Physicochemical Studies for Risk Identification, Assessment, and Characterization of Artisanal Barite Mining in Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Chemical Digestion, and Analytical Methods
2.2. Site Description
2.3. Characterization of Mine Water Sample
2.3.1. Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) Analysis of Water Samples
2.3.2. Heavy Metal Toxic Unit (TU) of Mine Water
2.4. Quantitative Risk Analysis and Calculation
2.4.1. Contamination Assessment
Geo-Accumulation Index (Igeo)
Contamination Factor (CF)
2.4.2. Health Risk Assessment and Chronic Daily Intake (CDI)
2.4.3. Exposure Assessment
2.5. Risk Characterization
2.5.1. Hazard Quotient (HQ)
2.5.2. Maximally Exposed Individual (MEI)
3. Results
3.1. Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) Analysis
3.2. Heavy Metal Toxic Unit (TU) Results
3.3. Hazard/Risk Assessment of Water Contaminated by Tailing Effluents and Barite Ponds
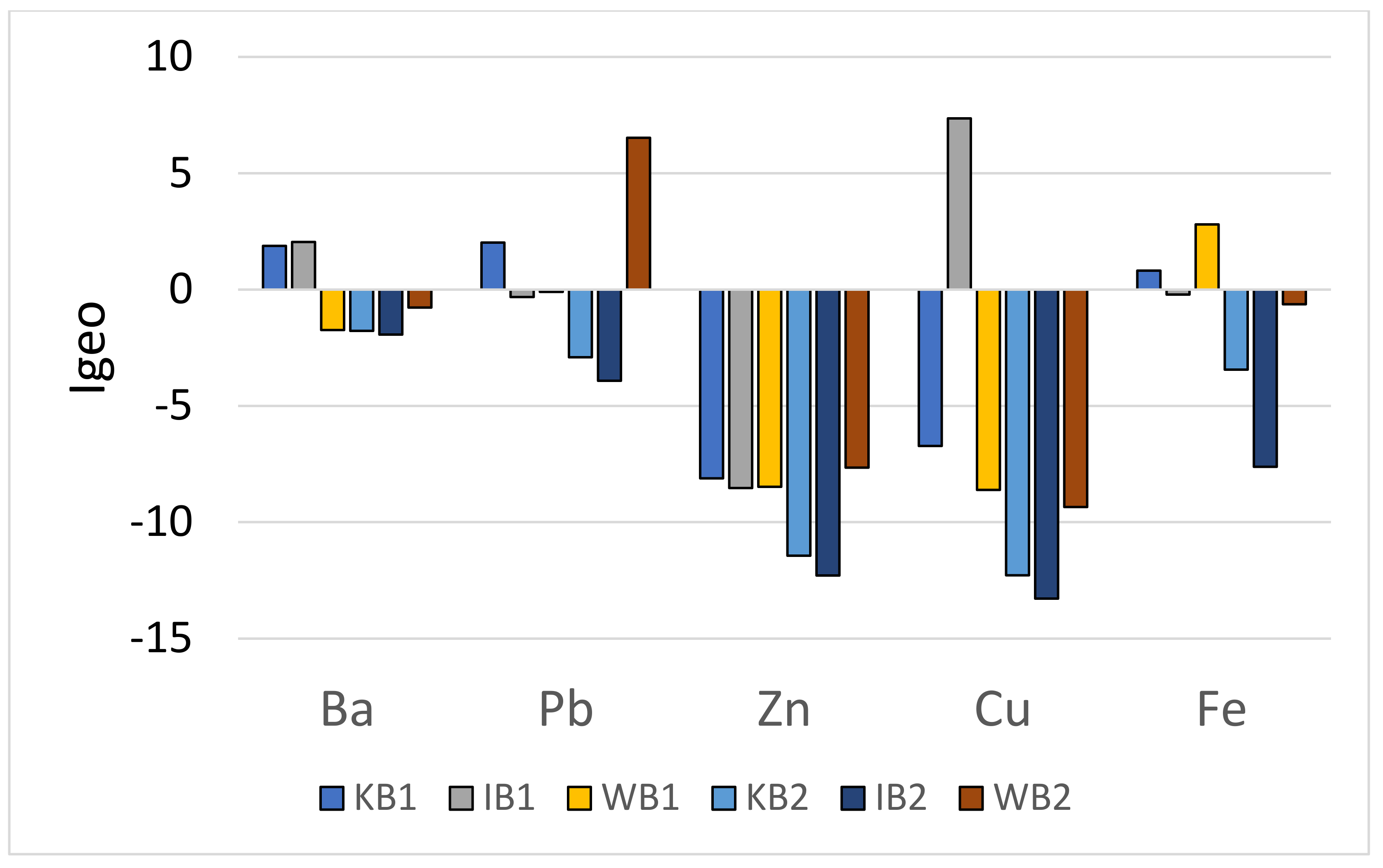
3.3.1. Geo-accumulation Index (Igeo)
3.3.2. Contamination Factor (CF)
3.4. Health Risk Assessment
3.4.1. Chronic Daily Intake (CDI)
3.4.2. Maximally Exposed Individual (MEI)
3.5. Hazard Exposure Assessment
3.6. Hazard Characterization
Hazard Quotient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Elements | References | |||
|---|---|---|---|---|
| Pb | 0.0004 | 0.0014 | 0.00042 | [46] |
| Ba | 0.003 | 0.07 | 0.000062 | [46,49] |
| Fe | 0.001 | 0.7 | 0.14 | [46] |
| Cd | 0.001 | 0.0005 | 0.000025 | [23,46] |
| Cu | 0.001 | 0.04 | 0.008 | [46] |
| Zn | 0.0006 | 0.03 | 0.06 | [46,72] |
| Elements | Inhalation RfD | Oral CSF | Dermal CSF | Inhalation CSF | References |
|---|---|---|---|---|---|
| Pb | NA | 0.0085 | NA | 420 | [47,49,72] |
| Ba | 0.0076 | ID | ID | ID | [23,46] |
| Fe | NA | NA | NA | NA | [46,47] |
| Cd | 0.000057 | NA | NA | 6.3 | [23,49] |
| Cu | NA | NA | NA | NA | [23,49] |
| Zn | NA | NA | NA | NA | [49,72] |
| Parameters | Unit | Child | Adult/Resident | Worker | References |
|---|---|---|---|---|---|
| Body weight (BW) | kg | 15 | 70 | 70 | [23,49] |
| Contact rate (CR) | L/day | 1.0 | 2.0 | 1.0 | [77] |
| Exposure factor (EF) | days/year | 350 | 350 | 250 | [49] |
| Exposure duration (ED) | years | 6 | 30 | 25 | [43,49] |
| Exposure time (ET) | days | 2190 | 10950 | [44,49] | |
| Exposure frequency (ER) | Days/year | 365 | 365 | 365 | [23,49] |
| Ingestion rate (IR or IR) | mg/day | 200 | 100 | [49] | |
| Inhalation rate (IRih) | m3/day | 10 | 20 | [49] | |
| Skin surface area (SA/EA) | cm2 | 2100 | 5800 | [49] | |
| Soil adherence factor (AF) | mg/cm2 | 0.2 | 0.07 | [43,49] | |
| Dermal adsorption factor (ABS) | none | 0.1 | 0.1 | [23,49] | |
| Dermal exposure (FE) | none | 0.61 | 0.61 | [49] | |
| Particulate emission factor | m3/mg | 1.3 × 109 | 1.3 × 109 | [49] | |
| (PEF) | |||||
| Conversion factor (CF) | kg/mg | 10−6 | 10−6 | [23,49] | |
| Average time (AT) | |||||
| For carcinogens | days | 365 × 70 | 365 × 70 | [49,77] | |
| For non-carcinogens | 365 × ED | 365 × ED | [49,77] | ||
| Samples/Standards | Zn (mg/L) | Cu (mg/L) | Fe (mg/L) | Ba (mg/L) | Pb (mg/L) |
|---|---|---|---|---|---|
| KB1 | 0.027 | 0.0185 | 0.7895 | 11.055 | 0.0305 |
| IB1 | 0.0205 | 0.012 | 0.389 | 12.4035 | 0.006 |
