Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Characteristics
2.2. Anaerobic Digestion (AD) Methods
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
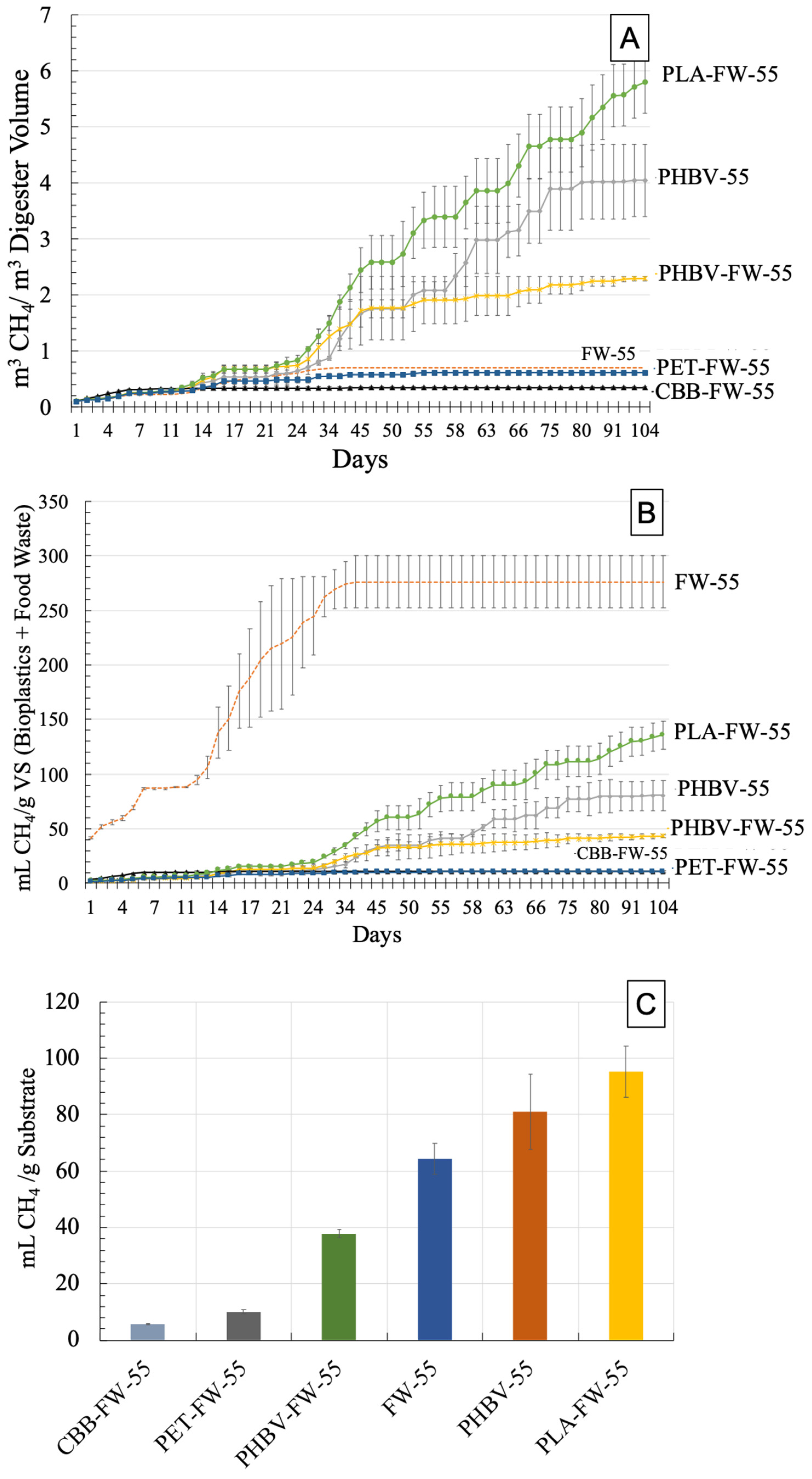
3.1. Cumulative Methane (CH4) Production—Thermophilic Digestion (55 °C)
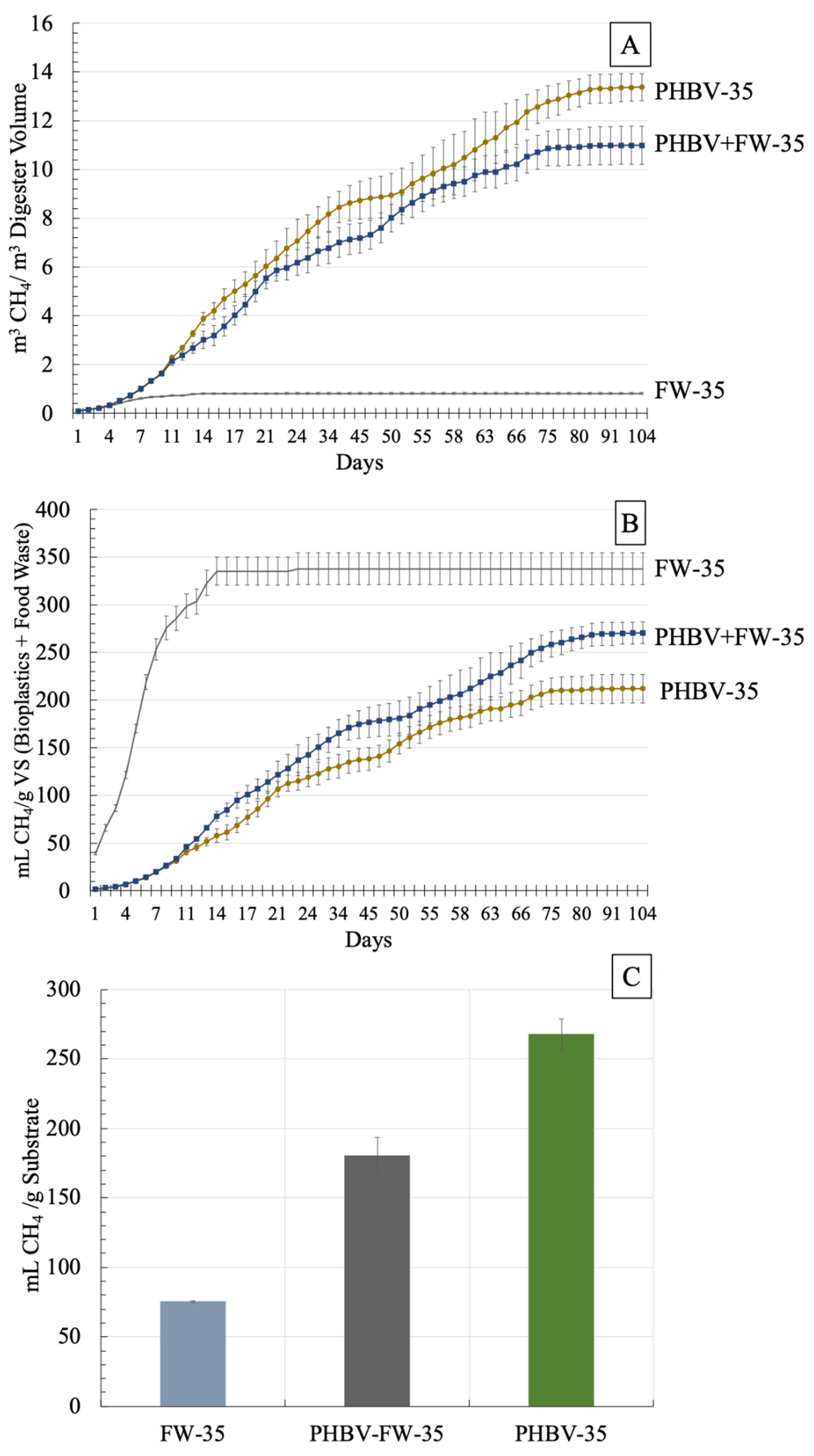
3.2. Cumulative Methane (CH4) Production—Mesophilic Digestion (35 °C)
3.3. VFA Concentration during Digestion
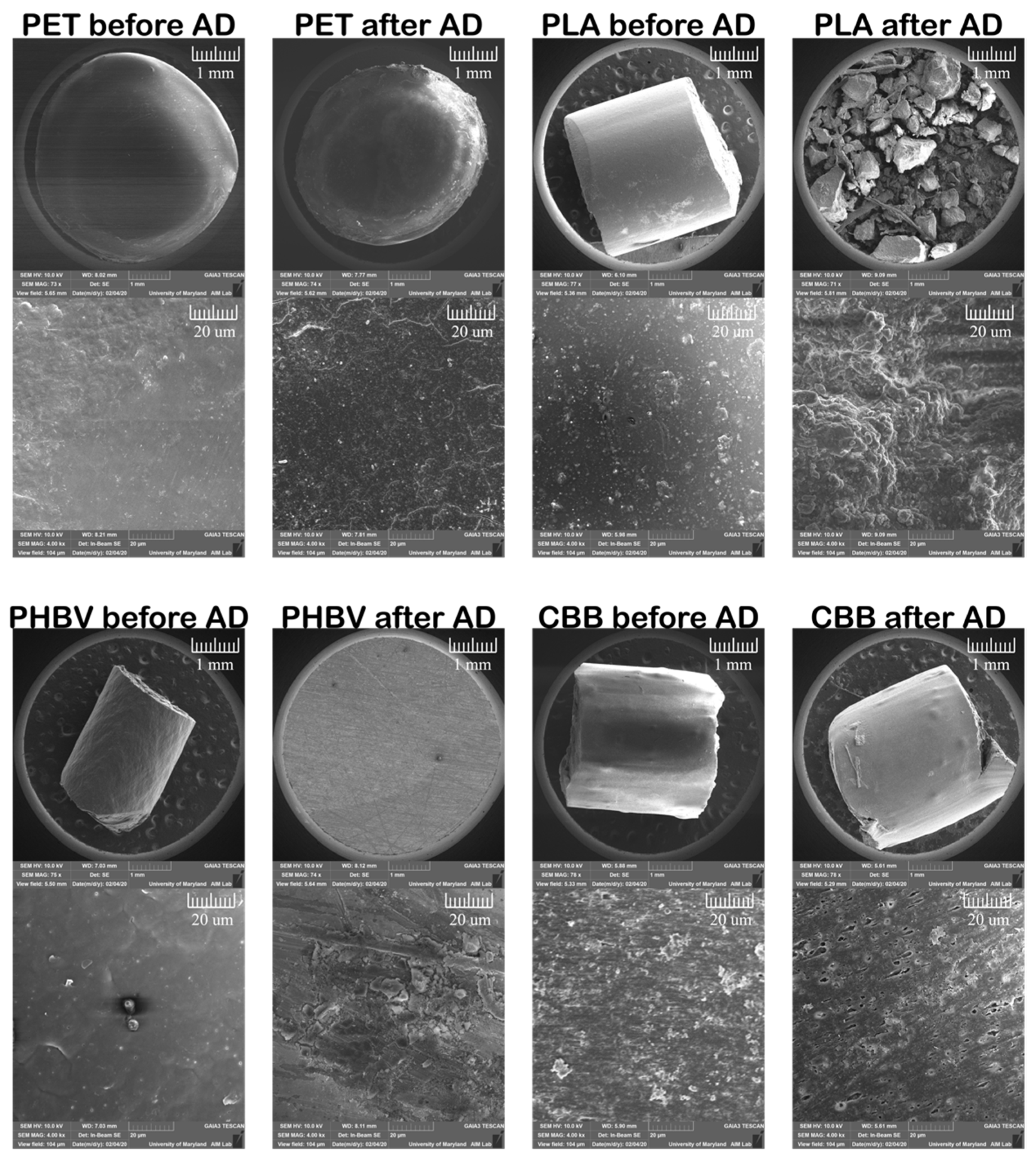
3.4. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masnadi, M.S.; El-Houjeiri, H.M.; Schunack, D.; Li, Y.; Englander, J.G.; Badahdah, A.; Monfort, J.-C.; Anderson, J.E.; Wallington, T.J.; Bergerson, J.A.; et al. Global carbon intensity of crude oil production. Science 2018, 361, 851–853. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Global Market for Bioplastics to Grow by 20 Percent—European Bioplastics e.V. Available online: https://www.european-bioplastics.org/global-market-for-bioplastics-to-grow-by-20-percent/ (accessed on 17 August 2021).
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Calabro’, P.S.; Folino, A.; Fazzino, F.; Komilis, D. Preliminary evaluation of the anaerobic biodegradability of three biobased materials used for the production of disposable plastics. J. Hazard. Mater. 2020, 390, 121653. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Basnett, P.; Ravi, S.; Roy, I. Natural bacterial biodegradable medical polymers: Polyhydroxyalkanoates. Sci. Princ. Biodegrad. Bioresorbable Med. Polym. Mater. Prop. 2017, 257–277. Available online: https://www.sciencedirect.com/science/article/pii/B9780081003725000088?via%3Dihub (accessed on 17 August 2021).
