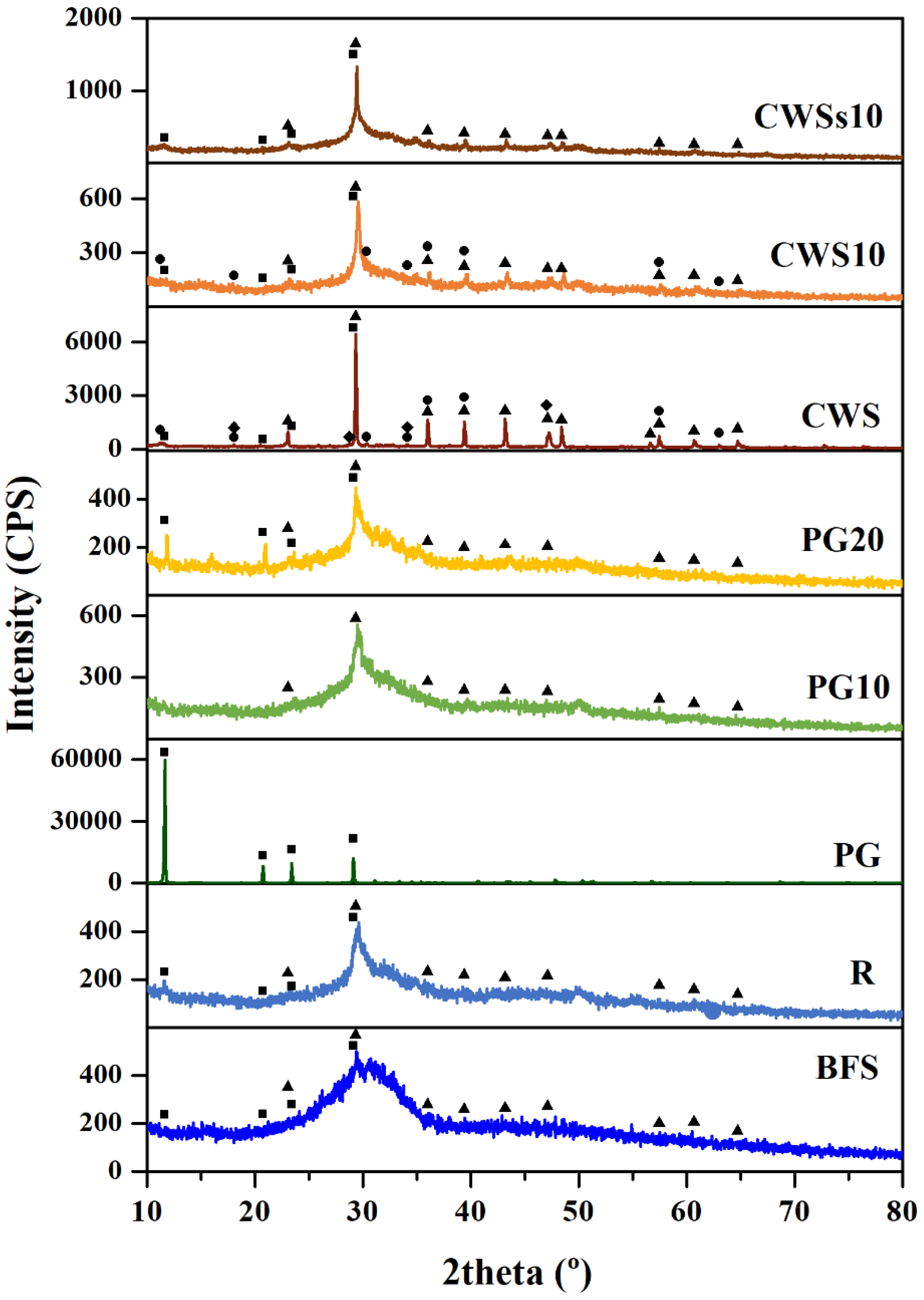
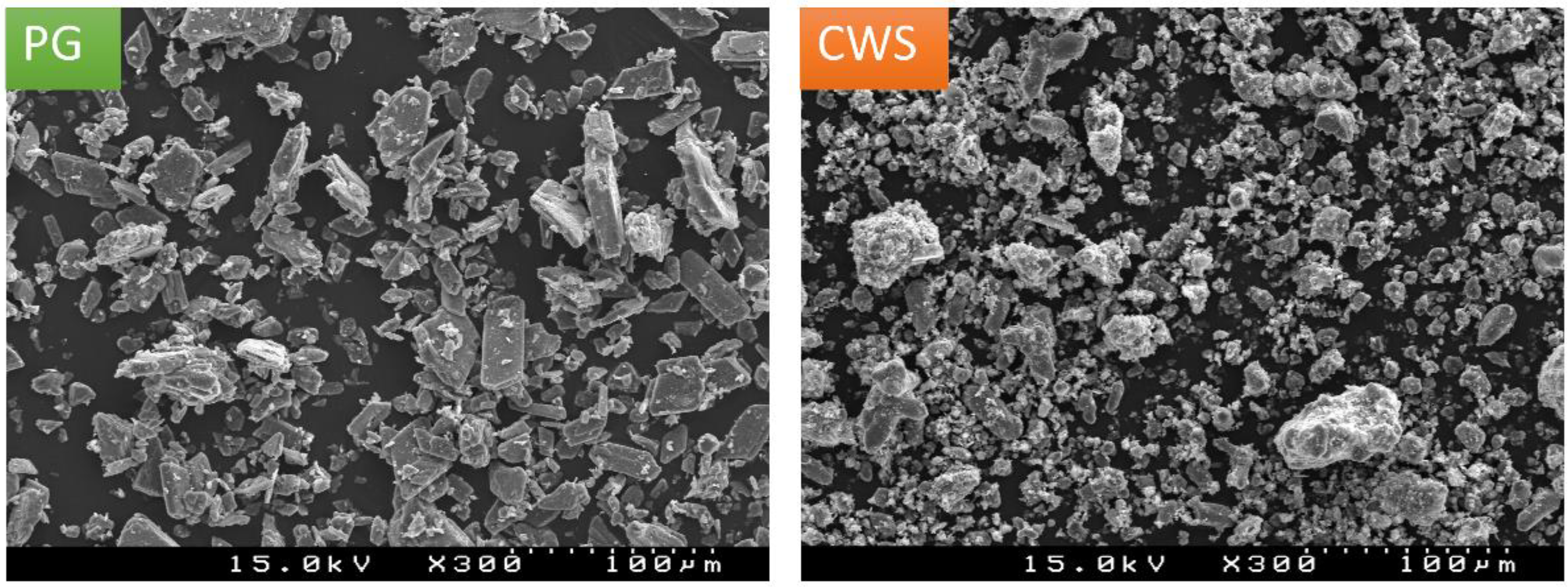
3.1. Raw Materials Characterization
3.2. Geopolymers Characterization
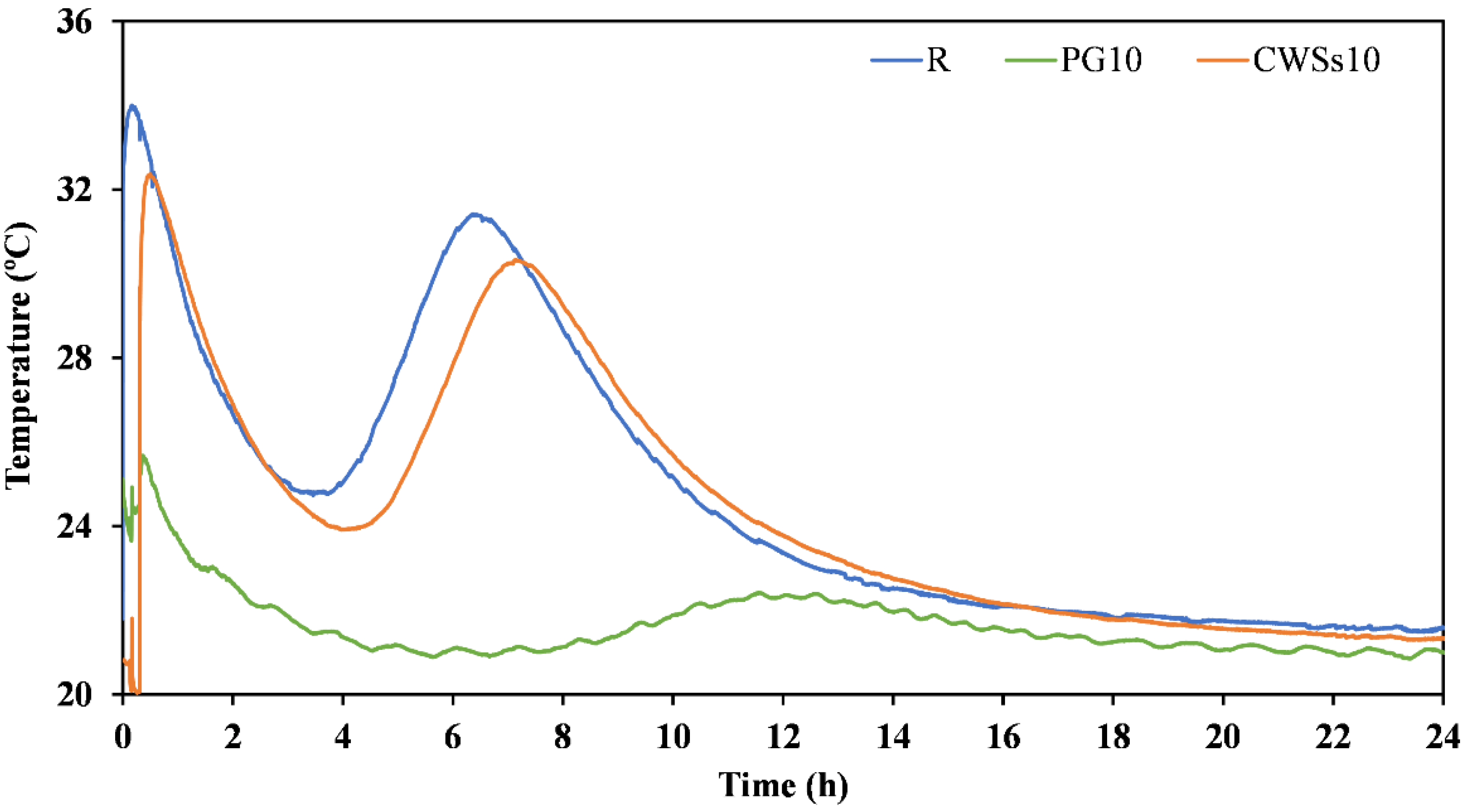
3.2.1. Fresh State
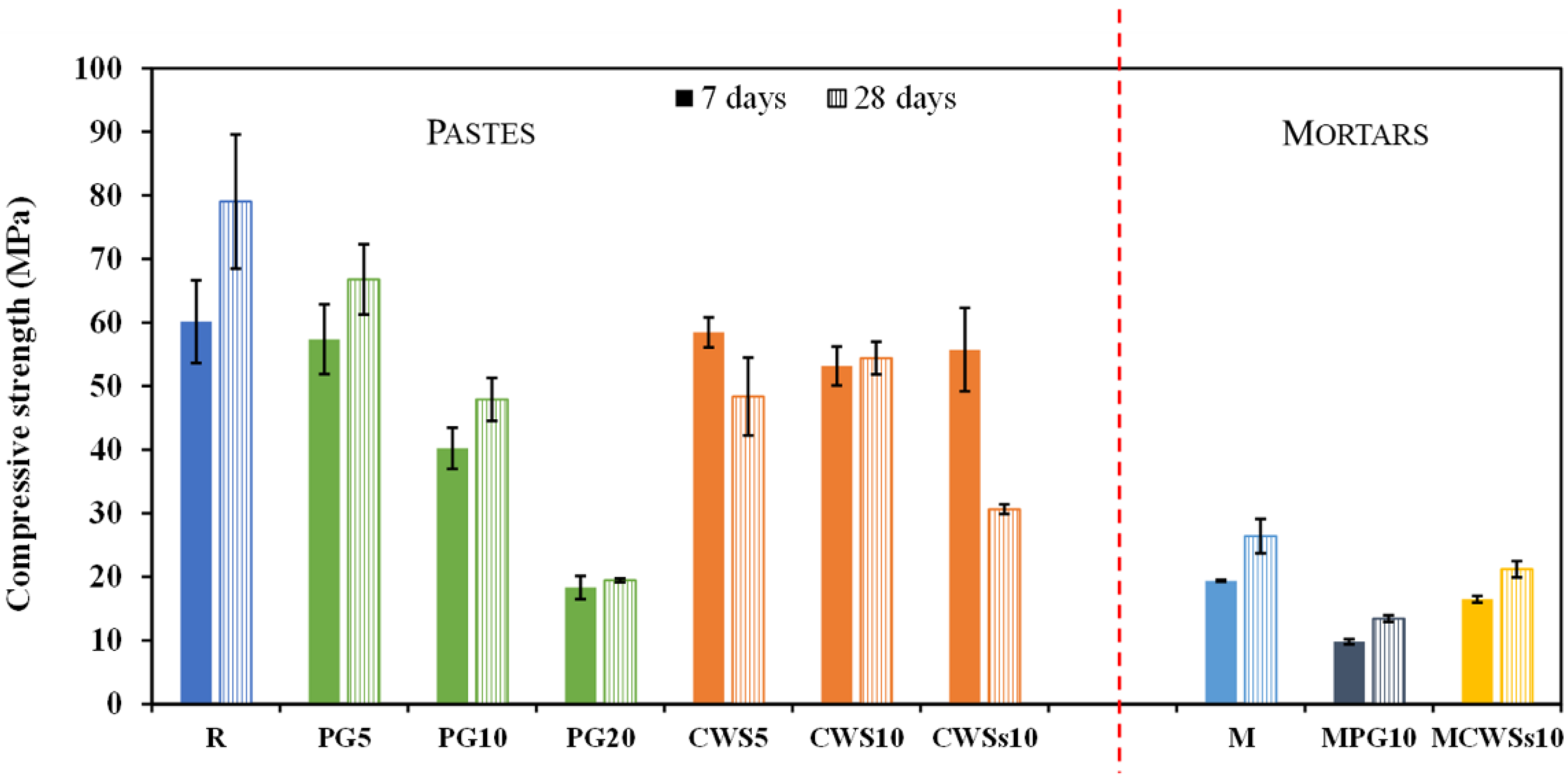
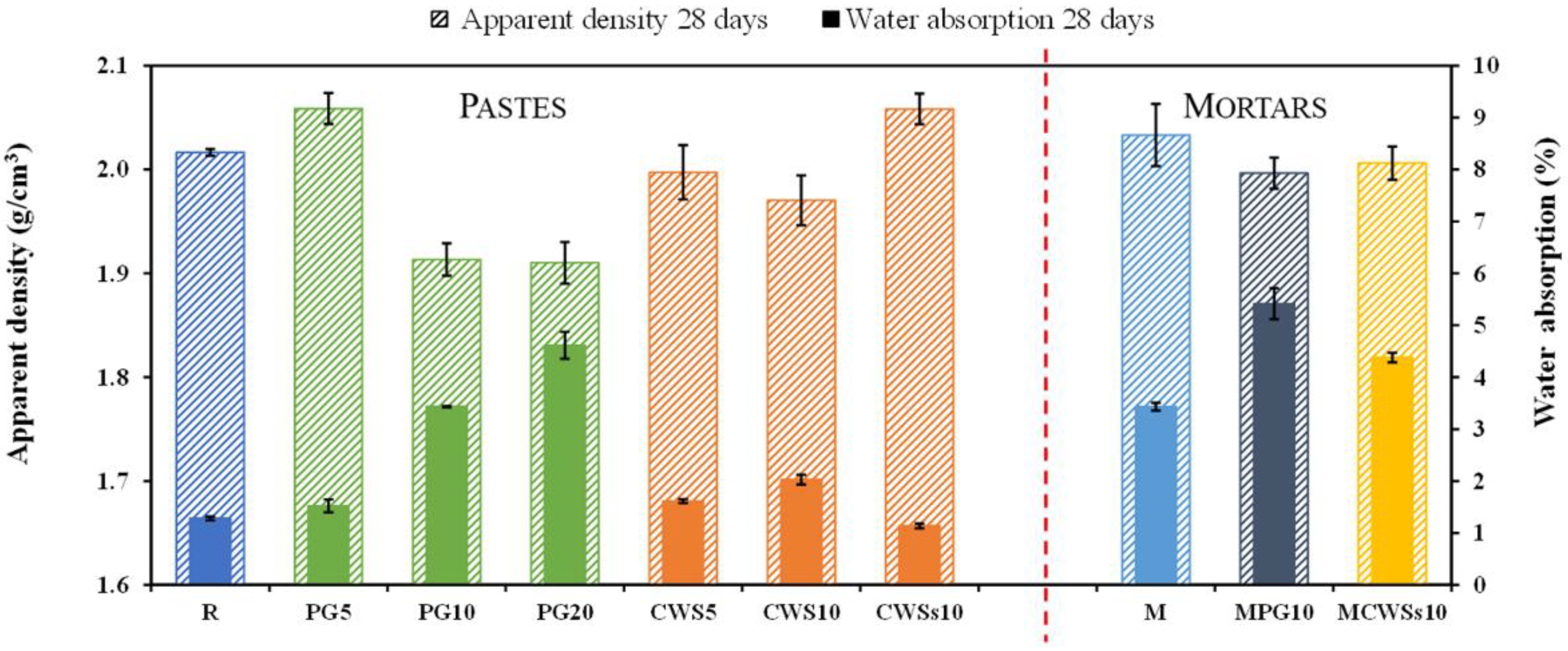
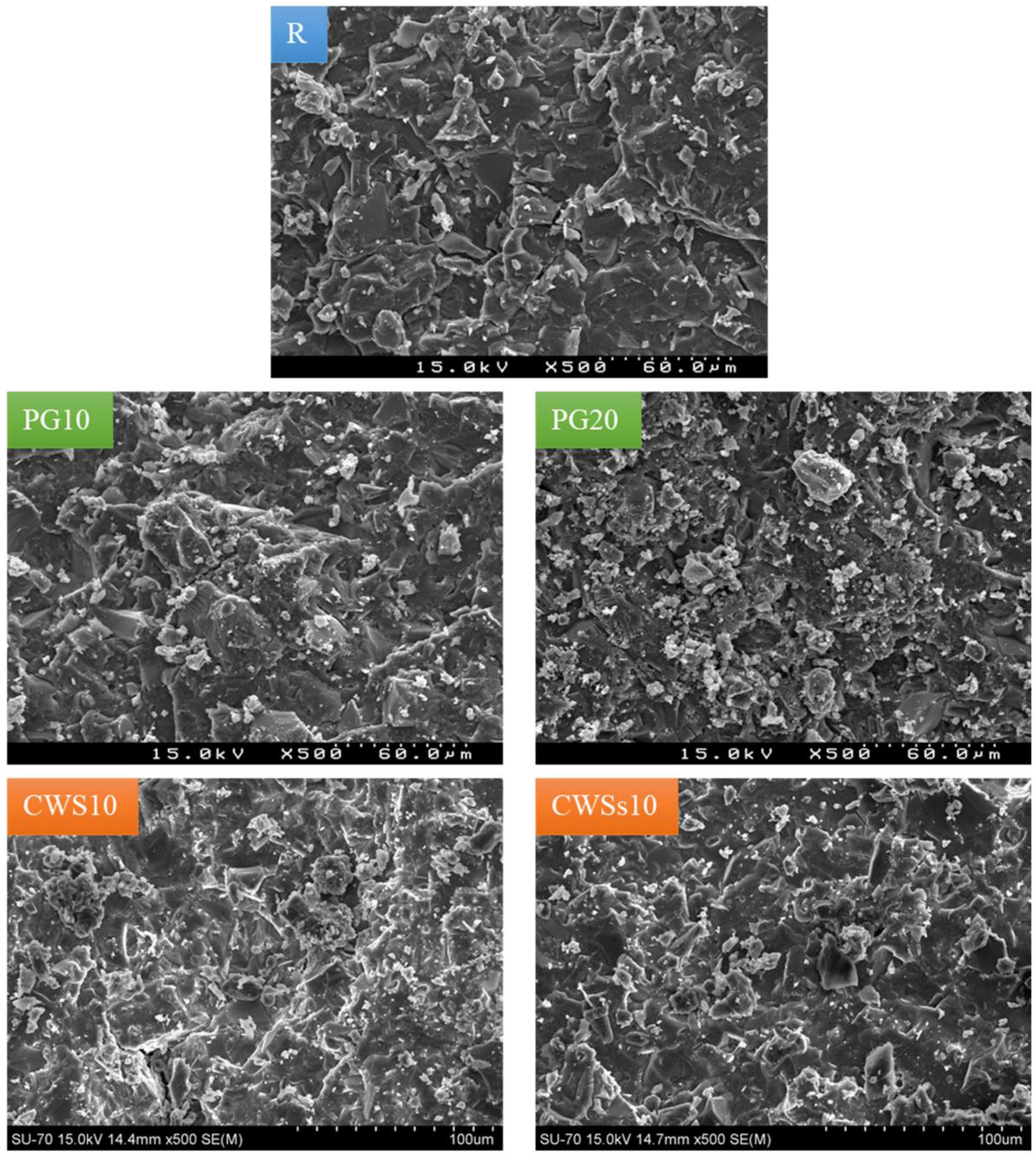
3.2.2. Hardened Samples
3.3. Leaching Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auping, W.L.; Pruyt, E.; Kwakkel, J.H. Analysing the Uncertain Future of Copper with Three Exploratory System Dynamics Models. In Proceedings of the International System Dynamics Conference, St. Gallen, Switzerland, 22–26 July 2012; pp. 1–25. [Google Scholar]
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating Global Copper Demand until 2100 with Regression and Stock Dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- International Copper Study Group (ICSG). The World Copper Factbook 2020; ICSG: Lisbon, Portugal, 2020. [Google Scholar]
- Khan, S.A.R.; Yu, Z.; Sharif, A.; Golpîra, H. Determinants of Economic Growth and Environmental Sustainability in South Asian Association for Regional Cooperation: Evidence from Panel ARDL. Environ. Sci. Pollut. Res. 2020, 27, 45675–45687. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.I.; Shih, X.H.; Ma, H. wen Secondary Copper Resource Recycling and Reuse: A Waste Input–Output Model. J. Clean. Prod. 2019, 239, 118142. [Google Scholar] [CrossRef]
- Atlantic Copper. Annual Environmental Report; Atlantic Copper: Huelva, Spain, 2017. [Google Scholar]
- Pérez-Moreno, S.M.; Gázquez, M.J.; Pérez-López, R.; Vioque, I.; Bolívar, J.P. Assessment of Natural Radionuclides Mobility in a Phosphogypsum Disposal Area. Chemosphere 2018, 211, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, J.P.; Martín, J.E.; García-Tenorio, R.; Pérez-Moreno, J.P.; Mas, J.L. Behaviour and Fluxes of Natural Radionuclides in the Production Process of a Phosphoric Acid Plant. Appl. Radiat. Isot. 2009, 67, 345–356. [Google Scholar] [CrossRef]
- Guerrero, J.L.; Gutiérrez-Álvarez, I.; Mosqueda, F.; Gázquez, M.J.; García-Tenorio, R.; Olías, M.; Bolívar, J.P. Evaluation of the Radioactive Pollution in the Salt-Marshes under a Phosphogypsum Stack System. Environ. Pollut. 2020, 258, 113729. [Google Scholar] [CrossRef]
