Product Characteristics of Sludge Pyrolysis and Adsorption Performance of Metals by Char
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sludge Microwave Pyrolysis
2.3. Sludge-Derived Adsorbent
2.4. Elemental Constituents
2.5. Organic Constituents of Liquid Oil
2.6. Pore Characteristics of Sludge-Derived Adsorbents
2.7. Metal Adsorption
2.8. Data Analysis
3. Results and Discussion
3.1. Sludge Pyrolysis and Mass Distribution
3.2. Elemental and Organic Constituents of Liquid Oil
3.3. Potential Use for Pyrolysis Char
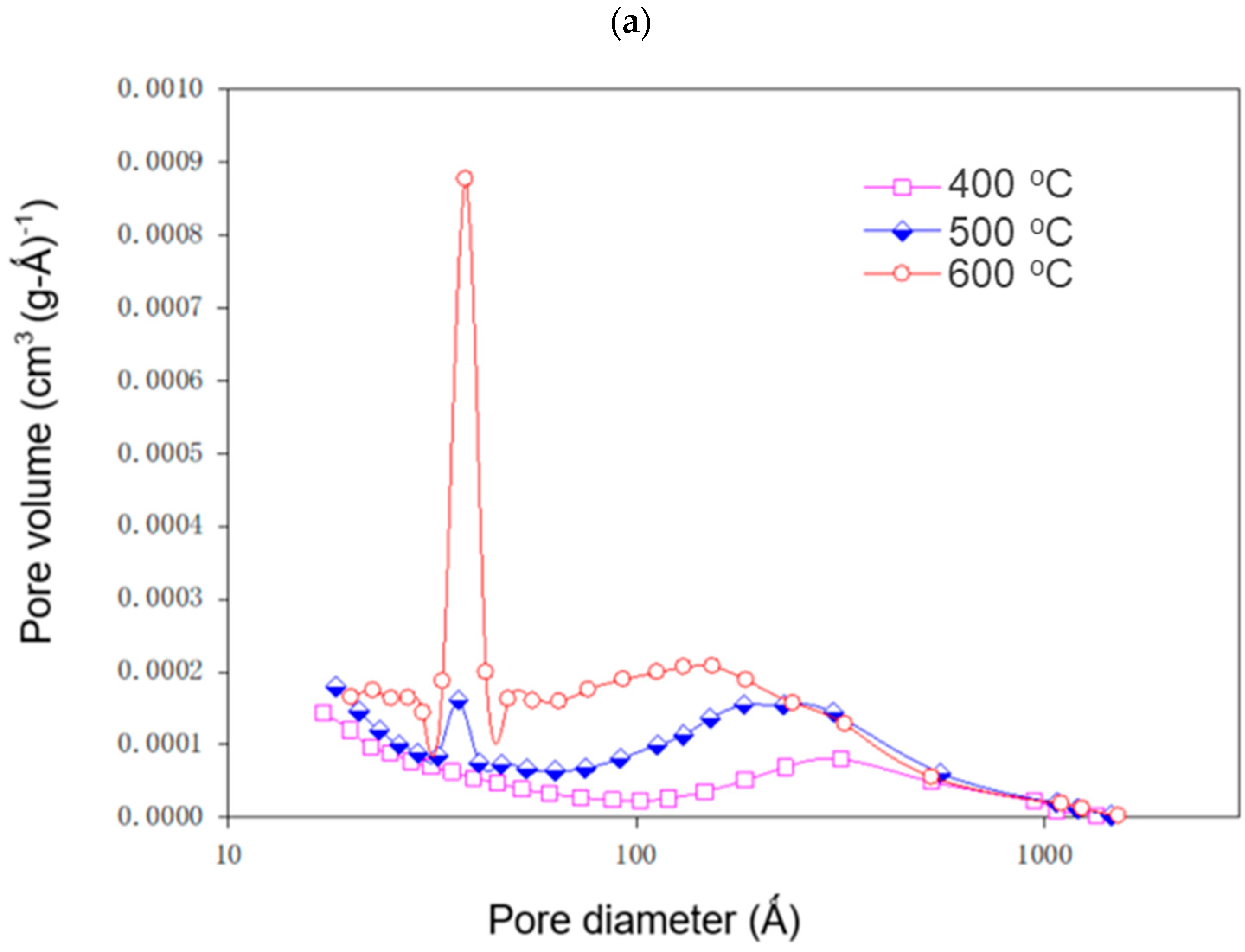
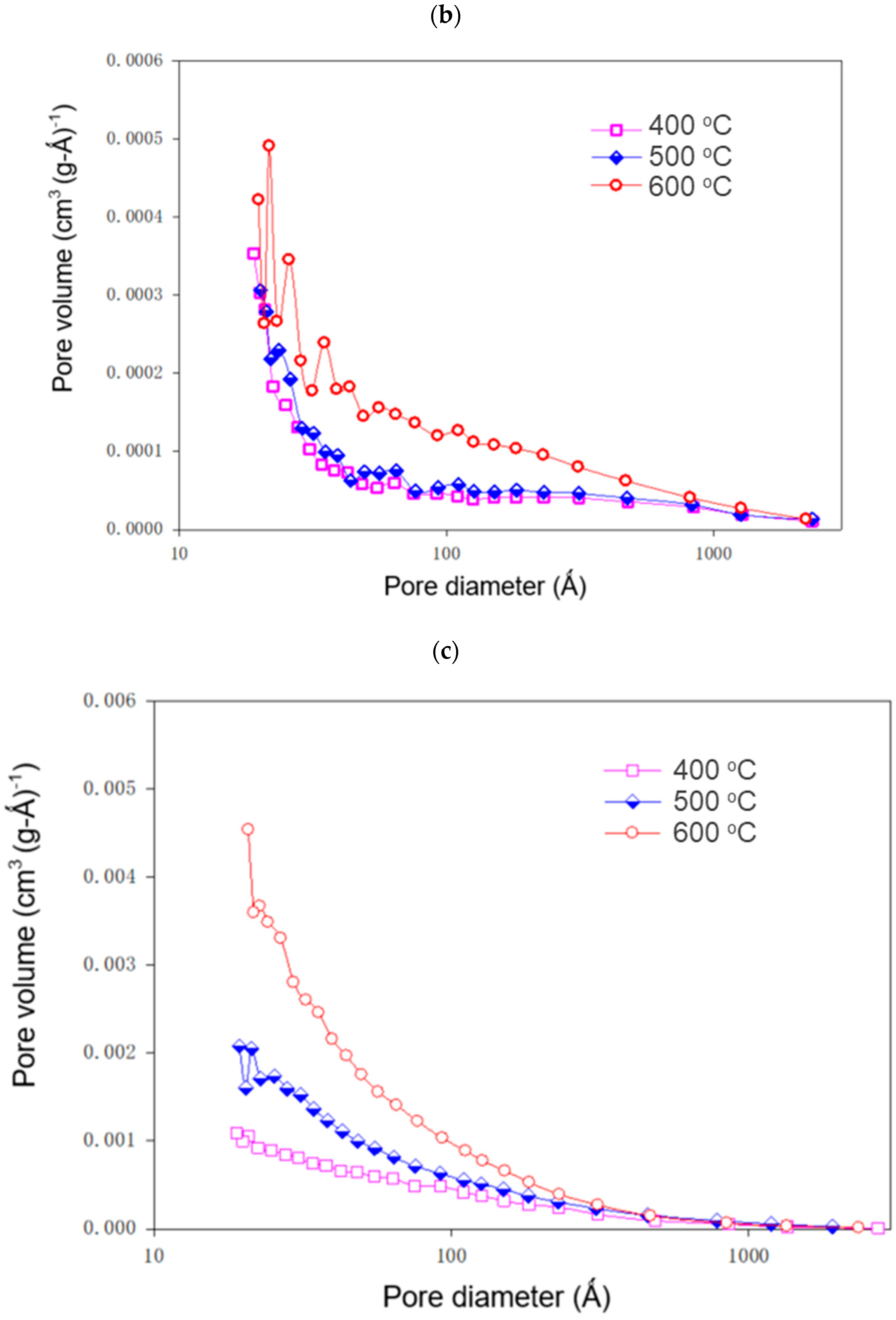
3.4. Characteristics of Sludge-Derived Adsorbents
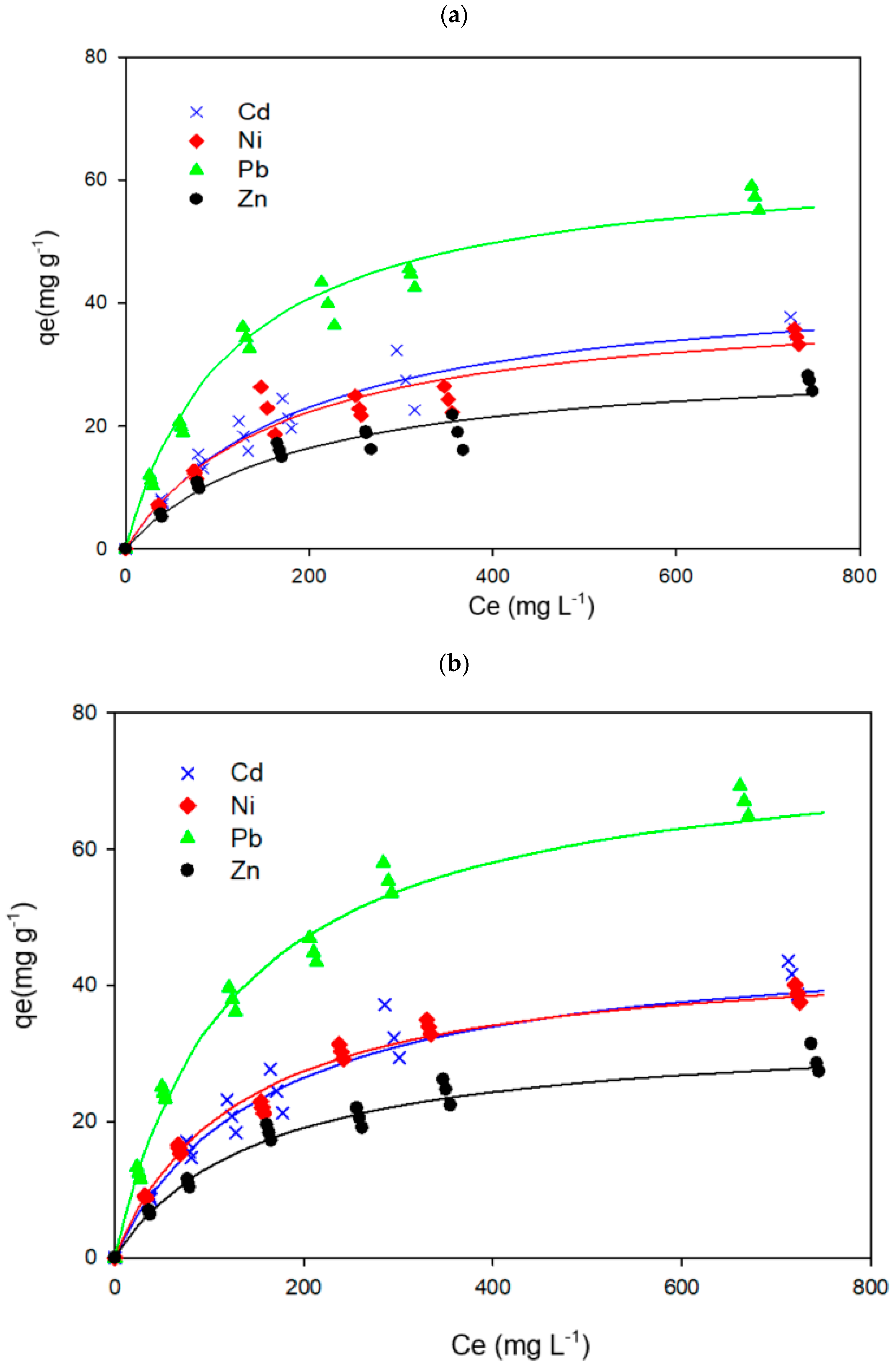
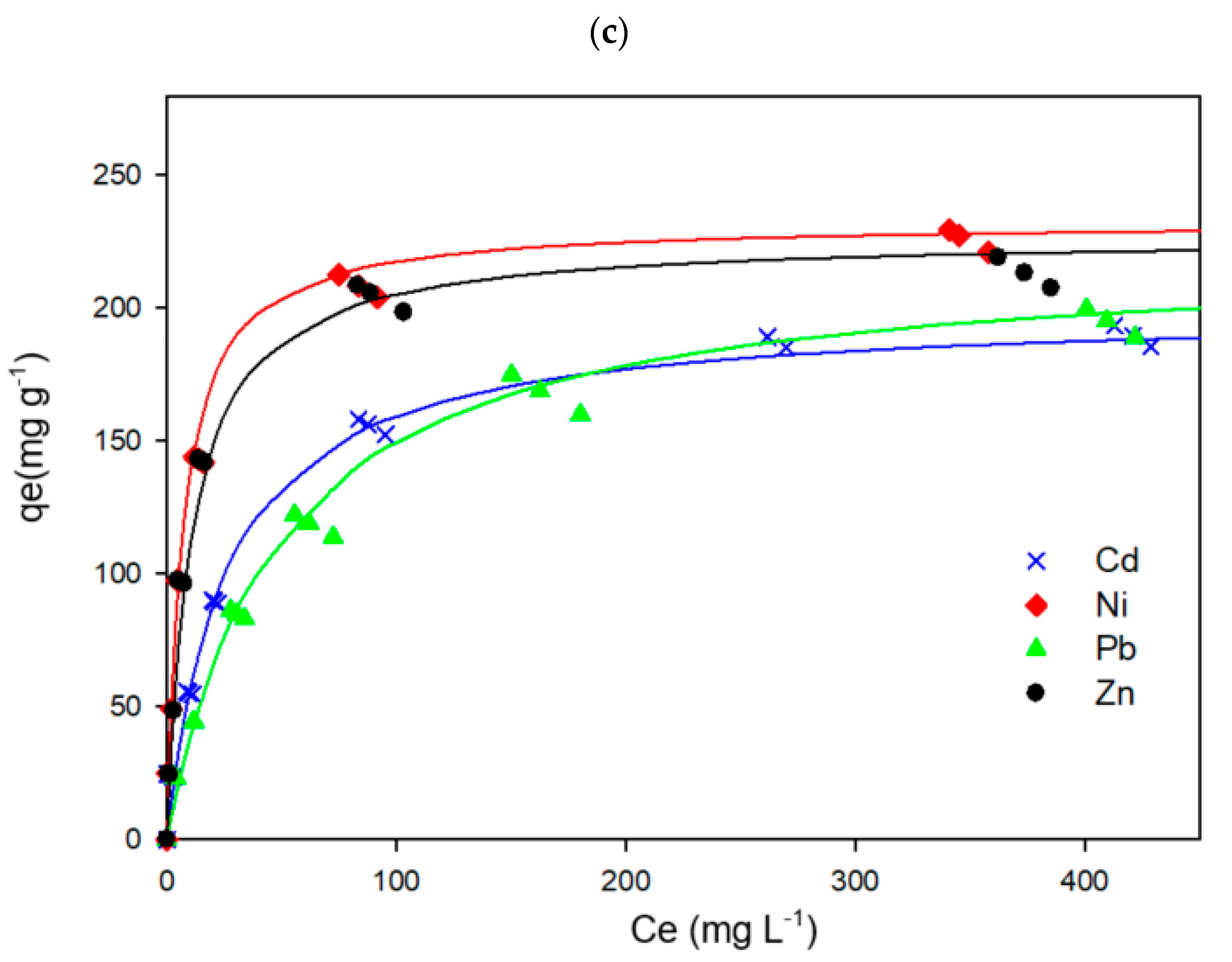
3.5. Metal Adsorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos, J.L.; Otero, L.; Franco, A.; Mosquera-Corral, A.; Roca, E. Ozonation strategies to reduce sludge production of a seafood industry WWTP. Bioresour. Technol. 2009, 100, 1069–1073. [Google Scholar] [CrossRef]
- Romero, P.; Coello, M.D.; Quiroga, J.M.; Aragon, C.A. Overview of sewage sludge minimisation: Techniques based on cell lysis-cryptic growth. Desal. Water Treat. 2013, 51, 5918–5933. [Google Scholar] [CrossRef]
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 2009, 88, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; Metcalf & Eddy Inc.; McGraw-Hill Higher Education: New York, NY, USA, 2003; ISBN 978-0070418783. [Google Scholar]
- Domínguez, A.; Menendez, J.A.; Inguanzo, M.; Pis, J.J. Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour. Technol. 2006, 97, 1185–1193. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, B.S.; Kim, J.S. Recovery of oils with high caloric value and low contaminant content by pyrolysis of digested and dried sewage sludge containing polymer flocculants. Energy Fuels 2008, 22, 1335–1340. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion: A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Microwave irradiation: A sustainable way for sludge treatment. Renew. Sustain. Energy Rev. 2013, 18, 288–305. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Hwang, I.H.; Ouchi, Y.; Matsuto, T. Characteristics of leachate from pyrolysis residue of sewage sludge. Chemosphere 2007, 68, 1913–1919. [Google Scholar] [CrossRef] [Green Version]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods: A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Kim, Y.; Parker, W.A. Technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; John Wiley & Sons: New York, NY, USA, 1987; ISBN 978-0-471-84802-8. [Google Scholar]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, F.; Qi, J.; Zhao, L.; Yan, F.; Li, C. Challenge of biodiesel production from sewage sludge catalyzed by KOH, KOH/activated carbon, and KOH/CaO. Front. Environ. Sci. Eng. 2017, 11, 3. [Google Scholar] [CrossRef]
- Nuithitikul, K.; Prasitturattanachai, W. Activity of sulfated aluminium-tin mixed oxides for the esterification of free fatty acids in crude palm oil. Int. J. Green Energy 2014, 11, 1097–1106. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Chu, C.M.; Chan, E.S.; Ravindra, P. Studies on design of heterogeneous catalysts for biodiesel production. Process Saf. Environ. Prot. 2013, 91, 131–144. [Google Scholar] [CrossRef]
- Juan, J.C.; Kartika, D.A.; Wu, T.Y.; Hin, T.Y. Biodiesel production from Jatropha oil by catalytic and non-catalytic approaches: An overview. Bioresour. Technol. 2011, 102, 452–460. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; de Yuso, A.M.; Nabarlatz, D. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.O.; Foo, K.Y.; Asif, M.; Hameed, B.H. Preparation of activated carbons from rambutan (Nepheliumlappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem. Eng. J. 2014, 250, 198–204. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Ros, A.; Fuente, E.; Montes-Morán, M.A.; Martin, M.J.; Linares-Solano, A. Further insights into the activation process of sewage sludge based precursors by alkaline hydroxides. Chem. Eng. J. 2008, 142, 168–174. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment: A review. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Brubauer, S.; Emmett, H.P.; Teller, E. Adsorption of gas in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lin, Q.H.; Cheng, H.; Chen, G.Y. Preparation and characterization of carbonaceous adsorbents from sewage sludge using a pilot-scale microwave heating equipment. J. Anal. Appl. Pyrolysis 2012, 93, 113–119. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Li, L.Y.; Gong, X.; Abida, O. Waste-to-resources: Exploratory surface modification of sludge-based activated carbon by nitric acid for heavy metal adsorption. Waste Manag. 2019, 87, 375–386. [Google Scholar] [CrossRef]
- ASTM D3173-02. Standard Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM D3174-02. Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM D3175-02. Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Barrett, E.P.; Jovner, L.G.; Halenda, P.P. The determination of pore volume and area distribution in porous substances I. computations of nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lin, B.; Wang, J.; Huang, Q.; Chi, Y. Effects of potassium hydroxide on the catalytic pyrolysis of oily sludge for high-quality oil product. Fuel 2017, 200, 124–133. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Stabile Fuel Emulsions and Method of Making. U.S. Patent 6,607,566, 2003.
- Fuel Comprising Emulsion between Water and a Liquid Hydrocarbon. U.S. Patent 7,018,433, 2006.
