An Integrated Medical-Psychological Approach in the Routine Care of Patients with Type 2 Diabetes: A Pilot Study to Explore the Clinical and Economic Sustainability of the Healthcare Intervention
Abstract
:1. Introduction
1.1. Psychological Conditions and Stress as Modifiable Risk Factors
1.2. Integrated Interventions Strategies for Persons with Type 2 Diabetes
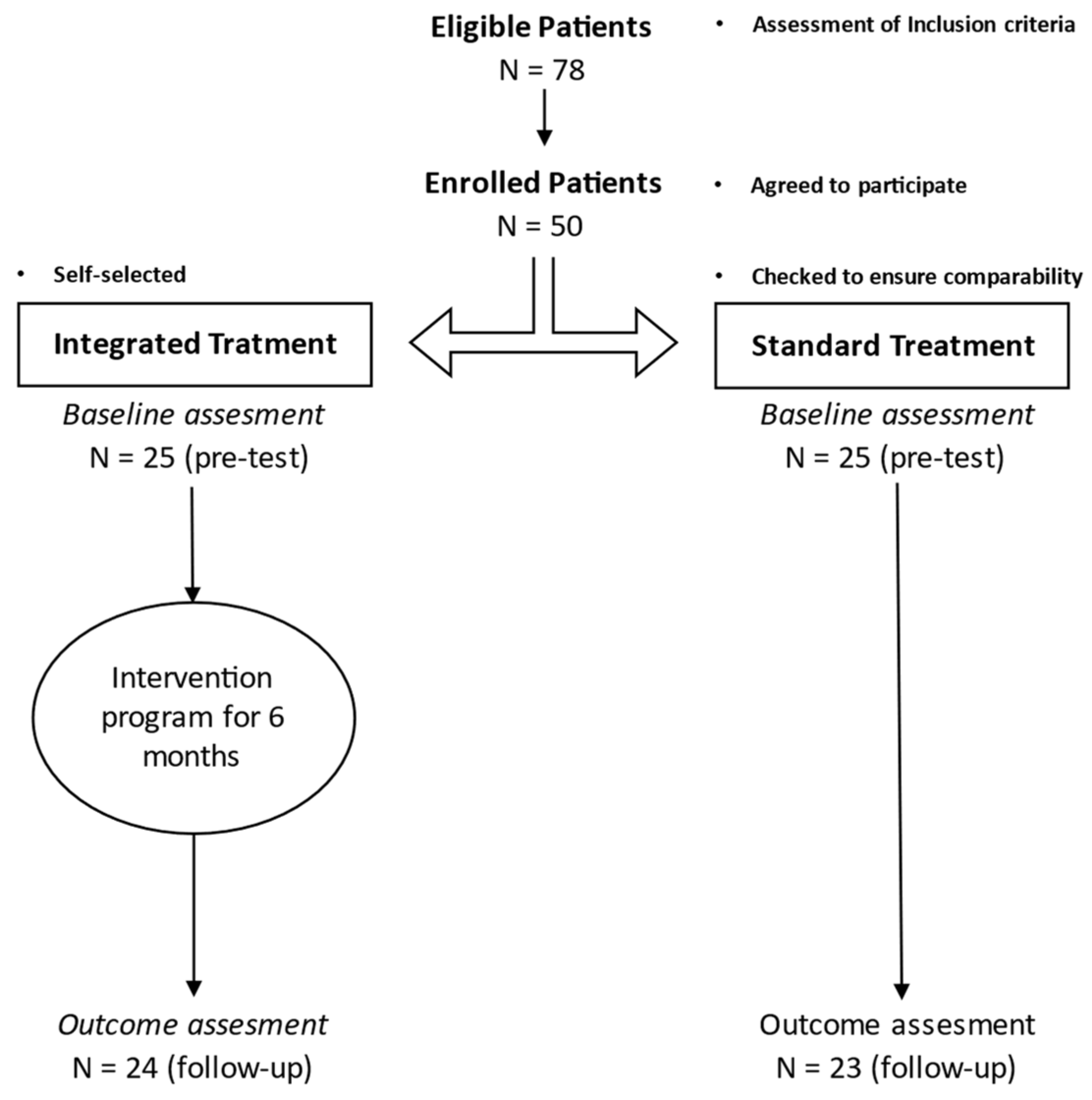
2. Materials and Methods
2.1. Study Design and Procedures
2.2. Participants
2.3. Interventions
2.4. Outcome Measures at Baseline and 6-Month Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics at Baseline
3.2. Patients Characteristics at Follow-Up
3.3. Longitudinal Change
3.4. Economic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of Chronic Disease in the 21st Century: Elimination of the Leading Preventable Causes of Premature Death and Disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef]
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 12 October 2021).
- IDF Diabetes Atlas, 9th ed. 2019. Available online: https://diabetesatlas.org/en/ (accessed on 12 October 2021).