| WB1 | 0.0215 | 0.005 | 3.1245 | 0.8915 | 0.007 |
| KB2 | 0.00275 | 0.0004 | 0.0415 | 0.659 | 0.001 |
| IB2 | 0.0015 | 0.0002 | 0.0023 | 0.8132 | 0.0005 |
| WB2 | 0.0373 | 0.003 | 0.2829 | 1.7500 | 0.6897 |
| WHO | 3.000 | 2.000 | 0.300 | 4.000 | 0.010 |
| EU | 3.000 | 2.000 | 0.300 | 0.700 | 0.010 |
| NIS | 3.000 | 2.000 | 0.300 | 0.700 | 0.010 |
| US EPA | 5.000 | 1.300–3.000 | 0.300 | 2.000–4.000 | 0.005 |
| CMHNS | NA | NA | 0.050 | NA | 0.010–1.000 |
| Elements Examined | Limit of Detection (LOD) (ppm) | Limit of Quantification (LOQ) (ppm) |
|---|---|---|
| Ba | 0.000693 | 0.29907 |
| Cu | 0.000693 | 0.30207 |
| Fe | 0.0001287 | 0.29312 |
| Pb | 0.000429 | 0.29804 |
| Zn | 0.000858 | 0.29508 |
References
- Amponsah-Tawiah, K.; Ntow, M.A.O.; Mensah, J. Occupational Health and Safety Management and Turnover Intention in the Ghanaian Mining Sector. Saf. Health Work 2016, 7, 12–17. [Google Scholar] [CrossRef]
- Bansah, K.J.; Yalley, A.B.; Dumakor-Dupey, N. The hazardous nature of small scale underground mining in Ghana. J. Sustain. Min. 2016, 15, 8–25. [Google Scholar] [CrossRef]
- Khanzode, V.V.; Maiti, J.; Ray, P.K. A methodology for evaluation and monitoring of recurring hazards in underground coal mining. Saf. Sci. 2011, 49, 1172–1179. [Google Scholar] [CrossRef]
- Afolayan, D.O.; Onwualu, A.P.; Eggleston, C.M.; Adetunji, A.R.; Tao, M.; Amankwah, R.K. Safe Mining Assessment of Artisanal Barite Mining Activities in Nigeria. Mining 2021, 1, 15. [Google Scholar] [CrossRef]
- Afolayan, D.O.; Adetunji, A.R.; Peter, A.; Oghenerume, O.; Amankwah, R.K. Characterization of barite reserves in Nigeria for use as weighting agent in drilling fluid. J. Pet. Explor. Prod. 2021, 11, 2157–2178. [Google Scholar] [CrossRef]
- Agwu, M.O.; Olele, H.E. Fatalities in the Nigerian construction industry: A case of poor safety culture. J. Econ. Manag. Trade 2014, 431–452. [Google Scholar] [CrossRef]
- Stemn, E. Analysis of injuries in the Ghanaian mining industry and priority areas for research. Saf. Health Work 2019, 10, 151–165. [Google Scholar] [CrossRef]
- Fayemi, H.E.K. Nigeria’ s Solid Minerals Sector: Alternative Investment Opportunities. Chatham House R. Inst. Int. Aff. 2016, 44, 1–9. [Google Scholar]
- Gottesfeld, P.; Tirima, S.; Anka, S.M.; Fotso, A.; Nota, M.M. Reducing lead and silica dust exposures in small-scale mining in northern Nigeria. Ann. Work Expo. Health 2019, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gottesfeld, P.; Andrew, D.; Dalhoff, J. Silica exposures in artisanal small-scale gold mining in Tanzania and implications for tuberculosis prevention. J. Occup. Environ. Hyg. 2015, 12, 647–653. [Google Scholar] [CrossRef]
- Adewumi, A.J.P.; Laniyan, T.A. Ecological and human health risks associated with metals in water from Anka Artisanal Gold Mining Area, Nigeria. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 307–326. [Google Scholar] [CrossRef]
- Oden, M.I. Barite veins in the Benue Trough: Field characteristics, the quality issue and some tectonic implications. Environ. Nat. Resour. Res. 2012, 2, 21. [Google Scholar] [CrossRef]
- Boamponsem, L.K.; Adam, J.I.; Dampare, S.B.; Owusu-Ansah, E.; Addae, G. Heavy metals level in streams of Tarkwa gold mining area of Ghana. J. Chem. Pharm. Res 2010, 2, 504–527. [Google Scholar]
