Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacterial Strain from the POME
2.2. Microorganism, Inoculum Preparation, and Growth Profile
2.3. Batch Fermentation of the Lysinibacillus sp. LC 556247
2.4. Analytical Methods and Characterization of POME
2.5. Statistical Analyses
3. Results and Discussion
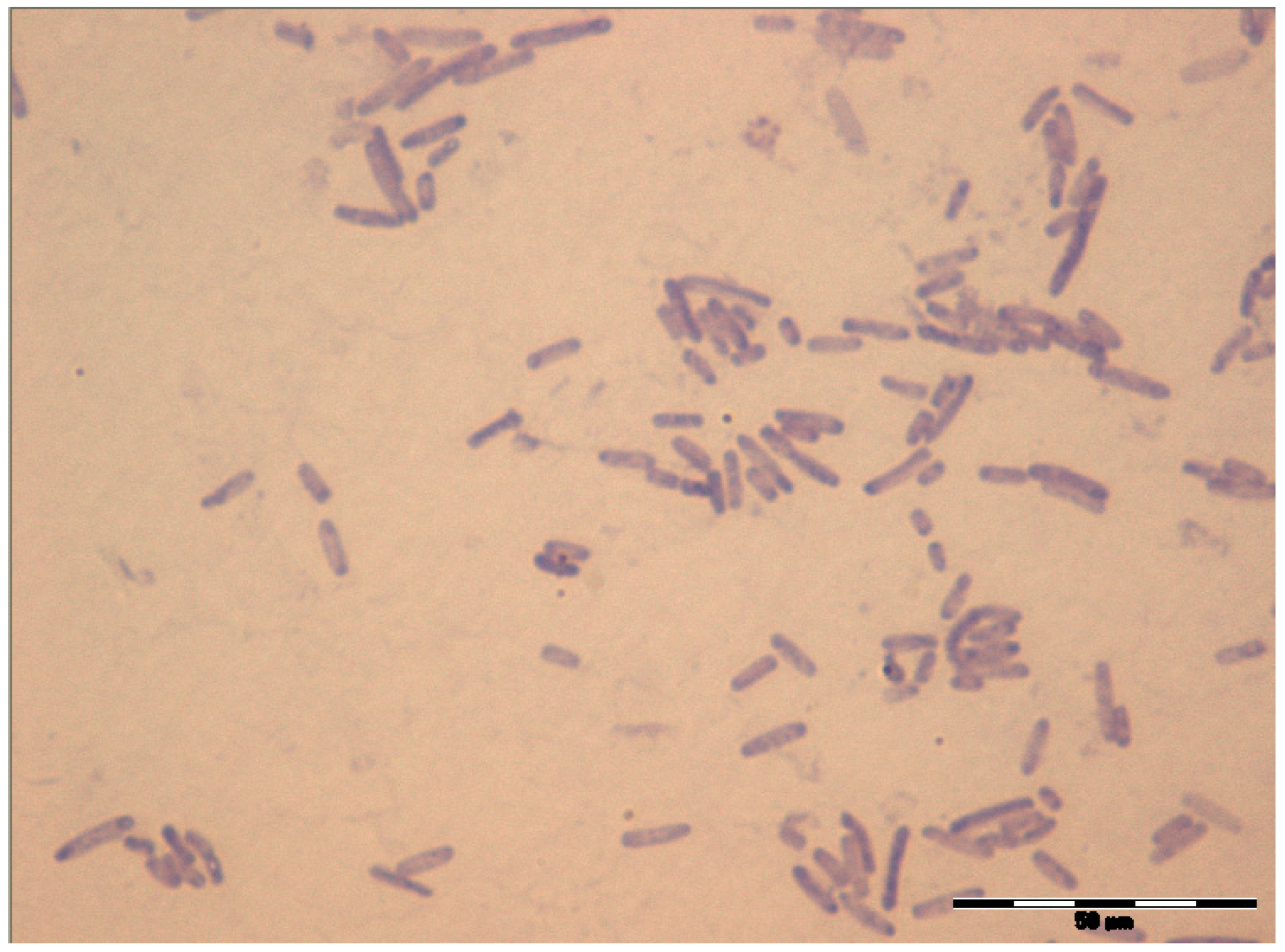
3.1. Strain Identification and General Characteristics of the Isolated Bacteria
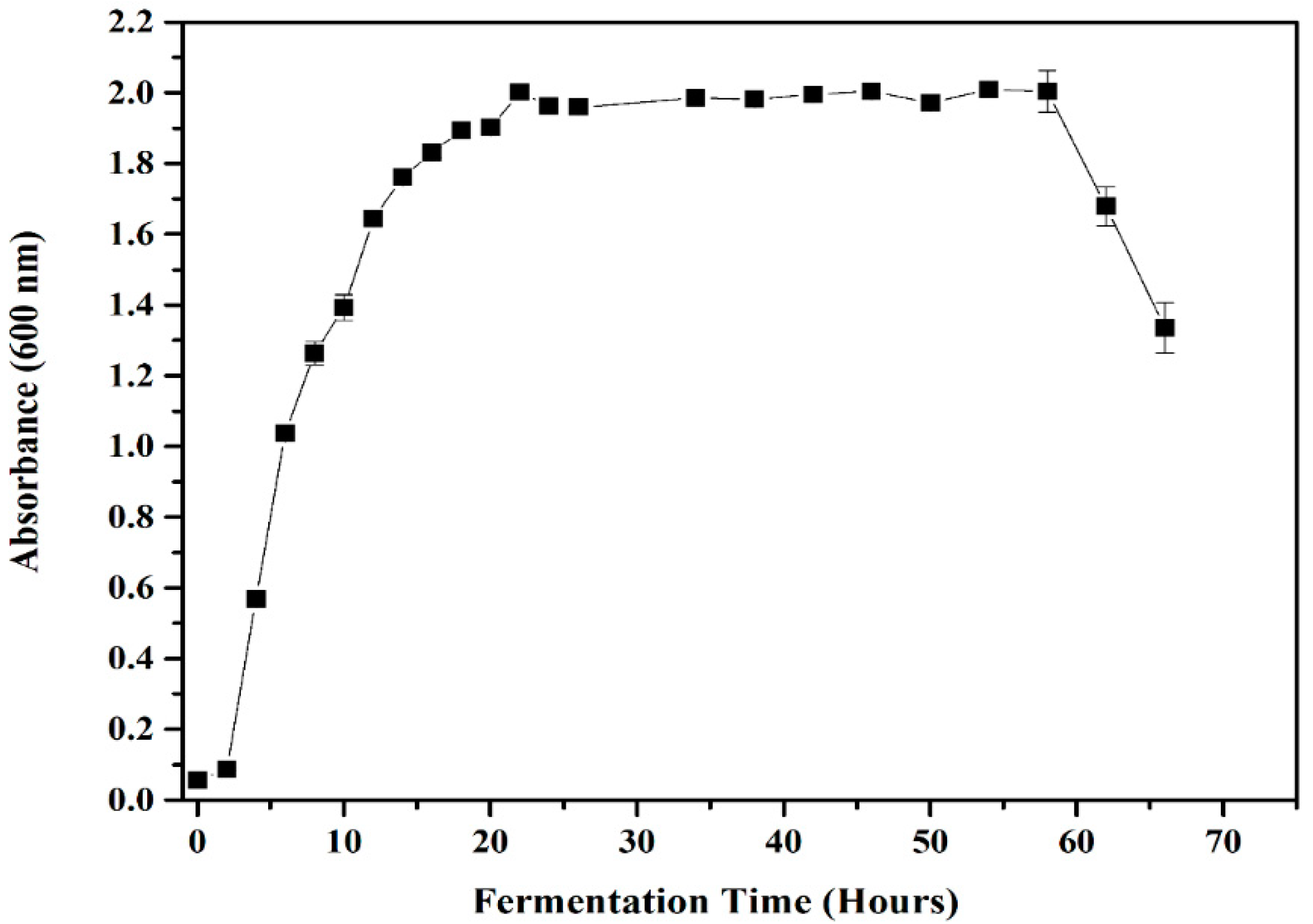
3.2. Growth Profiles of Lysinibacillus sp. LC 556247 and Colony-Forming Units (CFU)
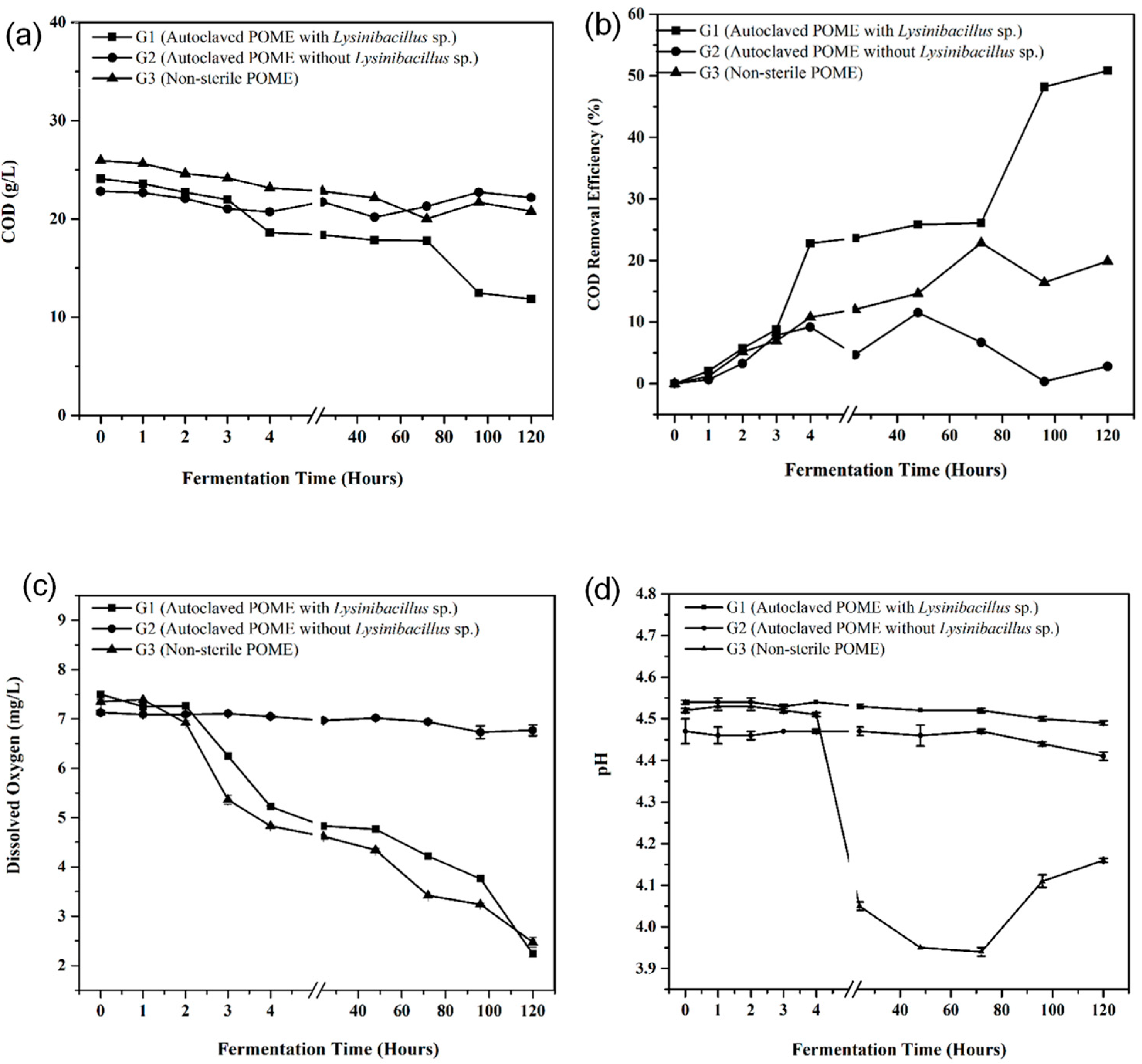
3.3. Batch Fermentation Utilizing POME as the Sole Carbon Source for Biomass Fuel Application
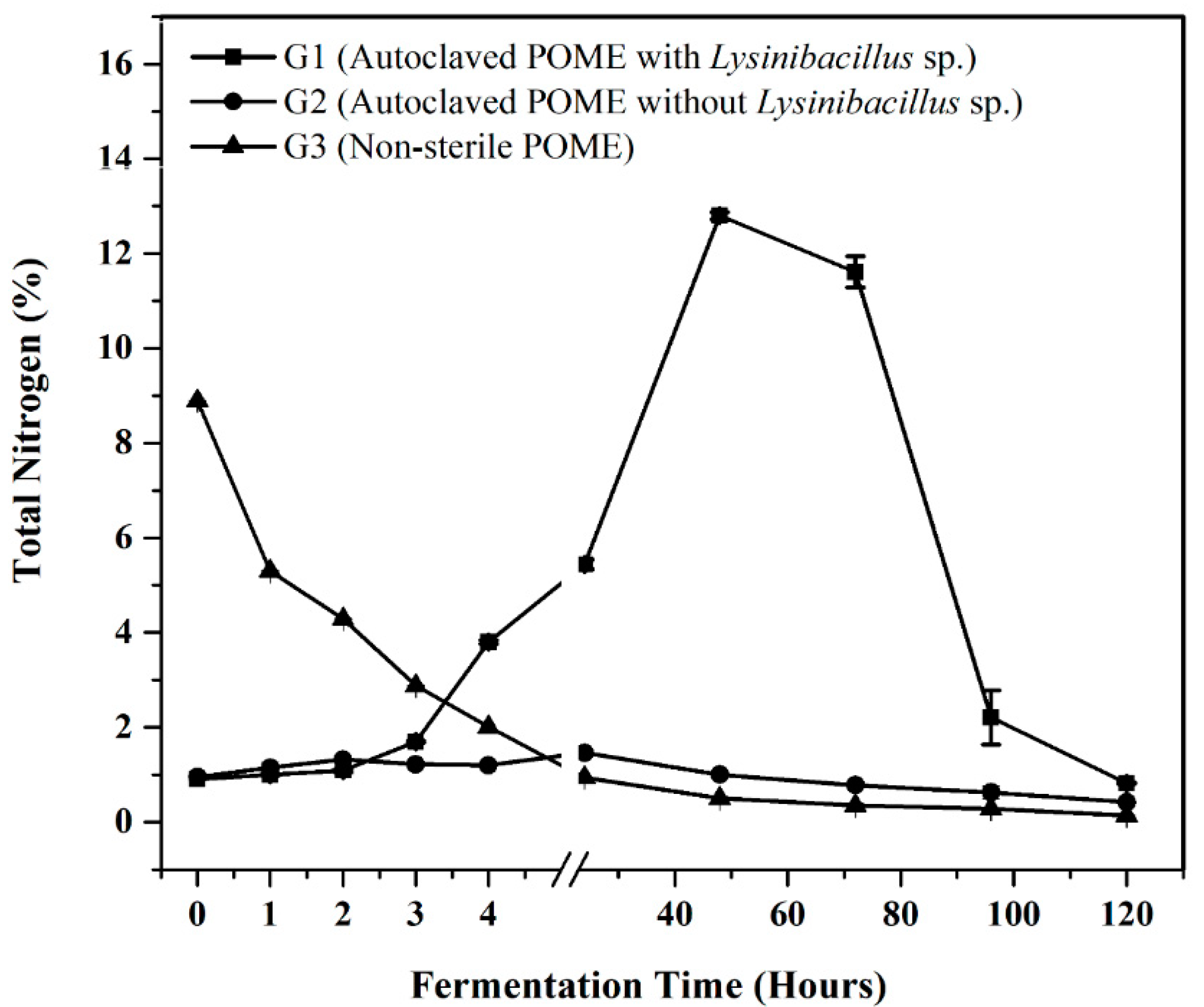
3.4. Biodegradation Study of POME Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.P.; Ibrahim, M.H.; Esa, N.; Iliyana, M.S. Composting of waste from palm oil mill: A sustainable waste management practice. Rev. Environ. Sci. Biotechnol. 2010, 9, 331–344. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F. The Oil Palm Wastes in Malaysia. In Biomass Now-Sustainable Growth Use; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Onoja, E.; Chandren, S.; Razak, F.I.A.; Mahat, N.A.; Wahab, R.A. Oil palm (Elaeis guineensis) biomass in Malaysia: The present and future prospects. Waste Biomass Valoriz. 2019, 10, 2099–2117. [Google Scholar] [CrossRef]
- Low, T.J.; Mohammad, S.; Sudesh, K.; Baidurah, S. Utilization of banana (Musa sp.) fronds extract as an alternative carbon source for poly (3-hydroxybutyrate) production by Cupriavidus necator H16. Biocatal. Agric. Biotechnol. 2021, 34, 102048. [Google Scholar] [CrossRef]
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of poly (3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal. Agric. Biotechnol. 2019, 17, 51–59. [Google Scholar] [CrossRef]