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the Current and Future Costs of Type 1 and Type 2 Diabetes in the UK, Including Direct Health Costs and Indirect Societal and Productivity Costs. Diabet. Med. 2012, 29, 855–862. [Google Scholar] [CrossRef]
- Pagano, E.; De Rosa, M.; Rossi, E.; Cinconze, E.; Marchesini, G.; Miccoli, R.; Vaccaro, O.; Bonora, E.; Bruno, G. The Relative Burden of Diabetes Complications on Healthcare Costs: The Population-Based CINECA-SID ARNO Diabetes Observatory. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 944–950. [Google Scholar] [CrossRef] [PubMed]
- McEwen, L.N.; Herman, W.H. Health Care Utilization and Costs of Diabetes. In Diabetes in America; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., et al., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018. [Google Scholar]
- Giorda, C.B.; Manicardi, V.; Diago Cabezudo, J. The Impact of Diabetes Mellitus on Healthcare Costs in Italy. Expert Rev. Pharm. Outcomes Res. 2011, 11, 709–719. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/Lifestyle Factors in the Pathogenesis and Prevention of Type 2 Diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Hackett, R.A.; Steptoe, A. Type 2 Diabetes Mellitus and Psychological Stress—a Modifiable Risk Factor. Nat. Rev. Endocrinol. 2017, 13, 547–560. [Google Scholar] [CrossRef]
- Marchini, F.; Caputo, A.; Napoli, A.; Balonan, J.T.; Martino, G.; Nannini, V.; Langher, V. Chronic Illness as Loss of Good Self: Underlying Mechanisms Affecting Diabetes Adaptation. Mediterr. J. Clin. Psychol. 2018, 6. [Google Scholar] [CrossRef]
- Martino, G.; Langher, V.; Cazzato, V.; Vicario, C.M. Editorial: Psychological Factors as Determinants of Medical Conditions. Front. Psychol. 2019, 10, 2502. [Google Scholar] [CrossRef] [Green Version]
- Chronic Disease Management. Available online: https://link.springer.com/chapter/10.1007/978-1-4419-6510-3_9 (accessed on 12 October 2021).
- Nobis, S.; Ebert, D.D.; Lehr, D.; Smit, F.; Buntrock, C.; Berking, M.; Baumeister, H.; Snoek, F.; Funk, B.; Riper, H. Web-Based Intervention for Depressive Symptoms in Adults with Types 1 and 2 Diabetes Mellitus: A Health Economic Evaluation. Br. J. Psychiatry 2018, 212, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Renn, B.N.; Obetz, V.; Feliciano, L. Comorbidity of Depressive Symptoms among Primary Care Patients with Diabetes in a Federally Qualified Health Center. J. Health Psychol. 2020, 25, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Deng, W. Psychosocial Intervention for Patients with Type 2 Diabetes Mellitus and Comorbid Depression: A Meta-Analysis of Randomized Controlled Trials. Neuropsychiatr. Dis. Treat. 2017, 13, 2681–2690. [Google Scholar] [CrossRef]
- Diabetes Mellitus as a Risk Factor for Depression. A Meta-Analysis of Longitudinal Studies—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0168822712004871?casa_token=1dhinJ-r5ykAAAAA:0XKNzaIV63Aoh0mOsMuPyCPzcUrCssRpfZRAqtmPZ91fJfcIyEhwRITLbT0p3HCtR7LeuueyjIg (accessed on 12 October 2021).
- Smith, K.J.; Deschênes, S.S.; Schmitz, N. Investigating the Longitudinal Association between Diabetes and Anxiety: A Systematic Review and Meta-Analysis. Diabet. Med. 2018, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, I.-C.; Lin, C.-H. Increased Risk of Diabetes in Patients with Anxiety Disorders: A Population-Based Study. J. Psychosom. Res. 2016, 86, 47–52. [Google Scholar] [CrossRef]
- Khambaty, T.; Callahan, C.M.; Perkins, A.J.; Stewart, J.C. Depression and Anxiety Screens as Simultaneous Predictors of 10-Year Incidence of Diabetes Mellitus in Older Adults in Primary Care. J. Am. Geriatr. Soc. 2017, 65, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góis, C.; Duarte, T.A.; Paulino, S.; Raposo, J.F.; do Carmo, I.; Barbosa, A. Depressive Symptoms Are Associated with Poor Glycemic Control among Women with Type 2 Diabetes Mellitus. BMC Res. Notes 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouwer, F.; Nefs, G.; Nouwen, A. Adverse Effects of Depression on Glycemic Control and Health Outcomes in People with Diabetes: A Review. Endocrinol. Metab. Clin. 2013, 42, 529–544. [Google Scholar] [CrossRef]