- Wang, C.; Liu, S.; Zhao, Q.; Deng, L.; Dong, S. Spatial variation and contamination assessment of heavy metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicol. Environ. Saf. 2012, 82, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hadzi, G.Y.; Ayoko, G.A.; Essumang, D.K.; Osae, S.K.D. Contamination impact and human health risk assessment of heavy metals in surface soils from selected major mining areas in Ghana. Environ. Geochem. Health 2019, 41, 2821–2843. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Laniyan, T.A. Contamination, sources and risk assessments of metals in media from Anka artisanal gold mining area, Northwest Nigeria. Sci. Total Environ. 2020, 718, 137235. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.F.; Akhter, M.; Mazumder, B.; Ferdous, A.; Hossain, M.D.; Dafader, N.C.; Ahmed, F.T.; Kundu, S.K.; Taheri, T.; Atique Ullah, A.K.M. Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk. J. Anal. Sci. Technol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Oluwatuyi, O.E.; Ajibade, F.O.; Ajibade, T.F.; Adelodun, B.; Olowoselu, A.S.; Adewumi, J.R.; Akinbile, C.O. Total concentration, contamination status and distribution of elements in a Nigerian State dumpsites soil. Environ. Sustain. Indic. 2020, 5, 100021. [Google Scholar] [CrossRef]
- Olaifa, F.E.; Olaifa, A.K.; Adelaja, A.A.; Owolabi, A.G. Heavy metal contamination of Clarias gariepinus from a lake and fish farm in Ibadan, Nigeria. Afr. J. Biomed. Res. 2004, 7, 145–148. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Anifowose, A.Y.; Olabode, F.O.; Laniyan, T.A. Hydrogeochemical characterization and vulnerability assessment of shallow groundwater in Basement Complex Area, Southwest Nigeria. Contemp. Trends Geosci. 2018, 7, 72–103. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, D.; Wang, Y. Characteristics of heavy metals in size-fractionated atmospheric particulate matters and associated health risk assessment based on the respiratory deposition. Environ. Geochem. Health 2021, 43, 285–299. [Google Scholar] [CrossRef]
- Dudek, M.; Tajduś, K.; Misa, R.; Sroka, A. Predicting of land surface uplift caused by the flooding of underground coal mines–A case study. Int. J. Rock Mech. Min. Sci. 2020, 132, 104377. [Google Scholar] [CrossRef]
- Gholami, A.; Tajik, R.; Atif, K.; Zarei, A.A.; Abbaspour, S.; Teimori-Boghsani, G.; Attar, M. Respiratory symptoms and diminished lung functions associated with occupational dust exposure among iron ore mine workers in iran. Open Respir. Med. J. 2020, 14, 1. [Google Scholar] [CrossRef]
- Laniyan, T.A.; Adewumi, A.J.P. Potential ecological and health risks of toxic metals associated with artisanal mining contamination in Ijero, southwest Nigeria. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2020, 55, 858–877. [Google Scholar] [CrossRef]
- Macdonald, K.; Lund, M.; Blanchette, M.; Mccullough, C. Regulation of artisanal small scale gold mining (ASGM) in Ghana and Indonesia as currently implemented fails to adequately protect aquatic ecosystems. In Proceedings of the International Mine Water Association (IMWA) Congress, Xuzhou, China, 8–22 August 2014; pp. 401–405. [Google Scholar]