- Sen, K.Y.; Baidurah, S. Renewable biomass feedstocks for production of sustainable biodegradable polymer. Curr. Opin. Green Sustain. Chem. 2020, 27, 1–6. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly (lactic acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.A.; Meng, T.K.; Serri, N.A.; Yusoff, S.B.; Shahrin, N.A.M.; Seng, K.Y.; Bakar, M.H.A.; Keong, L.C. Sustainable Biorefinery Concept for Industrial Bioprocessing. In Biorefinery Production Technologies for Chemicals and Energy, 1st ed.; Kuila, A., Mukhopadhyay, M., Eds.; Wiley & Sons: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 15–53. [Google Scholar]
- Karim, A.; Islam, M.A.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Microbial lipid accumulation through bioremediation of palm oil mill wastewater by Bacillus cereus. ACS Sustain. Chem. Eng. 2019, 7, 14500–14508. [Google Scholar] [CrossRef]
- Wakil, S.M.; Fasiku, S.A.; Adelabu, A.B.; Onilude, A.A. Production of bioethanol from spontaneous fermentation of palm oil mill effluent (POME). Researcher 2013, 5, 28–35. [Google Scholar]
- Zainal, B.S.; Zinatizadeh, A.A.; Chyuan, O.H.; Mohd, N.S.; Ibrahim, S. Effects of process, operational and environmental variables on biohydrogen production using palm oil mill effluent (POME). Int. J. Hydrogen Energy 2018, 43, 10637–10644. [Google Scholar] [CrossRef]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm Oil Mill Effluent Treatment Processes—A Review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Bergström, D.; Finell, M.; Gref, R. Effects of extractives on the physical characteristics of Scots pine sawdust fuel pellets. For. Prod. J. 2010, 60, 640–644. [Google Scholar] [CrossRef]
- Hassan, S.; Kee, L.S.; Al-Kayiem, H.H. Experimental study of palm oil mill effluent and oil palm frond waste mixture as an alternative biomass fuel. J. Eng. Sci. 2013, 8, 703–712. [Google Scholar]