- Gonzalez Heredia, T.; González-Ramírez, L.P.; Hernández-Corona, D.M.; Maciel-Hernández, E.A. Anxious Depression in Patients with Type 2 Diabetes Mellitus and Its Relationship with Medication Adherence and Glycemic Control. Glob. Public Health 2021, 16, 460–468. [Google Scholar] [CrossRef]
- Sumlin, L.L.; Garcia, T.J.; Brown, S.A.; Winter, M.A.; Garcia, A.A.; Brown, A.; Cuevas, H.E. Depression and Adherence to Lifestyle Changes in Type 2 Diabetes: A Systematic Review. Diabetes Educ. 2014, 40, 731–744. [Google Scholar] [CrossRef]
- Egede, L.E.; Gebregziabher, M.; Dismuke, C.E.; Lynch, C.P.; Axon, R.N.; Zhao, Y.; Mauldin, P.D. Medication Nonadherence in Diabetes: Longitudinal Effects on Costs and Potential Cost Savings from Improvement. Diabetes Care 2012, 35, 2533–2539. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Filippetti, G.; Schifano, I.; Aceto, P.; Tomai, M.; Lai, S.; Pierro, L.; Renzi, A.; Carnovale, A.; Maranghi, M. Psychological, Emotional and Social Impairments Are Associated with Adherence and Healthcare Spending in Type 2 Diabetic Patients: An Observational Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 749–754. [Google Scholar]
- McEwen, B.S.; Stellar, E. Stress and the Individual: Mechanisms Leading to Disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Rodriquez, E.J.; Kim, E.N.; Sumner, A.E.; Nápoles, A.M.; Pérez-Stable, E.J. Allostatic Load: Importance, Markers, and Score Determination in Minority and Disparity Populations. J. Urban Health 2019, 96, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Macit, M.S.; Acar-Tek, N. Evaluation of Nutritional Status and Allostatic Load in Adult Patients With Type 2 Diabetes. Can. J. Diabetes 2020, 44, 156–161. [Google Scholar] [CrossRef]
- Steptoe, A.; Hackett, R.A.; Lazzarino, A.I.; Bostock, S.; La Marca, R.; Carvalho, L.A.; Hamer, M. Disruption of Multisystem Responses to Stress in Type 2 Diabetes: Investigating the Dynamics of Allostatic Load. Proc. Natl. Acad. Sci. USA 2014, 111, 15693–15698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westley, K.V.; August, K.J.; Alger, M.R.; Markey, C.H. Main and Interactive Effects of Diabetes Distress and Stress from Life Events on Overall Psychological Distress. J. Health Psychol. 2021, 26, 312–318. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Gianaros, P.J. Central Role of the Brain in Stress and Adaptation: Links to Socioeconomic Status, Health, and Disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef] [Green Version]
- Kanapathy, J.; Bogle, V. The Effectiveness of Cognitive Behavioural Therapy for Depressed Patients with Diabetes: A Systematic Review. J. Health Psychol. 2019, 24, 137–149. [Google Scholar] [CrossRef]
- Wozniak, L.; Soprovich, A.; Rees, S.; Al Sayah, F.; Majumdar, S.R.; Johnson, J.A. Contextualizing the Effectiveness of a Collaborative Care Model for Primary Care Patients with Diabetes and Depression (Teamcare): A Qualitative Assessment Using RE-AIM. Can. J. Diabetes 2015, 39, S83–S91. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Zoppis, I.; Santoro, E.; Ceccarini, M.; Pietrabissa, G.; Manzoni, G.M.; Corti, S.; Borrello, M.; Giusti, E.M.; Cattivelli, R.; et al. Managing Chronic Pathologies with a Stepped MHealth-Based Approach in Clinical Psychology and Medicine. Front. Psychol. 2015, 6, 407. [Google Scholar] [CrossRef] [Green Version]
- Guérin, E.; Jaafar, H.; Amrani, L.; Prud’homme, D.; Aguer, C. Intervention Strategies for Prevention of Comorbid Depression Among Individuals With Type 2 Diabetes: A Scoping Review. Front. Public Health 2019, 7, 35. [Google Scholar] [CrossRef]