- Shively, W.; Bishop, P.; Gress, D.; Brown, T. Leaching Tests of Heavy Metals Stabilized with Portland Cement. J. Water Pollut. Control Fed. 1986, 58, 234–241. [Google Scholar]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of Heavy Metal in Cement-Based Solidification/Stabilisation: A Review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, G.Y. Evaluation the Solidification/Stabilization of Heavy Metals by Portland Cement. J. Ecol. Eng. 2019, 20, 91–100. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials, 1st ed.; Provis, J.L., van Deventer, J.S.J., Eds.; RILEM State-of-the-Art Reports; Springer: Dordrecht, The Netherlands, 2014; Volume 13, ISBN 978-94-007-7671-5. [Google Scholar]
- Minaříková, M.; Škvára, F. Fixation of Heavy Metals in Geopolymeric Materials Based on Brown Coal Fly Ash. Ceram.-Silik. 2006, 50, 200–207. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzeni, L. The Potential Use of Geopolymeric Materials To Immobilise Toxic Metals: Part I. Theory and Applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Bankowski, P.; Zou, L.; Hodges, R. Using Inorganic Polymer to Reduce Leach Rates of Metals from Brown Coal Fly Ash. Miner. Eng. 2004, 17, 159–166. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E.; Lachowski, E.E. Fixing Arsenic in Alkali-Activated Cementitious Matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N. Mechanisms of Heavy Metal Immobilisation Using Geopolymerisation Techniques—A Review. J. Adv. Concr. Technol. 2018, 16, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of Phosphogypsum on the Stability upon Firing Treatment of Alkali-Activated Slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-Activated Binders Based on Ground Granulated Blast Furnace Slag and Phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential Use of Phosphogypsum in Alkali-Activated Fly Ash under the Effects of Elevated Temperatures and Thermal Shock Cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of Red Mud and Pb/Zn Smelter Waste for the Synthesis of a Red Mud-Based Cementitious Material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Hu, X.; Tian, J.; Wang, J.; Wan, R.; Cui, X.; Zhou, X.; Liu, D. Adsorption of Si(OH)4 and Al(OH)4 onto Arsenopyrite Surface: Exploring the Sealing Feasibility of Geopolymer to Arsenopyrite. Miner. Eng. 2021, 170, 107017. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.F.; Yang, H.; Bao, C.J.; Wang, J.S.; Sun, Y.H.; Liu, D.W.; Shen, P.L.; Su, C. Red Mud-Metakaolin Based Cementitious Material for Remediation of Arsenic Pollution: Stabilization Mechanism and Leaching Behavior of Arsenic in Lollingite. J. Environ. Manag. 2021, 300, 113715. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Song, K. Il Assessment of CO2 Reduction of Alkali-Activated Concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable Concrete: Building a Greener Future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of Alkali and Silica Sources in One-Part Alkali-Activated Blast Furnace Slag Mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium Sulfoaluminate and Alkali-Activated Fly Ash Cements as Alternative to Portland Cement: Study on Chemical, Physical-Mechanical, and Durability Properties of Mortars with the Same Strength Class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A Review and Prospects for the Minerals Industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- CEN: European Committee for Standardization. EN 1015-3 Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table), Brussels; CEN: Brussels, Belgium, 1998; p. 9. [Google Scholar]
- CEN: European Committee for Standardization. EN 196-3 Methods of Testing Cement Part 3—Determination of Setting Times and Soundness; CEN: Brussels, Belgium, 2005; p. 3. [Google Scholar]
- CEN: European Committee for Standardization. EN 1015-11 Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; CEN: Brussels, Belgium, 1999; p. 15. [Google Scholar]
- CEN: European Committee for Standardization. EN 1015-18 Methods of Test for Mortar for Masonry Part 18—Determination of Water- Absorption Coefficient Due to Capillary Action of Hardened Mortar; CEN: Brussels, Belgium, 2002; p. 8. [Google Scholar]
- CEN: European Committee for Standardization. EN 12457-2 Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/Kg for Materials with Particle Size below 4 Mm (without or with Size Reduction); CEN: Brussels, Belgium, 2002; p. 30. [Google Scholar]