- Zeng, W.S.; Tang, S.Z.; Xiao, Q.H. Calorific values and ash contents of different parts of Masson pine trees in southern China. J. For. Res. 2014, 25, 779–786. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Xiao, Z.; Liang, J.; Li, H.; Cao, L.; Wang, H.; Chen, X.; Zeng, G. A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties. Energy Convers. Manag. 2016, 126, 509–515. [Google Scholar] [CrossRef]
- Gumisiriza, R.; Hawumba, J.F.; Okure, M.; Hensel, O. Biomass waste-to-energy valorisation technologies: A review case for banana processing in Uganda. Biotechnol. Biofuels 2017, 10, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Abdulrazik, A.; Elsholkami, M.; Elkamel, A.; Simon, L. Multi-products productions from Malaysian oil palm empty fruit bunch (EFB): Analyzing economic potentials from the optimal biomass supply chain. J. Clean. Prod. 2017, 168, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Sirrajudin, M.S.; Rasat, M.S.M.; Wahab, R.; Amini, M.H.M.; Mohamed, M.; Ahmad, M.I.; Moktar, J.; Ibrahim, M.A. Enhancing the energy properties of fuel pellets from oil palm fronds of agricultural residues by mixing with glycerin. ARPN J. Eng. Appl. Sci. 2016, 11, 6122–6127. [Google Scholar]
- Purwanto, W.W.; Supramono, D.; Fisafarani, H. Biomass waste and biomass pellets characteristics and their potential in Indonesia. In Proceedings of The 1st International Seminar on Fundamental and Application ISFAChE, Bali, Indonesia, 3–4 November 2010; p. C004-1. [Google Scholar]
- May, C.Y. The untapped potential of oil palm biomass and its potential applications: Challenges and Opportunities. In Proceedings of the Reach and Teach Friends of Industry Seminar, Putrajaya, Malaysia, 25 January 2011. [Google Scholar]