- Tomai, M.; Esposito, F.; Rosa, V. Psychologists in Italian Hospital Settings: An Exploratory Analysis of Hospital Physicians’ Representations and Demands of Psychological Intervention. Interdisciplinaria 2017, 34, 5–23. [Google Scholar] [CrossRef]
- Young-Hyman, D.; De Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial Care for People with Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef] [Green Version]
- Dezetter, A.; Briffault, X.; Lakhdar, C.B.; Kovess-Masfety, V. Costs and Benefits of Improving Access to Psychotherapies for Common Mental Disorders. J. Ment. Health Policy Econ. 2013, 16, 161–177. [Google Scholar]
- Knapp, M.; Wong, G. Economics and Mental Health: The Current Scenario. World Psychiatry 2020, 19, 3–14. [Google Scholar] [CrossRef]
- McGloin, H.; Devane, D.; McIntosh, C.D.; Winkley, K.; Gethin, G. Psychological Interventions for Treating Foot Ulcers, and Preventing Their Recurrence, in People with Diabetes. Cochrane Database Syst. Rev. 2021, CD012835. [Google Scholar] [CrossRef]
- Kok, J.L.A.; Williams, A.; Zhao, L. Psychosocial Interventions for People with Diabetes and Co-Morbid Depression. A Systematic Review. Int. J. Nurs. Stud. 2015, 52, 1625–1639. [Google Scholar] [CrossRef]
- Tovar, E.; Rayens, M.K.; Gokun, Y.; Clark, M. Mediators of Adherence among Adults with Comorbid Diabetes and Depression: The Role of Self-Efficacy and Social Support. J. Health Psychol. 2015, 20, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C. The Transtheoretical Approach. In Handbook of Psychotherapy Integration; Norcross, J.C., Goldfried, M.R., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 147–171. [Google Scholar]
- Chapman, A.; Liu, S.; Merkouris, S.; Enticott, J.C.; Yang, H.; Browning, C.J.; Thomas, S.A. Psychological Interventions for the Management of Glycemic and Psychological Outcomes of Type 2 Diabetes Mellitus in China: A Systematic Review and Meta-Analyses of Randomized Controlled Trials. Front. Public Health 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, T.A. The Empty Chair As a Tool to Promote Self-Awareness and Interaction in Groups. In 101 Interventions in Group Therapy; Fehr, S.S., Ed.; Taylor & Francis Group: Abingdon, UK, 2010; p. 381. [Google Scholar]
- Polster, E.; Polster, M. From the Radical Center: The Heart of Gestalt Therapy; Gestalt Press: Santa Cruz, CA, USA, 2000; ISBN 9780881633153. [Google Scholar]
- Ellis, A. The Revised ABC’s of Rational-Emotive Therapy (RET). J. Ration.-Emot. Cogn.-Behav. Ther. 1991, 9, 139–172. [Google Scholar] [CrossRef]
- Bandura, A. Modeling Theory: Some Traditions, Trends, and Disputes. In Recent Trends in Social Learning Theory; Parke, R.D., Ed.; Elsevier: Amsterdam, The Netherlands, 1972; pp. 35–61. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory (BDI-II); Pearson: London, UK, 1996; Volume 10. [Google Scholar]
- Spielberger, C.D. Inventaire d’Anxiété État-Trait: Forme Y; ECPA, les Éditions du Centre de Psychologie Appliquée: Paris, France, 1993; ISBN 2-7253-0003-7. [Google Scholar]
- Sirigatti, S.; Stefanile, C. CISS–Coping Inventory for Stressful Situation. Stanrdizzazione e Validazione Italiana; Giunti O.S. Organizzazioni Speciali: Firenze, Italy, 2009. [Google Scholar]
- The Twenty-Item Toronto Alexithymia Scale—I. Item Selection and Cross-Validation of the Factor Structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Gross, J.J.; John, O.P. Individual Differences in Two Emotion Regulation Processes: Implications for Affect, Relationships, and Well-Being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef]