- Gonçalves, M.; Vilarinho, I.S.; Capela, M.; Caetano, A.; Novais, R.M.; Labrincha, J.A.; Seabra, M.P. Waste-Based One-Part Alkali Activated Materials. Materials 2021, 14, 2911. [Google Scholar] [CrossRef] [PubMed]
- CEN: European Committee for Standardization. EN 197-1 Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; CEN: Brussels, Belgium, 2000; p. 38. [Google Scholar]
- Sambucci, M.; Sibai, A.; Valente, M. Recent Advances in Geopolymer Technology. A Potential Eco-Friendly Solution in the Construction Materials Industry: A Review. J. Compos. Sci. 2021, 5, 109. [Google Scholar] [CrossRef]
- Assi, L.N.; Eddie Deaver, E.; Ziehl, P. Effect of Source and Particle Size Distribution on the Mechanical and Microstructural Properties of Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Lancellotti, I.; Barbieri, L.; Leonelli, C. Use of Alkali-Activated Concrete Binders for Toxic Waste Immobilization; Woodhead Publishing Limited: Sawston, UK, 2015; ISBN 9781782422884. [Google Scholar]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of Chemical Admixtures on Properties of High-Calcium Fly Ash Geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A Review on Properties of Fresh and Hardened Geopolymer Mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Criado, M.; Walkley, B.; Ke, X.; Provis, J.L.; Bernal, S.A. Slag and Activator Chemistry Control the Reaction Kinetics of Sodium Metasilicate-Activated Slag Cements. Sustainability 2018, 10, 4709. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; van Riessen, A.; Chon, C.M. Benefits of Sealed-Curing on Compressive Strength of Fly Ash-Based Geopolymers. Materials 2016, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- CEN: European Committee for Standardization. EN 1992-1-1 Eurocode 2: Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings; CEN: Brussels, Belgium, 2004; Volume 1. [Google Scholar]