- Onoja, E.; Attan, N.; Chandren, S.; Razak, F.I.A.; Keyon, A.S.A.; Mahat, N.A.; Wahab, R.A. Insights into the physicochemical properties of the Malaysian oil palm leaves as an alternative source of industrial materials and bioenergy. Mal. J. Fund. Appl. Sci. 2017, 13, 623–631. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. A holistic approach to managing palm oil mill effluent (POME): Biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv. 2009, 27, 40–52. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Shimada, S. Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer—A case study of Malaysia. Biomass Bioenergy 2013, 51, 109–124. [Google Scholar] [CrossRef]
- Dererie, D.Y.; Trobro, S.; Momeni, M.H.; Hansson, H.; Blomqvist, J.; Passoth, V.; Schnürer, A.; Sandgren, M.; Ståhlberg, J. Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour. Technol. 2011, 102, 4449–4455. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Manan, Z.A.; Alwi, S.R.W.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Brijwani, K.; Bhargav, V.K.; Patil, R.T. Enhanced ethanol production via fermentation of rice straw with hydrolysate-adapted Candida tropicalis ATCC 13803. Process Biochem. 2010, 45, 1299–1306. [Google Scholar] [CrossRef]
- Zanetti, M.; Costa, C.; Greco, R.; Grigolato, S.; Ottaviani Aalmo, G.; Cavalli, R. How wood fuels’ quality relates to the standards: A class-modelling approach. Energies 2017, 10, 1455. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Piro, P.; Carbone, M.; Tomei, G. Assessing settleability of dry and wet weather flows in an urban area serviced by combined sewers. Water Air Soil Pollut. 2011, 214, 107–117. [Google Scholar] [CrossRef]
- Zhu, C.; Sun, G.; Chen, X.; Guo, J.; Xu, M. Lysinibacillus varians sp. nov., an endospore-forming bacterium with a filament-to-rod cell cycle. Int. J. Syst. Evol. Microbiol. 2014, 64, 3644–3649. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.-D.; Seo, M.-J.; Lim, S.-I.; Lee, S.-Y. Genome sequence of Lysinibacillus boronitolerans F1182, isolated from a traditional Korean fermented soybean product. J. Bacteriol. 