- Dubey, S.P.; Thakur, V.K.; Krishnaswamy, S.; Abhyankar, H.A.; Marchante, V.; Brighton, J.L. Progress in environmental-friendly polymer nanocomposite material from PLA: Synthesis, processing and applications. Vacuum 2017, 146, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknect, W. Comprehensive cellulose chemistry. Fundam. Anal. Methods 1999, 3. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Shrestha, A.; van-Eerten Jansen, M.C.A.A.; Acharya, B. Biodegradation of Bioplastic Using Anaerobic Digestion at Retention Time as per Industrial Biogas Plant and International Norms. Sustainability 2020, 12, 4231. [Google Scholar] [CrossRef]
- ENMAT. Technical Data Sheet & Processing Guide. ENMAT PHBV Resin (Y1000P). 2011. Available online: http://www.tianan-enmat.com/pdf/TDS_Y1000P_Dec2011.pdf (accessed on 1 March 2019).
- Biograde C 9550 WH › FKuR. Available online: https://fkur.com/en/brands/biograde-3/biograde-c-9550/ (accessed on 17 August 2021).
- Buzby, J.C.; Farah-Wells, H.; Hyman, J. The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States. Food Loss U. S. Sel. Anal. 2014, 1–42. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=43836 (accessed on 17 August 2021). [CrossRef] [Green Version]
- Moody, L.; Burns, R.; Wu-Haan, W.; Spajic, R. Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In Proceedings of the 44th Croatian and the 4th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2009. [Google Scholar]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and Limitations of Biochemical Methane Potential (BMP) Tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Reungsang, A.; Pattra, S.; Sittijunda, S. Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process. Energies 2012, 5, 4746–4757. [Google Scholar] [CrossRef] [Green Version]
- APHA Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005.
- Lansing, S.; Hülsemann, B.; Choudhury, A.; Schueler, J.; Lisboa, M.S.; Oechsner, H. Food waste co-digestion in Germany and the United States: From lab to full-scale systems. Resour. Conserv. Recycl. 2019, 148, 104–113. [Google Scholar] [CrossRef]
- Yuan, Q.; Sparling, R.; Oleszkiewicz, J.A. VFA generation from waste activated sludge: Effect of temperature and mixing. Chemosphere 2011, 82, 603–607. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation assessment of poly (lactic acid) filled with functionalized titania nanoparticles (PLA/TiO 2) under compost conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Benn, N.; Zitomer, D. Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Horváth, I.S. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-G.; Lee, B.; Jo, S.-Y.; Lee, J.-S.; Jun, H.-B. Control of accumulated volatile fatty acids by recycling nitrified effluent. J. Environ. Health Sci. Eng. 2018, 16, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Shin, P.K.; Kim, M.H.; Kim, J.M. Biodegradability of degradable plastics exposed to anaerobic digested sludge and simulated landfill conditions. J. Environ. Polym. Degrad. 1997, 5, 33–39. [Google Scholar]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic biodegradation tests of poly (lactic acid) under mesophilic and thermophilic conditions using a new evaluation system for methane fermentation in anaerobic sludge. Int. J. Mol. Sci. 2009, 10, 3824–3835. [Google Scholar] [CrossRef] [Green Version]