- Lin, K.H.; Lai, N.; Zeng, J.Y.; Chiang, H.L. Microwave-pyrolysis treatment of biosludge from a chemical industrial wastewater treatment plant for exploring product characteristics and potential energy recovery. Energy 2020, 199, 117446. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, E.; Kim, B.; Kim, S.; Lee, B.; Kim, M.; Jung, J.C. Preparation of activated carbon aerogel and its application to electrode material for electric double layer capacitor in organic electrolyte: Effect of activation temperature. Korean J. Chem. Eng. 2015, 32, 248–254. [Google Scholar] [CrossRef]
- Li, Z.; Deng, H.; Yang, L.; Zhang, G.; Li, Y.; Ren, Y. Influence of potassium hydroxide activation on characteristics and environmental risk of heavy metals in chars derived from municipal sewage sludge. Bioresour. Technol. 2018, 256, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.T.; Aleksić, R.; Perić-Grujić, A.A. Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Guzel, F.; Yakut, H.; Topal, G. Determination of kinetic and equilibrium parameters of the batch adsorption of Mn(II), Co(II), Ni(II) and Cu(II) from aqueous solution by black carrot (Daucuscarota L.) residues. J. Hazard. Mater. 2008, 153, 1275–1287. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef]
- Ding, Z.H.; Hu, X.; Wan, Y.S.; Wang, S.S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
| KOH/Sludge Ratio | 0 | 0.25 | 0.5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature °C | 400 | 500 | 600 | 400 | 500 | 600 | 400 | 500 | 600 |
| Mass fraction | |||||||||
| Char (wt.%) | 69 ± 3.5 | 63 ± 2.9 | 59 ± 2.1 | 64 ± 3.8 | 60 ± 3.2 | 54 ± 3.4 | 62 ± 4.2 | 55 ± 3.5 | 45 ± 3.1 |
| Liquid oil (wt.%) | 16 ± 1.4 | 17 ± 1.3 | 20 ± 1.6 | 14 ± 1.2 | 16 ± 1.5 | 17 ± 1.3 | 8.0 ± 0.6 | 13 ± 1.1 | 14 ± 1.2 |
| Gas (wt.%) | 15 ± 1.2 | 20 ± 1.7 | 21 ± 1.8 | 22 ± 1.6 | 24 ± 2.2 | 29 ± 2.3 | 30 ± 2.3 | 32 ± 2.5 | 41 ± 3.4 |