2012, 194, 5988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans Gen. Nov. Sp. Nov., and Transfer of Bacillus fusiformis to Lysinibacillus fusiformis Comb. Nov. and Bacillus sphaericus to Lysinibacillus sphaericus Comb. Nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Miwa, H.; Ahmed, I.; Yokota, A.; Fujiwara, T. Lysinibacillus parviboronicapiens sp. nov., a low-boron-containing bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2009, 59, 1427–1432. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-S.; Jung, Y.-T.; Park, S.; Oh, T.; Yoon, J.-H. Lysinibacillus xylanilyticus sp nov., a xylan-degrading bacterium isolated from forest humus. Int. J. Syst. Evol. Microbiol. 2009, 60, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Coorevits, A.; Dinsdale, A.E.; Heyrman, J.; Schumann, P.; Van Landschoot, A.; Logan, N.A.; De Vos, P. Lysinibacillus macroides sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 2012, 62, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.-L.; Huang, Y.; Liu, J.; Ma, L.; Mo, M.-H.; Li, W.-J.; Yang, F.X. Lysinibacillus mangiferahumi sp. nov., a new bacterium producing nematicidal volatiles. Antonie van Leeuwenhoek 2012, 102, 53–59. [Google Scholar] [CrossRef]
- Kim, J.-S.; Paek, W.; Styrak, I.; Park, I.-S.; Sin, Y.; Paek, J.; Park, K.-A.; Kim, H.; Kim, H.-L.; Chang, Y.-H. Description of Lysinibacillus sinduriensis sp. nov., and transfer of Bacillus massiliensis and Bacillus odysseyi to the genus Lysinibacillus as Lysinibacillus massiliensis comb. nov. and Lysinibacillus odysseyi comb. nov. with emended description of the genus Lysinibacillus. Int. J. Syst. Evol. Microbiol. 2011, 62, 2347–2355. [Google Scholar]