- Wallston, B.S.; Wallston, K.A.; Kaplan, G.D.; Maides, S.A. Development and Validation of the Health Locus of Control (HLC) Scale. J. Consult. Clin. Psychol. 1976, 44, 580–585. [Google Scholar] [CrossRef]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Wunder, J.; De Rosario, H.; De Rosario, M.H. Package ‘pwr’. Available online: https://cran.r-project.org/web/packages/pwr/pwr.pdf (accessed on 12 October 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Yanai, H.; Hirowatari, Y.; Yoshida, H. Diabetic Dyslipidemia: Evaluation and Mechanism. Glob. Health Med. 2019, 1, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Savelieff, M.G.; Callaghan, B.C.; Feldman, E.L. The Emerging Role of Dyslipidemia in Diabetic Microvascular Complications. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.-J.; Hsieh, Y.-T.; Yang, C.-M.; Yang, C.-H.; Lin, C.-L.; Wang, I.-J. Diabetic Retinopathy in Patients with Dyslipidemia: Development and Progression. Ophthalmol. Retin. 2018, 2, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Crispín-Trebejo, B.; Robles-Cuadros, M.C.; Bernabé-Ortiz, A. Association between Depression and Glycemic Control among Type 2 Diabetes Patients in Lima, Peru. Asia-Pac. Psychiatry 2015, 7, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrodimitris, S.D.; Endler, N.S. Coping, Control, and Adjustment in Type 2 Diabetes. Health Psychol. 2001, 20, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Shreck, E.; Psaros, C.; Safren, S.A. Distress and Type 2 Diabetes-Treatment Adherence: A Mediating Role for Perceived Control. Health Psychol. 2015, 34, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Quinn, L.; Park, C.; Martyn-Nemeth, P. Pathways of the Relationships among Eating Behavior, Stress, and Coping in Adults with Type 2 Diabetes: A Cross-Sectional Study. Appetite 2018, 131, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Shayeghian, Z.; Aguilar-Vafaie, M.E.; Besharat, M.A.; Amiri, P.; Parvin, M.; Gillani, K.R.; Hassanabadi, H. Self-Care Activities and Glycated Haemoglobin in Iranian Patients with Type 2 Diabetes: Can Coping Styles and Social Support Have a Buffering Role? Psychol. Health 2015, 30, 153–164. [Google Scholar] [CrossRef] [PubMed]
| N | M | SD | Min | Max | Range | |
|---|---|---|---|---|---|---|
| Physical Health | ||||||
| hBa1C (%) | 47 | 8.33 | 1.66 | 5 | 13 | 7 |
| Blood glucose (mg/dL) | 47 | 175.51 | 72.79 | 87 | 350 | 263 |
| Total Cholesterol (mg/dL) | 47 | 183.57 | 36.09 | 90 | 284 | 194 |
| HDL Cholesterol (mg/dL) | 47 | 103.94 | 28.89 | 32 | 100 | 68 |
| LDL Cholesterol (mg/dL) | 47 | 59.40 | 10.46 | 48 | 208 | 160 |
| Triglycerides (mg/dL) | 43 | 110.34 | 45.61 | 32 | 226 | 194 |
| Systolic Blood Pressure (mmHg) | 47 | 119.88 | 13.35 | 90 | 170 | 80 |
| Diastolic Blood Pressure (mmHg) | 47 | 75.31 | 9.02 | 60 | 100 | 40 |
| Lifestyle | ||||||
| Cigarettes per day (number) | 47 | 7.98 | 11.71 | 0 | 40 | 40 |
| Wine per day (glasses) | 37 | 0.78 | 1.27 | 0 | 5 | 5 |
| Alcohol per day (glasses) | 38 | 0.34 | 0.78 | 0 | 3 | 3 |
| Walk per day (minutes) | 47 | 25.76 | 31.30 | 0 | 120 | 120 |
| Physical activity per week (minutes) | 45 | 62.18 | 86.39 | 0 | 480 | 480 |
| BMI (kg/m2) | 42 | 30.84 | 593.00 | 20 | 46 | 26 |
| Mental Health | ||||||
| Depression | 41 | 11.43 | 8.87 | 0 | 37 | 37 |
| State Anxiety | 41 | 42.26 | 11.74 | 20 | 65 | 45 |
| Trait Anxiety | 41 | 44.42 | 10.40 | 23 | 65 | 42 |
| Health Locus of Control | 41 | 35.86 | 6.28 | 15 | 50 | 35 |
| Task-oriented Coping | 41 | 52.27 | 15.35 | 27 | 80 | 53 |
| Emotion-oriented Coping | 41 | 40.53 | 9.51 | 17 | 60 | 43 |
| Avoidance-oriented Coping | 41 | 41.09 | 9.61 | 24 | 61 | 37 |
| Difficulty Identifying Feelings | 41 | 15.77 | 5.42 | 7 | 24 | 17 |
| Difficulty Describing Feelings | 41 | 12.68 | 4.97 | 5 | 23 | 18 |
| Externally-oriented Thinking | 41 | 19.30 | 4.57 | 8 | 26 | 18 |
| Alexithymia | 41 | 48.02 | 12.13 | 20 | 68 | 48 |
| Cognitive Reappraisal | 37 | 4.75 | 1.18 | 2 | 7 | 5 |
| Suppression | 37 | 3.86 | 1.44 | 1 | 7 | 6 |
| Standard Treatment | Integrated Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Physical Health | N | M | SD | M | SD | t-Test | df | p | Cohen’s d |
| hBa1C (%) | 47 | 7.98 | (1.46) | 8.64 | (1.80) | −1.37 | 45 | 0.179 | 0.40 |