| Major element in oil | |||||||||
| N (wt.%) | 2.1 ± 0.15 | 2.0 ± 0.12 | 1.7 ± 0.11 | 1.5 ± 0.09 | 1.4 ± 0.09 | 1.2 ± 0.08 | 2.1 ± 0.10 | 1.2 ± 0.06 | 1.1 ± 0.05 |
| C (wt.%) | 80.7 ± 4.26 | 81.6 ± 5.05 | 77.1 ± 4.45 | 73.7 ± 3.83 | 74.3 ± 4.11 | 70.4 ± 3.77 | 77.9 ± 3.18 | 74.1 ± 3.32 | 66.5 ± 3.47 |
| S (wt.%) | 3.8 ± 0.12 | 1.5 ± 0.10 | 1.3 ± 0.09 | 0.9 ± 0.07 | 0.8 ± 0.06 | 0.9 ± 0.06 | 0.7 ± 0.04 | 0.8 ± 0.05 | 0.8 ± 0.06 |
| H (wt.%) | 3.9 ± 0.18 | 4.5 ± 0.28 | 4.2 ± 0.24 | 3.8 ± 0.27 | 3.6 ± 0.25 | 4.3 ± 0.22 | 4.1 ± 0.23 | 3.4 ± 0.21 | 3.9 ± 0.26 |
| Compounds | Formula | KOH/Sludge Ratio: 0 | KOH/Sludge Ratio: 0.5 | ||||
|---|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | 400 °C | 500 °C | 600 °C | ||
| Monoethyl toluene mixture | C9H12 | 6.87 ± 0.27 | 4.11 ± 0.21 | 5.68 ± 0.17 | *nd | *nd | *nd |
| Trimethylbenzene mixture | C9H12 | 5.38 ± 0.27 | 4.63 ± 0.32 | 5.71 ± 0.29 | *nd | *nd | *nd |
| Dicyclopentadiene | C10H12 | 3.47 ± 0.10 | 1.47 ± 0.04 | 1.77 ± 0.07 | *nd | *nd | *nd |
| Indene | C9H8 | 2.45 ± 0.15 | 2.72 ± 0.11 | 3.36 ± 0.24 | 2.18 ± 0.17 | 3.05 ± 0.27 | 3.37 ± 0.34 |
| Diethylbenzene mixture | C10H14 | 5.77 ± 0.23 | 2.26 ± 0.14 | 2.47 ± 0.21 | 2.41 ± 0.14 | 3.24 ± 0.16 | *nd |
| Monoethyl xylene mixture | C10H14 | 5.45 ± 0.27 | 5.46 ± 0.27 | 5.01 ± 0.32 | 6.23 ± 0.56 | 10.17 ± 0.71 | *nd |
| Pyrindan | C8H9N | *nd | *nd | *nd | *nd | *nd | 3.26 ± 0.39 |
| Indolene | C8H9N | *nd | *nd | *nd | *nd | *nd | 2.29 ± 0.21 |
| Tetramethyl benzene mixture | C10H14 | 5.54 ± 0.33 | 3.75 ± 0.15 | 3.84 ± 0.31 | 4.44 ± 0.36 | 5.01 ± 0.32 | *nd |
| 1+2+3-Methylindene | C10H10 | 7.64 ± 0.23 | 6.26 ± 0.38 | 7.37 ± 0.52 | 6.51 ± 0.33 | 8.21 ± 0.74 | 6.45 ± 0.45 |
| Naphthalene | C10H8 | 8.75 ± 0.35 | 6.23 ± 0.19 | 6.37 ± 0.33 | 6.42 ± 0.51 | 8.41 ± 0.34 | 12.40 ± 0.62 |