- Tomita, K.; Kuroki, Y.; Nagai, K. Isolation of thermophiles degrading poly (L-lactic acid). J. Biosci. Bioeng. 1999, 87, 752–755. [Google Scholar] [CrossRef]
- Pranamuda, H.; Tokiwa, Y.; Tanaka, H. Polylactide degradation by an Amycolatopsis sp. Appl. Environ. Microbiol. 1997, 63, 1637–1640. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
| Food Type | Food Waste (FW) Added (g) | Inclusion in Mixture (%) |
|---|---|---|
| Potato | 246 | 19.8 |
| White Bread | 132 | 10.7 |
| Apple | 94.5 | 7.62 |
| Banana | 56.7 | 4.57 |
| Chicken | 75.6 | 6.10 |
| Pork | 75.6 | 6.10 |
| Mixed Vegetables | 246 | 19.8 |
| Cheese | 56.7 | 4.57 |
| Cooked Rice | 37.8 | 3.05 |
| Water | 219 | 17.7 |
| Treatment | Inoculum (g) | Food Waste (g) | PHBV (g) | CBB (g) | PLA (g) | PET (g) |
|---|---|---|---|---|---|---|
| Inoculum-35 | 147.83 | 0 | 0 | 0 | 0 | 0 |
| FW-35 | 147.83 | 2.17 | 0 | 0 | 0 | 0 |
| PHBV-35 | 147.83 | 0 | 10 | 0 | 0 | 0 |
| PHBV-FW-35 | 147.83 | 2.17 | 10 | 0 | 0 | 0 |
| Inoculum-55 | 147.83 | 0 | 0 | 0 | 0 | 0 |
| FW-55 | 147.83 | 2.17 | 0 | 0 | 0 | 0 |
| PHBV-55 | 147.83 | 0 | 10 | 0 | 0 | 0 |
| PHBV-FW-55 | 147.83 | 2.17 | 10 | 0 | 0 | 0 |
| CBB-FW-55 | 147.83 | 2.17 | 0 | 10 | 0 | 0 |
| PLA-FW-55 | 147.83 | 2.17 | 0 | 0 | 10 | 0 |
| PET-FW-55 | 147.83 | 2.17 | 0 | 0 | 0 | 10 |
| Pre-Digestion Characteristics for in Each Treatment Reactor | |||
|---|---|---|---|
| Treatment | TS (g/kg) | VS (% TS) | pH |
| Inoculum | 9.70 ± 0.35 | 67.3 ± 0.5 | 7.52 ± 0.00 |
| FW | 12.9 ± 0.0 | 74.6 ± 0.0 | 7.53 ± 0.00 |
| PHBV | 72.2 ± 0.0 | 95.3 ± 0.0 | 7.55 ± 0.01 |
| PHBV + FW | 74.4 ± 0.0 | 95.3 ± 0.0 | 7.60 ± 0.02 |
| CBB + FW | 72.1 ± 0.0 | 61.1 ± 0.0 | 7.57 ± 0.00 |
| PLA + FW | 74.4 ± 0.0 | 79.0 ± 0.2 | 7.55 ± 0.02 |
| PET + FW | 74.6 ± 0.0 | 95.7 ± 0.0 | 7.58 ± 0.01 |
| Treatment | Cumulative CH4 (mL CH4/g VS of FW + BP) and (% of Total CH4 Production) | ||||
|---|---|---|---|---|---|
| 11 Days | 21 Days | 34 Days | 51 Days | 104 Days | |
| FW-55 | 88.7 ± 40 (32.1%) | 220 ± 60 (80.0%) | 274 ± 21 (99.1%) | 276 ± 24 (100%) | 276 ± 24 (100%) |
| PHBV-55 | 4.9 ± 0.1 (6.1%) | 10.6 ± 3.9 (13.2%) | 17.1 ± 2.6 (21.2%) | 34.7 ± 3.3 (43.1%) | 80.5 ± 13 (100%) |
| PHBV-FW-55 | 5.0 ± 0.1 (11.6%) | 12.8 ± 1.3 (29.5%) | 23.6 ± 3.6 (54.5%) | 33.4 ± 11 (77.2%) | 43.3 ± 1.2 (100%) |
| CBB-FW-55 | 10.2 ± 0.5 (95.5%) | 10.7 ± 0.7 (99.8%) | 10.7 ± 0.7 (100%) | 10.7 ± 0.8 (100%) | 10.7 ± 0.8 (100%) |
| PLA-FW-55 | 6.5 ± 0.3 (4.8%) | 15.6 ± 1.8 (11.5%) | 34.5 ± 2.9 (25.4%) | 63.8 ± 14 (47.0%) | 136 ± 13 (100%) |