| Blood glucose (mg/dL) | 47 | 176.68 | (76.38) | 174.48 | (71.05) | 0.1 | 45 | 0.919 | 0.03 |
| Total Cholesterol (mg/dL) | 47 | 174.68 | (33.04) | 191.4 | (37.49) | −1.61 | 45 | 0.114 | 0.47 |
| HDL Cholesterol (mg/dL) | 47 | 96.9 | (19.84) | 110.14 | (34.21) | −1.59 | 45 | 0.118 | 0.47 |
| LDL Cholesterol (mg/dL) | 47 | 60.62 | (12.91) | 58.33 | (15.00) | 0.56 | 45 | 0.581 | 0.16 |
| Triglycerides (mg/dL) | 43 | 110.5 | (33.47) | 110.22 | (53.35) | 0.02 | 41 | 0.984 | 0.01 |
| Systolic Blood Pressure (mmHg) | 47 | 121.78 | (12.73) | 118.2 | (13.91) | 0.92 | 44 | 0.364 | 0.27 |
| Diastolic Blood Pressure (mmHg) | 47 | 77.03 | (10.13) | 73.8 | (7.81) | 1.23 | 45 | 0.224 | 0.36 |
| Lifestyle | |||||||||
| Cigarettes per day (number) | 47 | 10.14 | (13.50) | 6.08 | (9.76) | 1.19 | 45 | 0.240 | 0.35 |
| Wine per day (glasses) | 37 | 0.33 | (0.72) | 1.09 | (1.48) | −1.84 | 35 | 0.075 | 0.61 |
| Alcohol per day (glasses) | 38 | 0.07 | (0.27) | 0.13 | (0.61) | −0.31 | 36 | 0.759 | 0.10 |
| Walk per day (minutes) | 47 | 20.45 | (28.36) | 30.42 | (33.55) | −1.09 | 45 | 0.281 | −0.32 |
| Physical activity per week (minutes) | 45 | 72.14 | (107.46) | 53.47 | (63.77) | 0.72 | 43 | 0.476 | 0.22 |
| BMI (kg/m2) | 42 | 23.23 | (5.04) | 29.50 | (6.41) | −1.33 | 40 | 0.191 | 0.41 |
| Mental Health | |||||||||
| Depression | 41 | 14.14 | (9.51) | 9.3 | (7.90) | 1.78 | 39 | 0.083 | 0.56 |
| State Anxiety | 41 | 46.95 | (9.54) | 38.58 | (12.16) | 2.4 | 39 | 0.021 | 0.75 |
| Trait Anxiety | 41 | 49.02 | (7.93) | 40.83 | (10.83) | 2.69 | 39 | 0.010 | 0.85 |
| Health Locus of Control | 41 | 37.68 | (6.00) | 34.43 | (6.24) | 1.68 | 39 | 0.100 | 0.53 |
| Task-oriented Coping | 41 | 49.79 | (16.06) | 54.22 | (14.83) | −0.92 | 39 | 0.365 | 0.29 |
| Emotion-oriented Coping | 41 | 39.43 | (8.99) | 41.39 | (10.00) | −0.65 | 39 | 0.519 | 0.20 |
| Avoidance-oriented Coping | 41 | 38.71 | (9.40) | 42.96 | (9.57) | −1.42 | 39 | 0.163 | 0.45 |
| Difficulty Identifying Feelings | 41 | 16.47 | (5.60) | 15.23 | (5.34) | 0.72 | 39 | 0.475 | 0.23 |
| Difficulty Describing Feelings | 41 | 13.62 | (4.44) | 11.95 | (5.33) | 1.06 | 39 | 0.294 | 0.34 |
| Externally-oriented Thinking | 41 | 20.73 | (3.95) | 18.18 | (4.79) | 1.82 | 39 | 0.076 | 0.57 |
| Alexithymia | 41 | 51.42 | (11.30) | 45.36 | (12.33) | 1.62 | 39 | 0.114 | 0.51 |
| Cognitive Reappraisal | 37 | 4.7 | (1.29) | 4.78 | (1.14) | −0.18 | 35 | 0.859 | 0.06 |
| Suppression | 37 | 4.29 | (1.40) | 3.61 | (1.43) | 1.41 | 35 | 0.168 | 0.48 |
| Standard Treatment | Integrated Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Physical Health | N | M | SD | M | SD | t-Test | df | p | Cohen’s d |
| hBa1C (%) | 45 | 6.88 | (0.91) | 7.11 | (0.96) | −0.8 | 43 | 0.426 | 0.24 |
| Blood glucose (mg/dL) | 47 | 124.76 | (30.97) | 116.31 | (20.86) | 1.11 | 45 | 0.273 | 0.32 |
| Total Cholesterol (mg/dL) | 47 | 160.95 | (26.05) | 176.99 | (22.72) | −2.25 | 45 | 0.029 | 0.66 |
| HDL Cholesterol (mg/dL) | 46 | 87.14 | (19.00) | 94.01 | (22.18) | −1.12 | 44 | 0.270 | 0.33 |
| LDL Cholesterol (mg/dL) | 46 | 52.33 | (13.66) | 61.54 | (15.36) | −2.13 | 44 | 0.039 | 0.63 |
| Triglycerides (mg/dL) | 43 | 136.44 | (54.65) | 97.2 | (46.23) | 2.54 | 41 | 0.015 | 0.79 |
| Systolic Blood Pressure (mmHg) | 46 | 120.32 | (9.62) | 114.97 | (6.22) | 2.27 | 44 | 0.028 | 0.67 |
| Diastolic Blood Pressure (mmHg) | 47 | 73.45 | (7.98) | 69.6 | (6.76) | 1.79 | 45 | 0.080 | 0.52 |
| Lifestyle | |||||||||
| Cigarettes per day (number) | 47 | 4.41 | (6.99) | 2.21 | (4.12) | 1.34 | 45 | 0.188 | 0.39 |
| Wine per day (glasses) | 38 | 0.20 | (0.56) | 0.26 | (0.54) | −0.33 | 36 | 0.740 | 0.11 |
| Alcohol per day (glasses) | 38 | 0.07 | (0.27) | 1.25 | (6.12) | −0.72 | 36 | 0.479 | 0.24 |
| Walk per day (minutes) | 47 | 40.5 | (31.89) | 61.93 | (54.64) | −1.61 | 45 | 0.114 | 0.47 |