| 1-Methylnaphthalene | C11H10 | 8.76 ± 0.53 | 5.54 ± 0.33 | 5.32 ± 0.21 | 9.95 ± 0.63 | 11.20 ± 0.22 | 5.39 ± 0.43 |
| 2-Methylnaphthalene | C11H10 | 5.95 ± 0.30 | 4.20 ± 0.13 | 3.92 ± 0.24 | 7.37 ± 0.52 | 7.82 ± 0.63 | 9.26 ± 1.02 |
| 4-Phenylbutyronitrile | C10H11N | *nd | *nd | *nd | *nd | *nd | 3.19 ± 0.29 |
| Biphenyl | C12H10 | 1.74 ± 0.07 | 1.81 ± 0.07 | 1.66 ± 0.13 | 1.91 ± 0.17 | 1.93 ± 0.13 | 3.48 ± 0.21 |
| 1+2-Ethylnaphthalene | C12H12 | *nd | *nd | *nd | 3.52 ± 0.32 | 3.02 ± 0.12 | 1.28 ± 0.12 |
| Dimethylnaphthalene mixture | C12H12 | 11.53 ± 0.69 | 10.24 ± 0.51 | 7.74 ± 0.23 | 10.11 ± 0.71 | 10.56 ± 0.95 | 3.05 ± 0.15 |
| Biphenylene | C12H8 | *nd | *nd | *nd | 2.37 ± 0.12 | 2.30 ± 0.25 | *nd |
| 2+3+4-Phenyltoluene | C13H12 | 2.81 ± 0.20 | 2.95 ± 0.15 | 2.53 ± 0.18 | 4.16 ± 0.33 | 3.74 ± 0.37 | 5.32 ± 0.21 |
| Trimethylnaphthalene mixture | C13H14 | 1.78 ± 0.05 | 2.77 ± 0.19 | 1.89 ± 0.08 | 0.94 ± 0.07 | 0.73 ± 0.04 | *nd |
| 1,3-Diphenylpropane | C15H16 | *nd | *nd | *nd | *nd | *nd | 10.85 ± 0.87 |
| Phenanthrene | C14H10 | 1.11 ± 0.06 | 2.24 ± 0.09 | 1.97 ± 0.16 | 3.74 ± 0.34 | 3.37 ± 0.27 | 0.93 ± 0.07 |
| Sum (%) | 84.90 ± 4.25 | 66.64 ± 4.66 | 66.61 ± 3.83 | 72.26 ± 6.48 | 82.76 ± 7.97 | 70.88 ± 6.74 | |
| KOH/Sludge Ratio | Temperature (°C) | Heat Value (MJ kg−1) | Energy Content (MJ kg-drb−1 *) | Energy Recovery (%) | |||
|---|---|---|---|---|---|---|---|
| Oil | Char | Oil | Char | Oil+Char | Char | ||
| 400 | 41.39 ± 2.48 | 12.15 ± 1.34 | 6.62 ± 0.86 | 8.38 ± 0.75 | 95 ± 7.6 | 53 ± 7.4 | |
| 0 | 500 | 40.93 ± 3.27 | 11.58 ± 1.39 | 6.96 ± 0.57 | 7.29 ± 1.02 | 90 ± 4.5 | 46 ± 5.1 |
| 600 | 40.86 ± 2.04 | 11.07 ± 0.77 | 8.17 ± 0.98 | 6.64 ± 0.53 | 94 ± 8.5 | 42 ± 3.8 | |
| 400 | 39.38 ± 4.33 | 11.93 ± 0.72 | 5.51 ± 0.72 | 7.63 ± 0.96 | 84 ± 10.1 | 49 ± 2.9 | |