- Njinga, R.L.; Tshivhase, V.M. Major chemical carcinogens in drinking water sources: Health implications due to illegal gold mining activities in Zamfara State-Nigeria. Expo. Health 2019, 11, 47–57. [Google Scholar] [CrossRef]
- Ochelebe, I.; Nkebem, G.E.; Kudamnya, E.A. Assessment of Heavy Metals Concentration and Enrichment Levels in Soils around Quarries and Barite Mine Sites in Part of Akamkpa and Biase Area, Southeastern Nigeria. J. Geosci. Environ. Prot. 2020, 8, 107. [Google Scholar] [CrossRef]
- Ajiboye, Y.; Isinkaye, M.O.; Khanderkar, M.U. Spatial distribution mapping and radiological hazard assessment of groundwater and soil gas radon in Ekiti State, Southwest Nigeria. Environ. Earth Sci. 2018, 77, 545. [Google Scholar] [CrossRef]
- Ajiboye, Y.; Badmus, O.G.; Ojo, O.D.; Isinkaye, M.O. Measurement of Radon Concentration and Radioactivity in Soil Samples of Aramoko, Ekiti State, Nigeria. Int. J. Public Health Res. 2016, 4, 37–41. [Google Scholar]
- Aluko, T.; Njoku, K.; Adesuyi, A.; Akinola, M. Health risk assessment of heavy metals in soil from the iron mines of Itakpe and Agbaja, Kogi State, Nigeria. Pollution 2018, 4, 527–538. [Google Scholar]
- Anka, S.A.; Bello, T.S.; Waziri, A.F.; Muhammad, A.S.; Bello, I.; Nasiru, A.M. Environmental effect of lead combination of mining communities in Zamfara State, Nigeria: A review. J. Biol. Today’s World 2020, 9, 1–3. [Google Scholar]
- Donoghue, A.M. Occupational health hazards in mining: An overview. Occup. Med. 2004, 54, 283–289. [Google Scholar] [CrossRef]
- Dike, C.G.; Oladele, B.O.; Olubi, O.E.; Ife-Adediran, O.O.; Aderibigbe, A. Ecological and radiological hazards due to natural radioactivity and heavy metals in soils of some selected mining sites in Nigeria. Hum. Ecol. Risk Assess. Int. J. 2019, 26, 1428–1438. [Google Scholar] [CrossRef]
- Adewumi, A.J.P.; Laniyan, T.A.; Xiao, T.; Liu, Y.; Ning, Z. Exposure of children to heavy metals from artisanal gold mining in Nigeria: Evidences from bio-monitoring of hairs and nails. Acta Geochim. 2020, 39, 451–470. [Google Scholar] [CrossRef]
- Daburum, N.H.; Songden, S.D.; Mangset, E.W. Assessment of Radiation Dose with Excess Life Cancer Risk of Mining Dumpsites of Wase, Plateau State, Nigeria. Afr. J. Med. Phy. Biomed. Eng. Sci. 2019, 6, 47–54. [Google Scholar]
- Laniyan, T.A.; Adewumi, A.J. Evaluation of contamination and ecological risk of heavy metals associated with cement production in Ewekoro, Southwest Nigeria. J. Health Pollut. 2020, 10, 200306. [Google Scholar] [CrossRef]
- Adamu, C.I.; Nganje, T.; Edet, A. Hydrochemical assessment of pond and stream water near abandoned barite mine sites in parts of Oban massif and Mamfe Embayment, Southeastern Nigeria. Environ. Earth Sci. 2014, 71, 3793–3811. [Google Scholar] [CrossRef]
- Adamu, C.I.; Nganje, T.N.; Edet, A. Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ. Nanotechnol. Monit. Manag. 2015, 3, 10–21. [Google Scholar] [CrossRef]
- Ebunu, A.I.; Olanrewaju, Y.A.; Ogolo, O.; Adetunji, A.R.; Onwualu, A.P. Barite as an Industrial Mineral in Nigeria: Occurrence, Utilization, Challenges and Future Prospects. Heliyon 2021, 7, e07365. [Google Scholar] [CrossRef] [PubMed]