- Yahya, Z.; Abdullah, M.M.A.B.; Talib, S.Z.A.; Razak, R.A. Comparative Study on Early Strength of Sodium Hydroxide (NaOH) Activated Fly Ash Based Geopolymer. AIP Conf. Proc. 2017, 1887, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Vilarinho, I.S.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge. Sustainability 2021, 13, 1999. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Manguri, S.; Nassani, D.E.; Oleiwi, S.M. Effect of Aggregate Properties on the Mechanical and Absorption Characteristics of Geopolymer Mortar. Eng. Sci. Technol. Int. J. 2017, 20, 1642–1652. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Labrincha, J.A. Upcycling Unexplored Dregs and Biomass Fly Ash from the Paper and Pulp Industry in the Production of Eco-Friendly Geopolymer Mortars: A Preliminary Assessment. Constr. Build. Mater. 2018, 184, 464–472. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Natural Pozzolana Addition Effect on Compressive Strength and Capillary Water Absorption of Mortar. Energy Procedia 2017, 139, 689–695. [Google Scholar] [CrossRef]
- Saeli, M.; Senff, L.; Seabra, M.P.; Labrincha, J.A. Alkali-Activated Fly Ash-Based Mortars for Green Applications in Architecture and Civil Engineering. Int. J. Struct. Civ. Eng. Res. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Mádai, F.; Kristály, F.; Mucsi, G. Microstructure, Mineralogy and Physical Properties of Ground Fly Ash Based Geopolymers. Ceram.-Silik. 2015, 59, 70–79. [Google Scholar]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 2028109. [Google Scholar] [CrossRef] [Green Version]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Aguiar, J.B.; Jesus, C. Durability Performance of Fly Ash Based One-Part Geopolymer Mortars. Key Eng. Mater. 2015, 634, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía De Gutierrez, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Buruberri, L.H.; Tobaldi, D.M.; Caetano, A.; Seabra, M.P.; Labrincha, J.A. Evaluation of Reactive Si and Al Amounts in Various Geopolymer Precursors by a Simple Method. J. Build. Eng. 2019, 22, 48–55. [Google Scholar] [CrossRef]