| 0.25 | 500 | 36.05 ± 1.85 | 11.03 ± 1.43 | 5.76 ± 0.63 | 6.62 ± 0.73 | 79 ± 4.7 | 42 ± 5.2 |
| 600 | 30.03 ± 2.14 | 10.63 ± 0.53 | 5.12 ± 0.36 | 5.74 ± 0.52 | 69 ± 7.6 | 37 ± 3.3 | |
| 400 | 37.32 ± 4.48 | 10.83 ± 1.32 | 2.98 ± 0.33 | 6.71 ± 1.02 | 62 ± 5.6 | 43 ± 5.6 | |
| 0.5 | 500 | 34.05 ± 3.06 | 9.93 ± 0.89 | 4.43 ± 0.42 | 5.46 ± 0.65 | 66 ± 7.3 | 35 ± 4.9 |
| 600 | 30.01 ± 3.88 | 9.43 ± 0.67 | 4.02 ± 0.48 | 4.24 ± 0.63 | 53 ± 6.4 | 27 ± 2.4 | |
| KOH/Sludge Ratio | Temperature (°C) | Ni | Cu | Zn | As | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|
| Dried Raw Sludge | 616.7 ± 45.9 | 124.3 ± 6.1 | 2859.4 ± 217.9 | 16.6 ± 2.2 | 15.5 ± 1.9 | 168.2 ± 21.2 | 174.8 ± 7.2 | |
| 0 (acid washing) | ||||||||
| 400 °C | 357.9 ± 27.4 | 86.3 ± 10.2 | 1148.9 ± 60.3 | 12.4 ± 0.8 | 8.2 ± 0.4 | 122.2 ± 7.2 | 135.7 ± 7.4 | |
| 500 °C | 393.7 ± 23.5 | 93.2 ± 3.9 | 1202.6 ± 78.5 | 13.3 ± 0.9 | 8.8 ± 0.8 | 131.5 ± 9.5 | 139.2 ± 10.2 | |
| 600 °C | 388.5 ± 27.6 | 99.8 ± 12.2 | 1280.3 ± 116.4 | 14.5 ± 1.2 | 9.1 ± 0.7 | 138.6 ± 17.7 | 144.6 ± 16.3 | |
| 0.5 (acid washing) | ||||||||
| 400 °C | 263.4 ± 33.5 | 93.4 ± 5.7 | 923.2 ± 104.7 | 13.7 ± 0.7 | 9.3 ± 1.1 | 115.1 ± 13.1 | 123.5 ± 15.7 | |
| 500 °C | 302.5 ± 24.8 | 102.7 ± 6.4 | 939.6 ± 101.8 | 14.5 ± 1.1 | 9.7 ± 1.3 | 119.4 ± 8.6 | 128.7 ± 7.4 | |
| 600 °C | 311.7 ± 14.3 | 110.5 ± 8.1 | 1006.5 ± 84.3 | 15.5 ± 1.3 | 10.3 ± 0.8 | 123.7 ± 15.3 | 133.4 ± 5.8 | |
| KOH/Sludge Ratio | Temperature °C | BET Surface Area (m2 g−1) | TPV (cm3 g−1) × 102 | MV (cm3 g−1) × 103 | PD (Å) |
|---|---|---|---|---|---|
| 400 | 8.43 ± 0.75 | 5.36 ± 0.21 | 1.67 ± 0.05 | 254.14 ± 14.63 | |
| 0 | 500 | 16.12 ± 0.58 | 9.75 ± 0.83 | 1.02 ± 0.04 | 241.82 ± 17.09 |
| 600 | 25.11 ± 1.49 | 10.89 ± 0.61 | 0.56 ± 0.05 | 173.52 ± 9.27 | |
| 400 | 11.22 ± 0.56 | 7.68 ± 0.73 | 0.10 ± 0.01 | 273.83 ± 25.17 | |