- Brenniman, G.R.; Namekata, T.; Kojola, W.H.; Carnow, B.W.; Levy, P.S. Cardiovascular disease death rates in communities with elevated levels of barium in drinking water. Environ. Res. 1979, 20, 318–324. [Google Scholar] [CrossRef]
- Drochioiu, G.; Surleva, A.; Ilieva, D.; Tudorachi, L.; Necula, R. Heavy metal toxicity around a closed barite mine in tarnita-romania. Int. Multidiscip. Sci. GeoConference SGEM 2016, 2, 525–532. [Google Scholar]
- Szeleg, E.; Janeczek, J.; Metelski, P. Native selenium as a byproduct of microbial oxidation of distorted pyrite crystals: The first occurrence in the Carpathians. Geol. Carpathica 2013, 64, 231. [Google Scholar] [CrossRef]
- Brenniman, G.R.; Levy, P.S. Epidemiological study of barium in Illinois drinking water supplies. Adv. Mod. Toxicol. 1984, 9, 231–249. [Google Scholar]
- WHO. Barium in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Melekestseva, I.Y.; Tret’yakov, G.A.; Nimis, P.; Yuminov, A.M.; Maslennikov, V.V.; Maslennikova, S.P.; Kotlyarov, V.A.; Beltenev, V.E.; Danyushevsky, L.V.; Large, R. Barite-rich massive sulfides from the Semenov-1 hydrothermal field (Mid-Atlantic Ridge, 13 30.87′ N): Evidence for phase separation and magmatic input. Mar. Geol. 2014, 349, 37–54. [Google Scholar] [CrossRef]
- NIS-554-2015 Nigerian Standard for Drinking Water Quality Technical Committee for Standard for Drinking Water Quality; ICS 13.060; Standards Organisation of Nigeria: Lagos, Nigeria, 2015.
- US EPA. Guidelines for Water Reuse, US Environmental Protection Agency; EPA/625/R-04/108; US EPA: Washington, DC, USA, 2004. [Google Scholar]
- US EPA. Fact Sheet PFOA & PFOS Drinking Water Health Advisories; EPA 800-F-16-003; US EPA: Washington, DC, USA, 2016. [Google Scholar]
- United States Environmental Protection Agency. Provisional Peer Reviewed Toxicity Values for Iron and Compounds; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- WHO. Barium and barium compounds. In Barium and Barium Compounds; WHO: Geneva, Switzerland, 2001; p. 52. [Google Scholar]
- Labe, N.A.; Ogunleye, P.O.; Ibrahim, A.A. Field occurrence and geochemical characteristics of the baryte mineralization in Lessel and Ihugh areas, Lower Benue Trough, Nigeria. J. Afr. Earth Sci. 2018, 142, 207–217. [Google Scholar] [CrossRef]
- Aladesanmi, A.O.; Ogundana, A.K.; Olowookere, A.A.; Jenakumo, L. Geological Characterization of Azara Barite Mineralization, Middle Benue Trough Nigeria. J. Environ. Earth. Sci. 2018, 8, 44–46. [Google Scholar]
- Department of Environmental Affairs. GHG Inventory for South Africa (2000–2010). S. Afr. Gov. 2014, 46, 114–116. [Google Scholar]
- Offodile, M.E. A Review of the Geology of the Cretaceous of the Benue Valley; Elizabethan Publ.: Lagos, Nigeria, 1976. [Google Scholar]
- Salawu, N.B.; Orosun, M.M.; Adebiyi, L.S.; Abdulraheem, T.Y.; Dada, S.S. Existence of subsurface structures from aeromagnetic data interpretation of the crustal architecture around Ibi, Middle Benue, Nigeria. SN Appl. Sci. 2020, 2, 427. [Google Scholar] [CrossRef]
- Gabriel, A.T.; Yusuf, M.B.; Bwadi, B.E.; Clement, Y.G. Morphometric Analysis and Flash Floods Assessment of River Taraba Basin in Taraba State, Nigeria. Eur. Sci. J. ESJ 2020, 16, 158–163. [Google Scholar] [CrossRef]