- Hakem Aziz, I.; Mustafa Al Bakri Abdullah, M.; Arif Anuar Mohd Salleh, M.; Victor Sandu, A. The Incorporation of Sodium Hydroxide (NaOH) Concentration and CaO-Si Components on Ground Granulated Blast Furnace Slag Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012005. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical Activation of Fly Ash: Effect on Reaction, Structure and Properties of Resulting Geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of Synthesis Parameters on the Mechanical Properties of Fly Ash-Based Geopolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and Hardened Properties of One-Part Fly Ash-Based Geopolymer Binders Cured at Room Temperature: Effect of Slag and Alkali Activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y. The Evaluation of the Heavy Metal Leaching Behavior of Mswi-Fa Added Alkali-Activated Materials Bricks by Using Different Leaching Test Methods. Int. J. Environ. Res. Public Health 2019, 16, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keulen, A.; van Zomeren, A.; Dijkstra, J.J. Leaching of Monolithic and Granular Alkali Activated Slag-Fly Ash Materials, as a Function of the Mixture Design. Waste Manag. 2018, 78, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union Directive. 2003/33/EC of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Off. J. Eur. Communities 2003, 11, 27–49. [Google Scholar]
- US EAP. SW-846 Test Method 1311: Toxicity Characteristic Leaching Procedure; US EAP: Washington, DC, USA, 1992.
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, Physical and Structural Characterisation of Geopolymer Matrixes Synthesised from Coal (Co-)Combustion Fly Ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]





| Specimen Name | Mixture Proportion (g) | |||||
|---|---|---|---|---|---|---|
| BFS | Waste | Sodium metasilicate | Sand | Water/Binder | ||
| Pastes | R | 100 | - | 10 | - | 0.38 |
| PG5 | 95 | 5 | 0.37 | |||
| PG10 | 90 | 10 | 0.36 | |||
| PG20 | 80 | 20 | 0.38 | |||
| CWS5 | 95 | 5 | 0.38 | |||
| CWS10 | 90 | 10 | 0.39 | |||
| CWSs10 | 90 | 10 | 0.38 | |||
| Mortars | M | 100 | - | 10 | 400 | 0.71 |
| MPG10 | 90 | 10 | 0.71 | |||
| MCWSs10 | 90 | 10 | 0.71 | |||
| BFS | PG | CWS | BFS | PG | CWS | ||
|---|---|---|---|---|---|---|---|
| Major Components | wt. % | Minor Components | wt. % | ||||
| Al2O3 | 9.1 | 0.40 | 0.40 | As | - | - | 3.4 |
| CaO | 47 | 40 | 69 | Ba | 0.06 | 0.02 | 0.80 |
| Fe2O3 | 0.33 | 0.23 | 15 | Ce | 0.04 | - | - |