| 0.25 | 500 | 12.89 ± 0.94 | 8.19 ± 0.37 | 0.14 ± 0.01 | 254.46 ± 22.89 |
| 600 | 22.29 ± 1.52 | 12.21 ± 0.53 | 0.20 ± 0.01 | 219.16 ± 10.95 | |
| 400 | 63.42 ± 2.78 | 22.62 ± 0.97 | 1.18 ± 0.08 | 142.65 ± 5.35 | |
| 0.5 | 500 | 101.26 ± 4.42 | 31.09 ± 2.10 | 1.52 ± 0.07 | 122.83 ± 6.63 |
| 600 | 174.71 ± 10.29 | 39.27 ± 2.21 | 2.05 ± 0.09 | 89.91 ± 7.19 |
| KOH/Sludge Ratio | Metal Solution | K (L mmol−1) | qm (mmol g−1) | r2 |
|---|---|---|---|---|
| 0 | Cd(II) | 0.607 | 0.395 | 0.969 |
| Pb(II) | 1.580 | 0.309 | 0.964 | |
| Ni(II) | 0.353 | 0.673 | 0.975 | |
| Zn(II) | 0.362 | 0.478 | 0.973 | |
| 0.25 | Cd(II) | 0.704 | 0.423 | 0.967 |
| Pb(II) | 1.668 | 0.367 | 0.956 | |
| Ni(II) | 0.449 | 0.772 | 0.988 | |
| Zn(II) | 0.430 | 0.514 | 0.983 | |
| 0.5 | Cd(II) | 4.439 | 1.774 | 0.982 |
| Pb(II) | 4.307 | 1.068 | 0.985 | |
| Ni(II) | 8.473 | 3.962 | 0.995 | |
| Zn(II) | 6.128 | 3.472 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-H.; Tsai, J.-H.; Chou, Z.-W.; Chiang, H.-L. Product Characteristics of Sludge Pyrolysis and Adsorption Performance of Metals by Char. Sustainability 2021, 13, 12125. https://doi.org/10.3390/su132112125
Lin K-H, Tsai J-H, Chou Z-W, Chiang H-L. Product Characteristics of Sludge Pyrolysis and Adsorption Performance of Metals by Char. Sustainability. 2021; 13(21):12125. https://doi.org/10.3390/su132112125
Chicago/Turabian StyleLin, Kuo-Hsiung, Jiun-Horng Tsai, Zhi-Wei Chou, and Hung-Lung Chiang. 2021. "Product Characteristics of Sludge Pyrolysis and Adsorption Performance of Metals by Char" Sustainability 13, no. 21: 12125. https://doi.org/10.3390/su132112125
APA StyleLin, K.-H., Tsai, J.-H., Chou, Z.-W., & Chiang, H.-L. (2021). Product Characteristics of Sludge Pyrolysis and Adsorption Performance of Metals by Char. Sustainability, 13(21), 12125. https://doi.org/10.3390/su132112125