| PET-FW-55 | 5.0 ± 0.2 (46.9%) | 8.6 ± 1.2 (80.5%) | 9.9 ± 0.9 (92.7%) | 10.3 ± 0.8 (96.1%) | 10.7 ± 0.9 (100%) |
| FW-35 | 299 ± 13 (88.4%) | 335 ± 15 (99.2%) | 338 ± 17 (100%) | 338 ± 17 (100%) | 338 ± 17 (100%) |
| PHBV-35 | 46.0 ± 1.9 (17.0%) | 122 ± 14 (45.1%) | 165 ± 14 (61.1%) | 184 ± 19 (67.9%) | 271 ± 11 (100%) |
| PHBV-FW-35 | 41.2 ± 3.4 (19.4%) | 107 ± 8.3 (50.3%) | 131 ± 13 (61.6%) | 161 ± 11 (76.0%) | 212 ± 15 (100%) |
| Pre-BMP | ||||||
|---|---|---|---|---|---|---|
| Treatment | pH | Acetic Acid (mg/L) | Propionic Acid (mg/L) | Butyric Acid (mg/L) | Valeric Acid (mg/L) | Total VFA (mg/L) |
| PHBV | 7.55 | 811 ± 7.3 | 309 ± 2.5 | 176 ± 4.3 | 20.2 ± 0.75 | 1316 ± 11 |
| PHBV-FW | 7.60 | 754 ± 6.8 | 267 ± 12 | 164 ± 6.3 | 65.6 ± 56 | 1250 ± 69 |
| CBB-FW | 7.57 | 4316 ± 16 | 153 ± 4.7 | 932 ± 27 | 2.65 ± 2.65 | 5405 ± 34 |
| PLA-FW | 7.55 | 813 ± 5.9 | 264 ± 1.9 | 153 ± 1.7 | 5.04 ± 2.5 | 1234 ± 11 |
| PET-FW | 7.58 | 815 ± 19 | 310 ± 4.1 | 210 ± 20 | 11.7 ± 0.28 | 1350 ± 35 |
| FW | 7.53 | 826 ± 5.1 | 173 ± 5.4 | 226 ± 8.4 | 18.5 ± 2.7 | 1244 ± 3 |
| Post-BMP Thermophilic (55 °C) Digestion | ||||||
| Treatment | pH | Acetic Acid (mg/L) | Propionic Acid (mg/L) | Butyric Acid (mg/L) | Valeric Acid (mg/L) | Total VFA (mg/L) |
| PHBV | 4.56 | 2153 ± 458 | 111 ± 26 | 2229 ± 242 | 119 ± 0.85 | 4613 ± 592 |
| PHBV-FW | 8.79 | 236 ± 12 | 69.8 ± 18.5 | 161 ± 2 | 114 ± 80 | 582 ± 85 |
| CBB-FW | 5.20 | 9154 ± 248 | 403 ± 54 | 2141 ± 172 | 98.9 ± 22.9 | 11,797 ± 175 |
| PLA-FW | 8.49 | 431 ± 132 | 115 ± 23 | 81.7 ± 53.9 | 27.6 ± 3.7 | 657 ± 99 |
| FW | 8.48 | 196 ± 9 | 45.5 ± 0.4 | 0 ± 0.0 | 55.9 ± 11.2 | 298 ± 5 |
| PET-FW | 8.42 | 201 ± 9 | 49.2 ± 5.4 | 0 ± 0.0 | 13.7 ± 1.2 | 264 ± 14 |
| Post-BMP Mesophilic (35 °C) Digestion | ||||||
| Treatment | pH | Acetic Acid (mg/L) | Propionic Acid (mg/L) | Butyric Acid (mg/L) | Valeric Acid (mg/L) | Total VFA (mg/L) |
| PHBV | 8.73 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 9.4 ± 1.0 | 9.4 ± 1.0 |
| PHBV-FW | 8.72 | 0 ± 0.0 | 61.7 ± 16.6 | 82.1 ± 41.5 | 111 ± 31 | 255 ± 73 |
| FW | 8.58 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 18.5 ± 2.2 | 18.5 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachod, B.; Keller, E.; Hassanein, A.; Lansing, S. Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion. Sustainability 2021, 13, 13295. https://doi.org/10.3390/su132313295
Nachod B, Keller E, Hassanein A, Lansing S. Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion. Sustainability. 2021; 13(23):13295. https://doi.org/10.3390/su132313295
Chicago/Turabian StyleNachod, Benjamin, Emily Keller, Amro Hassanein, and Stephanie Lansing. 2021. "Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion" Sustainability 13, no. 23: 13295. https://doi.org/10.3390/su132313295
APA StyleNachod, B., Keller, E., Hassanein, A., & Lansing, S. (2021). Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion. Sustainability, 13(23), 13295. https://doi.org/10.3390/su132313295