| Physical activity per week (minutes) | 47 | 146.93 | (136.19) | 160.98 | (128.06) | −0.36 | 45 | 0.717 | 0.11 |
| BMI (kg/m2) | 41 | 31.69 | (4.02) | 29.39 | (4.32) | 1.74 | 39 | 0.090 | 0.55 |
| Mental Health | |||||||||
| Depression | 41 | 17.45 | (9.37) | 6.3 | (5.16) | 4.85 | 39 | 0.000 | 1.53 |
| State Anxiety | 41 | 43.31 | (10.20) | 34.4 | (7.26) | 3.27 | 39 | 0.002 | 1.03 |
| Trait Anxiety | 41 | 47.58 | (8.57) | 35.5 | (6.20) | 5.24 | 39 | 0.000 | 1.65 |
| Health Locus of Control | 41 | 35.51 | (5.43) | 35.6 | (4.38) | −0.06 | 39 | 0.951 | −0.02 |
| Task-oriented Coping | 41 | 47.85 | (13.89) | 60.33 | (10.88) | −3.23 | 39 | 0.003 | −1.02 |
| Emotion-oriented Coping | 41 | 39.08 | (7.86) | 35.25 | (5.90) | 1.78 | 39 | 0.082 | 0.56 |
| Avoidance-oriented Coping | 41 | 39.91 | (5.27) | 43.17 | (7.83) | −1.51 | 39 | 0.138 | −0.48 |
| Difficulty Identifying Feelings | 41 | 16.11 | (5.40) | 12.56 | (1.89) | 2.95 | 39 | 0.005 | 0.93 |
| Difficulty Describing Feelings | 41 | 11.44 | (3.78) | 10.22 | (1.34) | 1.43 | 39 | 0.160 | 0.45 |
| Externally-oriented Thinking | 41 | 22.11 | (5.98) | 18.44 | (3.73) | 2.40 | 39 | 0.021 | 0.76 |
| Alexithymia | 41 | 49.66 | (13.34) | 41.22 | (5.48) | 2.76 | 39 | 0.009 | 0.87 |
| Cognitive Reappraisal | 23 | 5.06 | (1.37) | 5.06 | (1.45) | −0.01 | 21 | 0.993 | 0.00 |
| Suppression | 23 | 4.31 | (0.78) | 3.77 | (0.81) | 1.63 | 21 | 0.118 | 0.68 |
| Outcome | Treatment | Pre-Post Change | 95% Confidence Interval | t | p-Level (Two-Tailed) |
|---|---|---|---|---|---|
| hBa1C (%) | Integrated | −1.48 | [−2.03; −0.92] | −5.37 | 0.000 |
| Standard | −1.10 | [−1.68; −0.53] | −3.88 | 0.001 | |
| Blood glucose (mg/dL) | Integrated | −58.17 | [−86.31; −30.03] | −4.16 | 0.000 |
| Standard | −51.91 | [−81.91; −21.92] | −3.49 | 0.001 | |
| Total Cholesterol (mg/dL) | Integrated | −14.41 | [−25.38; −3.44] | −2.65 | 0.011 |
| Standard | −13.73 | [−25.42; −2.03] | −2.36 | 0.022 | |
| HDL Cholesterol (mg/dL) | Integrated | −16.13 | [−16.13; −6.25] | −3.29 | 0.002 |
| Standard | −10.55 | [−10.55; 0.16] | −1.98 | 0.053 | |
| LDL Cholesterol (mg/dL) | Integrated | 3.21 | [−3.62; −3.62] | 0.95 | 0.349 |
| Standard | −8.52 | [−15.92; −15.92] | −2.32 | 0.025 | |
| Triglycerides (mg/dL) | Integrated | −13.01 | [−28.94; 2.91] | −1.65 | 0.106 |
| Standard | 25.94 | [7.18; 44.71] | 2.79 | 0.008 | |
| Systolic Blood Pressure (mmHg) | Integrated | −3.22 | [−8.57; 2.12] | −1.21 | 0.231 |
| Standard | −1.48 | [−7.27; 4.30] | −0.52 | 0.608 | |
| Diastolic Blood Pressure (mmHg) | Integrated | −4.20 | [−7.50; −0.90] | −2.56 | 0.014 |
| Standard | −3.58 | [−7.10; −0.07] | −2.05 | 0.046 | |
| Cigarettes per day (number) | Integrated | −3.88 | [−7.50; −0.73] | −2.48 | 0.017 |
| Standard | −5.73 | [−7.10; −2.38] | −3.44 | 0.001 | |
| Wine per day (glasses) | Integrated | −0.83 | [−1.31; −0.34] | −3.46 | 0.001 |
| Standard | −0.13 | [−0.72; 0.46] | −0.46 | 0.650 | |
| Alcohol per day (glasses) | Integrated | 0.79 | [−1.07; 2.66] | 0.86 | 0.395 |
| Standard | −0.07 | [−2.51; 2.37] | −0.06 | 0.953 | |
| Walk per day (minutes) | Integrated | 31.51 | [18.68; 44.33] | 4.95 | 0.000 |
| Standard | 20.05 | [6.37; 33.72] | 2.95 | 0.005 | |
| Physical activity per week (minutes) | Integrated | 109.54 | [73.12; 145.96] | 6.06 | 0.000 |
| Standard | 77.63 | [38.71; 116.54] | 4.02 | 0.000 | |
| BMI (kg/m2) | Integrated | −0.47 | [−2.01; 1.07] | −0.61 | 0.543 |
| Standard | −0.54 | [−2.29; 1.21] | −0.63 | 0.535 | |
| Depression | Integrated | −3.00 | [−6.46; 0.46] | −1.76 | 0.087 |
| Standard | 3.31 | [−0.60; 7.22] | 1.71 | 0.095 | |
| State Anxiety | Integrated | −4.18 | [−9.27; 0.91] | −1.66 | 0.104 |
| Standard | −3.64 | [−9.39; 2.10] | −1.28 | 0.207 | |
| Trait Anxiety | Integrated | −5.33 | [−9.33; −1.32] | −2.69 | 0.010 |
| Standard | −1.44 | [−5.96; 3.09] | −0.64 | 0.525 | |
| Health Locus of Control | Integrated | 1.34 | [−1.53; 3.87] | 0.88 | 0.386 |
| Standard | 1.51 | [−5.23; 0.88] | −1.44 | 0.157 | |