| K2O | 0.58 | 0.03 | - | Cl | 0.02 | - | 0.36 |
| MgO | 6.7 | 0.04 | 3.6 | Cu | - | 0.01 | 0.65 |
| MnO | 0.21 | - | 0.09 | F | - | 3.3 | - |
| Na2O | 0.23 | 0.13 | 0.24 | Pb | - | - | 0.29 |
| P2O5 | 0.01 | 0.95 | 0.14 | Sb | - | - | 0.06 |
| SiO2 | 33 | 2.4 | 2.3 | Sr | 0.06 | 0.08 | 0.05 |
| SO3 | 1.7 | 52 | 3.8 | Zn | - | - | 0.34 |
| TiO2 | 0.80 | 0.04 | - | Zr | 0.02 | 0.01 | - |
| LOI | 0.20 | 2.4 | 36 | ||||
| R | PG5 | PG10 | PG20 | CWS5 | CWS10 | CWSs10 | M | MPG10 | MCWSs10 | NHM | HM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Leached Concentration (mg/kg) | |||||||||||
| As | - | - | - | - | 13.4 ± 0.3 | 40 ± 3 | 2.01 ± 0.02 | - | - | 34.8 ± 0.2 | 2 | 25 |
| Ba | - | - | - | - | - | - | - | - | - | - | 100 | 300 |
| Br | 0.10 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.1 | 0.11 ± 0.01 | - | - | - | 0.11 ± 0.01 | 0.04 ± 0.01 | - | - | - |
| Ca | 25 ± 2 | 49 ± 18 | 12 ± 1 | 117 ± 1 | 27 ± 3 | 22 ± 2 | 19.3 ± 0.2 | 9.2 ± 0.2 | 45.0 ± 0.2 | 10.7 ± 0.2 | - | - |
| Cd | - | - | - | - | - | - | - | - | - | - | 1 | 5 |
| Cl | 6.7 ± 0.1 | 14.4 ± 0.4 | - | - | 17.0 ± 0.3 | 24 ± 1 | 8.8 ± 0.3 | - | - | 1.5 ± 0.3 | 15,000 | 25,000 |
| Cr | 0.05 ± 0.01 | - | - | - | - | - | - | - | - | - | 10 | 70 |
| Cu | - | - | - | - | 0.07 ± 0.01 | 0.14 ± 0.01 | 0.03 ± 0.01 | - | - | 0.42 ± 0.02 | 50 | 100 |
| Fe | 0.68 ± 0.04 | 0.27 ± 0.01 | 0.3 ± 0.1 | 1.21 ± 0.01 | 0.32 ± 0.03 | 0.20 ± 0.01 | 0.26 ± 0.01 | 0.13 ± 0.02 | 0.11 ± 0.01 | 0.49 ± 0.02 | - | - |
| Hg | - | 0.09 ± 0.02 | 0.10 ± 0.04 | 0.14 ± 0.01 | - | - | - | - | - | - | 0.2 | 2 |
| K | 105 ± 3 | 98 ± 2 | 51 ± 7 | 51 ± 1 | 103 ± 1 | 106 ± 6 | 74.2 ± 0.4 | 18.5 ± 0.4 | 32.1 ± 0.2 | 12.4 ± 0.3 | - | - |
| Mo | - | - | - | - | - | - | - | - | - | - | 10 | 30 |
| Ni | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | - | 0.02 ± 0.01 | - | - | - | - | 10 | 40 |
| Pb | - | - | - | - | - | - | - | - | - | - | 10 | 50 |
| Rb | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.30 ± 0.01 | 0.42 ± 0.01 | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.33 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.06 ± 0.01 | - | - |
| S | - | 372 ± 24 | 880 ± 220 | 853 ± 4 | 83 ± 3 | 90 ± 9 | 23 ± 1 | - | 647 ± 2 | 70 ± 1 | - | - |