- Ankidawa, A.A.; Ishaku, J.M.; Ahmadu, S.P. Hydrogeological and engineering investigations of gully sites in Zing and environs, Northeastern Nigeria. Arid Zone J. Eng. Technol. Environ. 2020, 16, 337–350. [Google Scholar]
- Diloha, I.I.; Udom, G.J.; Nwankwoala, H.O. Application of Aquifer Parameters in Evaluating Groundwater Potential of Logo Area, Benue State, Nigeria. Int. J. Environ. Sci. Nat. Resour. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Gyang, J.D.; Ashano, E.C. Effects of Mining on Water Quality and the Environment: A Case Study of Parts of the Jos Plateau, North Central Nigeria. Pac. J. Sci. Technol. 2010, 11, 631–639. [Google Scholar]
- Akujieze, C.N.; Coker, S.J.L.; Oteze, G.E. Groundwater in Nigeria—A millennium experience—Distribution, practice, problems and solutions. Hydrogeol. J. 2003, 11, 259–274. [Google Scholar] [CrossRef]
- JohnPaul, A.A.; Ayodeji, L.T.; Tangfu, X.; Ning, Z.; Liu, Y. Toxicity, uptake, potential ecological and health risks of Thallium (Tl) in environmental media around selected artisanal mining sites in Nigeria. Int. J. Environ. Anal. Chem. 2020, 1–22. [Google Scholar] [CrossRef]
- Vandi, K.D.; Ntekim, E.E.; Ahmed, H.A. Petrochemistry and manganese mineralization potentials of the calc-alkaline granitolds from Northeastern Hawal Massif_North-Eastern Ngeria. Proc. Sci. Forum J. Pure Appl. Sci. 2019, 16, 18–42. [Google Scholar]
- Ogundipe, I.E. Thermal and chemical variations of the Nigerian Benue trough lead-zinc-barite-fluorite deposits. J. Afr. Earth Sci. 2017, 132, 72–79. [Google Scholar] [CrossRef]
- Ekwueme, B.N.; Akpeke, G.B.; Ephraim, B.E. The chemcial composition and industrial quality of barite mineralization in Calabar Flank, Oban Massif, Mamfe Enbayment and Obudu Plateau, SouthEastern Nigeria. Glob. J. Geol. Sci. 2015, 13, 53–66. [Google Scholar] [CrossRef][Green Version]
- Ekwueme, B.N.; Akpeke, G.B. Occurrence and distribution of barite mineralization in Cross River State, south-eastern Nigeria. Glob. J. Geol. Sci. 2012, 10, 85–98. [Google Scholar]
- EL-Nafaty, J.M.; Garba, I.; Baba, S. Geochemical Investigation of the Barite-Copper Mineralization in Gulani Area, Upper Benue Trough, Northeastern Nigeria. J. Min. Geol. 2015, 51, 179–199. [Google Scholar]
- Aliyu, A.S.; Ibrahim, U.; Akpa, C.T.; Garba, N.N.; Ramli, A.T. Health and ecological hazards due to natural radioactivity in soil from mining areas of Nasarawa State, Nigeria. Isotopes Environ. Health Stud. 2015, 51, 448–468. [Google Scholar] [CrossRef] [PubMed]
- Obaje, N.G.; Wehner, H.; Scheeder, G.; Abubakar, M.B.; Jauro, A. Hydrocarbon prospectivity of Nigeria’s inland basins: From the viewpoint of organic geochemistry and organic petrology. Am. Assoc. Pet. Geol. Bull. 2004, 88, 325–353. [Google Scholar] [CrossRef]
- Obaje, N.G.; Attah, D.O.; Opeloye, S.A.; Moumouni, A. Geochemical evaluation of the hydrocarbon prospects of sedimentary basins in Northern Nigeria. Geochem. J. 2006, 40, 227–243. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Cui, B.; Zhang, K.; Wang, Q.; Liu, X.; Gao, H.; Huang, L. Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environ. Pollut. 2011, 159, 817–824. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Quick Guide to Drinking Water Sample Collection, 2nd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2016; pp. 6–20.