- Duan, Y.-Q.; He, S.-T.; Li, Q.-Q.; Wang, M.-F.; Wang, W.-Y.; Zhe, W.; Cao, Y.-H.; Mo, M.-H.; Zhai, Y.-L.; Li, W.-J. Lysinibacillus tabacifolii sp. nov., a novel endophytic bacterium isolated from Nicotiana tabacum leaves. J. Microbiol. 2013, 51, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Jang, Y.-H.; Hamada, M.; Ahn, J.-H.; Weon, H.-Y.; Suzuki, K.; Wang, K.-S.; Kwon, S.-W. Lysinibacillus chungkukjangi sp. nov., isolated from Chungkukjang, Korean fermented soybean food. J. Microbiol. 2013, 51, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, H.; Zhang, J.; Hu, G.; Zhang, J.; He, J.; Huang, X. Lysinibacillus fluoroglycofenilyticus sp. nov., a bacterium isolated from fluoroglycofen contaminated soil. Antonie van Leeuwenhoek 2015, 107, 157–164. [Google Scholar] [CrossRef]
- Sun, J.-Q.; Xu, L.; Wu, X.-L. Lysinibacillus alkalisoli sp. nov., isolated from saline-alkaline soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 67–71. [Google Scholar] [CrossRef]
- Ouoba, L.I.I.; Mbozo, A.B.V.; Thorsen, L.; Anyogu, A.; Nielsen, D.S.; Kobawila, S.C.; Sutherland, J.P. Lysinibacillus louembei sp. nov., a spore-forming bacterium isolated from Ntoba Mbodi, alkaline fermented leaves of cassava from the Republic of the Congo. Int. J. Syst. Evol. Microbiol. 2015, 65, 4256–4262. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y.; Chen, F.; Zheng, S.; Wang, G. Lysinibacillus manganicus sp. nov., isolated from manganese mining soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 3568–3573. [Google Scholar] [CrossRef] [Green Version]
- Melnick, R.L.; Suárez, C.; Bailey, B.A.; Backman, P.A. Isolation of endophytic endospore-forming bacteria from Theobroma cacao as potential biological control agents of cacao diseases. Biol. Control. 2011, 57, 236–245. [Google Scholar] [CrossRef]
- Hii, K.L.; Yeap, S.P.; Mashitah, M.D. Cellulase production from palm oil mill effluent in Malaysia: Economical and technical perspectives. Eng. Life Sci. 2012, 12, 7–28. [Google Scholar] [CrossRef]