| SO42− | - | 1100 ± 70 | 2600 ± 660 | 2500 ± 12 | 250 ± 9 | 271 ± 27 | 70 ± 3 | - | 1900 ± 6 | 210 ± 3 | 20,000 | 50,000 |
| Sb | - | - | - | - | - | - | - | - | - | - | 0.7 | 5 |
| Se | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | - | 0.08 ± 0.01 | 0.21 ± 0.01 | 0.01 ± 0.01 | - | - | 0.20 ± 0.01 | 0.5 | 7 |
| Sr | 0.08 ± 0.01 | 0.17 ± 0.04 | 0.08 ± 0.01 | 1.51 ± 0.02 | - | 0.11 ± 0.01 | - | - | 0.16 ± 0.01 | - | - | - |
| Ti | 0.99 ± 0.01 | 0.11 ± 0.02 | 0.17 ± 0.03 | 0.16 ± 0.02 | 0.10 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.02 | - | - | - | - | - |
| V | 0.14 ± 0.04 | 0.13 ± 0.02 | 0.14 ± 0.01 | - | 0.06 ± 0.02 | 0.19 ± 0.01 | - | - | - | - | - | - |
| Zn | 0.01 ± 0.01 | - | - | - | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | - | - | 0.06 ± 0.01 | 50 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz-Gómez, D.C.; Vilarinho, I.S.; Pérez-Moreno, S.M.; Carvalheiras, J.; Guerrero, J.L.; Novais, R.M.; Seabra, M.P.; Ríos, G.; Bolívar, J.P.; Labrincha, J.A. Immobilization of Hazardous Wastes on One-Part Blast Furnace Slag-Based Geopolymers. Sustainability 2021, 13, 13455. https://doi.org/10.3390/su132313455
Paz-Gómez DC, Vilarinho IS, Pérez-Moreno SM, Carvalheiras J, Guerrero JL, Novais RM, Seabra MP, Ríos G, Bolívar JP, Labrincha JA. Immobilization of Hazardous Wastes on One-Part Blast Furnace Slag-Based Geopolymers. Sustainability. 2021; 13(23):13455. https://doi.org/10.3390/su132313455
Chicago/Turabian StylePaz-Gómez, Daniela Carolina, Inês Silveirinha Vilarinho, Silvia M. Pérez-Moreno, João Carvalheiras, José Luis Guerrero, Rui Miguel Novais, Maria Paula Seabra, Guillermos Ríos, Juan Pedro Bolívar, and João António Labrincha. 2021. "Immobilization of Hazardous Wastes on One-Part Blast Furnace Slag-Based Geopolymers" Sustainability 13, no. 23: 13455. https://doi.org/10.3390/su132313455
APA StylePaz-Gómez, D. C., Vilarinho, I. S., Pérez-Moreno, S. M., Carvalheiras, J., Guerrero, J. L., Novais, R. M., Seabra, M. P., Ríos, G., Bolívar, J. P., & Labrincha, J. A. (2021). Immobilization of Hazardous Wastes on One-Part Blast Furnace Slag-Based Geopolymers. Sustainability, 13(23), 13455. https://doi.org/10.3390/su132313455