- International Organization for Standardization. Activities Relating to Drinking Water and Wastewater Services: Guidelines for the Management of Drinking Water Utilities and for the Assessment of Drinking Water Services; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario; Queen’s Printer Ontario: Toronto, ON, Canada, 1993; pp. 20–23. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Muhammad, S.; Tahir Shah, M.; Khan, S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 2011, 98, 334–343. [Google Scholar] [CrossRef]
- Shah, I.; Khan, T.; Hanif, M.; Shah, A.; Siddiqui, S.; Khattak, S.A. Environmental aspects of selected heavy and trace elements of Cherat Coal deposits. J. Himal. Earth Sci. 2016, 49, 77–85. [Google Scholar]
- Nazaroff, W.W.; Alvarez-Cohen, L. Environmental Engineering Science; John Willey & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- United States Environmental Protection Agency. Alternative Disinfectants and Oxidants Guidance Manual; USEPA: Washington, DC, USA, 1999.
- Rapant, S.; Fajčíková, K.; Khun, M.; Cvečková, V. Application of health risk assessment method for geological environment at national and regional scales. Environ. Earth Sci. 2011, 64, 513–521. [Google Scholar] [CrossRef]
- Nazaroff; Alvarez-Cohen. Risk Assessment. Risk Manag. 2001, 24, 396–397, 568–573. [Google Scholar]
- United States Environmental Protection Agency. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A); EPA/540/1-89/002; U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- The Risk Assessment Information System. Available online: https://rais.ornl.gov/tools/rais_chemical_risk_guide.html (accessed on 8 May 2021).
- DNR Arsenic in Drinking Water. 2017. Available online: https://dnr.wi.gov/files/pdf/pubs/dg/dg0062.pdf (accessed on 15 September 2021).
- Otoijamun, I.; Kigozi, M.; Abdulraman, S.O.; Adetunji, A.R.; Onwualu, A.P. Fostering the Sustainability of Artisanal and Small-Scale Mining (ASM) of Barite in Nasarawa State, Nigeria. Sustainability 2021, 13, 5917. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Mitchener, M. Life-term studies in rats: Effects of aluminum, barium, beryllium, and tungsten. J. Nutr. 1975, 105, 421–427. [Google Scholar] [CrossRef]
- Zdanowicz, J.A.; Featherstone, J.D.B.; Espeland, M.A.; Curzon, M.E.J. Inhibitory effect of barium on human caries prevalence. Community Dent. Oral Epidemiol. 1987, 15, 6–9. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yavuz, E.; Demir, S.; Patat, Ş. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Çiçek, B.; İnanç, N.; Zararsız, G.; Öztürk, A. Multivariate statistical analysis of data and ICP-MS determination of heavy metals in different brands of spices consumed in Kayseri, Turkey. Food Anal. Methods 2018, 11, 2407–2418. [Google Scholar] [CrossRef]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Ishaya, S. Dry Season Water Quality Assessment and Its Health Implications in Ankpa Town, Kogi State, Nigeria. Environ. Rev. 2019, 7, 1. [Google Scholar]
- Fry, K.L.; Wheeler, C.A.; Gillings, M.M.; Flegal, A.R.; Taylor, M.P. Anthropogenic contamination of residential environments from smelter As, Cu and Pb emissions: Implications for human health. Environ. Pollut. 2020, 262, 114235. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Sahakyan, L.; Maghakyan, N.; Saghatelyan, A. Combination of compositional data analysis and machine learning approaches to identify sources and geochemical associations of potentially toxic elements in soil and assess the associated human health risk in a mining city. Environ. Pollut. 2020, 261, 114210. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ríos, M.D.L.; Juárez-Pérez, C.A.; Rendón-Gandarilla, F.J.; Talavera-Mendoza, O.; Aguilar-Madrid, G. Elevated blood Lead levels in children associated with living near mining waste sites in Guerrero/Mexico. Environments 2017, 4, 41. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochem. Source Antioxid. Role Dis. Prev. BoD Books Demand 2018, 7, 49–74. [Google Scholar]
- EPA. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2; United States Environmental Protection Agency: Washington, DC, USA, 2016; pp. 1–6.