| Task-oriented Coping | Integrated | 6.12 | [−0.82; 13.05] | 1.78 | 0.082 |
| Standard | −1.94 | [−9.78; 5.90] | −0.50 | 0.620 | |
| Emotion-oriented Coping | Integrated | −6.14 | [−10.33; −1.95] | −2.96 | 0.005 |
| Standard | −0.35 | [−5.09; 4.39] | −0.15 | 0.881 | |
| Avoidance-oriented Coping | Integrated | 0.21 | [−4.51; 4.93] | 0.09 | 0.929 |
| Standard | 1.20 | [−4.13; 6.53] | 0.45 | 0.652 | |
| Difficulty Identifying Feelings | Integrated | −4.14 | [−10.38; 2.10] | −1.34 | 0.187 |
| Standard | −1.76 | [−8.81; 5.29] | −0.51 | 0.616 | |
| Difficulty Describing Feelings | Integrated | −2.67 | [−5.36; 0.01] | −2.01 | 0.051 |
| Standard | −0.36 | [−3.39; 2.68] | −0.24 | 0.814 | |
| Externally-oriented Thinking | Integrated | −1.73 | [−3.74; 0.28] | −1.74 | 0.089 |
| Standard | −2.18 | [−4.45; 0.09] | −1.94 | 0.060 | |
| Alexithymia | Integrated | −1.73 | [−3.74; 0.28] | −1.74 | 0.089 |
| Standard | −2.18 | [−4.45; 0.09] | −1.94 | 0.060 | |
| Cognitive Reappraisal | Integrated | 0.20 | [−0.54; 0.94] | 0.56 | 0.579 |
| Standard | 0.43 | [−0.36; 1.22] | 1.13 | 0.270 | |
| Suppression | Integrated | 0.05 | [−0.68; 0.77] | 0.13 | 0.897 |
| Standard | 0.05 | [−0.73; 0.83] | 0.13 | 0.898 |
| Standard Treatment | Integrated Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | M | SD | t-Test | df | p | Cohen’s d | |
| Ophthalmic referrals needed | 41 | 0.94 | 0.24 | 0.83 | 0.39 | 1.14 | 39 | 0.261 | 0.36 |
| Cost of Ophthalmic examination | 41 | 60.44 | 15.08 | 52.87 | 24.8 | 1.14 | 39 | 0.261 | 0.36 |
| Cardiology referrals needed | 41 | 1.33 | 0.84 | 0.78 | 0.42 | 2.74 | 39 | 0.009 | 0.86 |
| Cost of Cardiology referrals | 41 | 85.33 | 53.77 | 50.09 | 26.99 | 2.74 | 39 | 0.009 | 0.86 |
| Diabetological referrals needed | 41 | 2.06 | 0.24 | 1.48 | 0.51 | 4.43 | 39 | <0.001 | 1.39 |
| Cost of Diabetological referrals | 41 | 131.56 | 15.08 | 94.61 | 32.69 | 4.43 | 39 | <0.001 | 1.39 |
| Total Blood tests performed | 50 | 2.63 | 0.99 | 2.1 | 0.57 | 2.34 | 48 | 0.024 | 0.66 |
| Total Diagnostic exams preformed | 50 | 4.35 | 0.85 | 3.19 | 0.79 | 4.97 | 48 | <0.001 | 1.41 |
| Visits to the general practitioner | 50 | 1.66 | 1.58 | 0.46 | 0.78 | 3.41 | 48 | 0.001 | 0.97 |
| Total Visits | 41 | 4.33 | 0.84 | 3.09 | 0.85 | 4.69 | 39 | <0.001 | 1.48 |
| Total Costs | 41 | 277.33 | 53.77 | 197.57 | 54.28 | 4.69 | 39 | <0.001 | 1.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lastretti, M.; Tomai, M.; Visalli, N.; Chiaramonte, F.; Tambelli, R.; Lauriola, M. An Integrated Medical-Psychological Approach in the Routine Care of Patients with Type 2 Diabetes: A Pilot Study to Explore the Clinical and Economic Sustainability of the Healthcare Intervention. Sustainability 2021, 13, 13182. https://doi.org/10.3390/su132313182
Lastretti M, Tomai M, Visalli N, Chiaramonte F, Tambelli R, Lauriola M. An Integrated Medical-Psychological Approach in the Routine Care of Patients with Type 2 Diabetes: A Pilot Study to Explore the Clinical and Economic Sustainability of the Healthcare Intervention. Sustainability. 2021; 13(23):13182. https://doi.org/10.3390/su132313182
Chicago/Turabian StyleLastretti, Mara, Manuela Tomai, Natalia Visalli, Francesco Chiaramonte, Renata Tambelli, and Marco Lauriola. 2021. "An Integrated Medical-Psychological Approach in the Routine Care of Patients with Type 2 Diabetes: A Pilot Study to Explore the Clinical and Economic Sustainability of the Healthcare Intervention" Sustainability 13, no. 23: 13182. https://doi.org/10.3390/su132313182
APA StyleLastretti, M., Tomai, M., Visalli, N., Chiaramonte, F., Tambelli, R., & Lauriola, M. (2021). An Integrated Medical-Psychological Approach in the Routine Care of Patients with Type 2 Diabetes: A Pilot Study to Explore the Clinical and Economic Sustainability of the Healthcare Intervention. Sustainability, 13(23), 13182. https://doi.org/10.3390/su132313182