- Moosavi, S.M.; Ghassabian, S. Linearity of calibration curves for analytical methods: A review of criteria for assessment of method reliability. In Calibration and Validation of Analytical Methods: A Sampling of Current Approaches; IntechOpen Ltd.: London, UK, 2018; pp. 109–127. [Google Scholar]
- CORESTA. Technical Guide for Setting Method LOD and LOQ Values for the Determination of Metals in E-Liquid and E-Vapour Aerosol by ICP-MS; Cooperation Centre for Scientific Research Relative to Tobacco: Paris, France, 2020; pp. 1–9. [Google Scholar]




| Heavy Metals | Severe-Effect Level (SEL) | TU (mg/L) | Standards |
|---|---|---|---|
| Zn | 820 | 3.0–5.0 | EU, NIS, US EPA [45,62,70] |
| Cd | 10 | 0.001–0.005 | EU, NIS, US EPA [53,64,65,66,69] |
| Cu | 110 | 1.3–3.0 | EU, NIS, US EPA [45,71] |
| Ba | Not yet reported | 0.7–4.0 | EU, NIS, US EPA [46] |
| Fe | 4 | 0.3 | EU, NIS, US EPA [46,62,71] |
| Pb | 250 | 0.005–0.01 | EU, NIS, US EPA [62,71,72] |
| Heavy Metals | Maximum Metallic Conc in Media (ppm) | SEL | Toxic Unit (TU) | % TU |
|---|---|---|---|---|
| Zinc | 0.0805 | 820 | 0.0000975 | 0.010 |
| Copper | 0.0185 | 110 | 0.000168 | 0.017 |
| Iron | 3.1245 | 4 | 0.78135 | 79.750 |
| Lead | 1.516 | 250 | 0.0061 | 0.62 |
| Cadmium | 0.000 | 10 | 0.000 | 0.00 |
| Barium | 12.4035 | 64.6 | 0.192 | 19.6 |
| Elements | Ba | Pb | Zn | Cu | Fe |
|---|---|---|---|---|---|
| (CF) (dimensionless) | |||||
| KB1 | 5.528 | 6.100 | 5.4 × 10−3 | 1.4 × 10−2 | 2.630 |
| IB1 | 6.202 | 1.200 | 4.1 × 10−3 | 3.8 × 10−3 | 1.297 |
| WB1 | 0.446 | 1.400 | 4.3 × 10−3 | 3.0 × 10−4 | 10.415 |
| KB2 | 0.330 | 0.200 | 5.5 × 10−4 | 7.7 × 10−4 | 0.138 |
| IB2 | 0.407 | 0.100 | 3.0 × 10−4 | ------- | 0.008 |
| WB2 | 0.875 | 137.940 | 7.46 × 10−3 | 2.3 × 10−3 | 0.943 |
| Samples | Ba | Pb | Zn | Cu | Fe |
|---|---|---|---|---|---|
| (CDI) Adult (mg kg−1 d−1) | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| (CDI) Child (mg kg−1 d−1) | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| (MEI) Resident (mg kg−1 d−1) | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| (MEI) Worker (mg kg−1 d−1) | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| Elements | Ba | Pb | Zn | Cu | Fe |
|---|---|---|---|---|---|
| EXPing (mg kg−1 d−1) Adult | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| EXPing (mg L−1 kg−1 d−1) Child | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| EXPderm (mg kg−1 d−1) Adult | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| EXPderm (mg kg−1 d−1) Child | |||||
| KB1 | |||||
| IB1 | |||||
| WB1 | |||||
| KB2 | |||||
| IB2 | |||||
| WB2 | |||||
| Samples | Hazard Quotient (HQ) | Hazard Index | ||||
|---|---|---|---|---|---|---|
| Ba | Pb | Zn | Cu | Fe | (HI) | |
| (HQ) Adult | ||||||
| KB1 | ||||||
| IB1 | ||||||
| WB1 | ||||||
| KB2 | ||||||
| IB2 | ||||||
| WB2 | ||||||
| (HQ) Child | ||||||
| KB1 | ||||||
| IB1 | ||||||
| WB1 | ||||||
| KB2 | ||||||
| IB2 | ||||||
| WB2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolayan, D.O.; Eggleston, C.M.; Onwualu, A.P.; Adetunji, A.R.; Tao, M.; Amankwah, R.K. Physicochemical Studies for Risk Identification, Assessment, and Characterization of Artisanal Barite Mining in Nigeria. Sustainability 2021, 13, 12982. https://doi.org/10.3390/su132312982
Afolayan DO, Eggleston CM, Onwualu AP, Adetunji AR, Tao M, Amankwah RK. Physicochemical Studies for Risk Identification, Assessment, and Characterization of Artisanal Barite Mining in Nigeria. Sustainability. 2021; 13(23):12982. https://doi.org/10.3390/su132312982
Chicago/Turabian StyleAfolayan, David Oluwasegun, Carrick McAfee Eggleston, Azikiwe Peter Onwualu, Adelana Rasak Adetunji, Mingjiang Tao, and Richard Kwasi Amankwah. 2021. "Physicochemical Studies for Risk Identification, Assessment, and Characterization of Artisanal Barite Mining in Nigeria" Sustainability 13, no. 23: 12982. https://doi.org/10.3390/su132312982
APA StyleAfolayan, D. O., Eggleston, C. M., Onwualu, A. P., Adetunji, A. R., Tao, M., & Amankwah, R. K. (2021). Physicochemical Studies for Risk Identification, Assessment, and Characterization of Artisanal Barite Mining in Nigeria. Sustainability, 13(23), 12982. https://doi.org/10.3390/su132312982