- Gamaralalage, D.; Sawai, O.; Nunoura, T. Degradation behavior of palm oil mill effluent in Fenton oxidation. J. Hazard. Mater. 2019, 364, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, R.L. Lag phase is a dynamic, organized, adaptive, and evolvable period that prepares bacteria for cell division. J. Bacteriol. 2019, 201, e00697-18. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M. Bacterial Growth. In Environmental Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press Incorporation: San Diego, CA, USA, 2000; pp. 37–54. [Google Scholar]
- Iwuagwu, J.O.; Ugwuanyi, J.O. Treatment and valorization of palm oil mill effluent through production of food grade yeast biomass. J. Waste Manag. 2014, 2014, 439071. [Google Scholar] [CrossRef] [Green Version]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Nwuche, C.O.; Aoyagi, H.; Ogbonna, J.C. Treatment of palm oil mill effluent by a microbial consortium developed from compost soils. Int. Sch. Res. Not. 2014, 2014, 762070. [Google Scholar] [CrossRef]
- Bala, J.D.; Lalung, J.; Al-Gheethi, A.A.S.; Hossain, K.; Ismail, N. Microbiota of palm oil mill wastewater in Malaysia. Trop. Life Sci. Res. 2018, 29, 131–163. [Google Scholar] [CrossRef]
- Soleimaninanadegani, M.; Manshad, S. Enhancement of biodegradation of palm oil mill effluents by local isolated microorganisms. Int. Sch. Res. Not. 2014, 2014, 727049. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Biological treatment of anaerobically digested palm oil mill effluent (POME) using a Lab-Scale Sequencing Batch Reactor (SBR). J. Environ. Manag. 2010, 91, 1738–1746. [Google Scholar] [CrossRef]
- Al-Mutairi, N.Z. Aerobic selectors in slaughterhouse activated sludge systems: A preliminary investigation. Bioresour. Technol. 2009, 100, 50–58. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Lam, M.K.; Leong, W.H.; Chuah, L.F.; Yusup, S.; Setiabudi, H.D.; Tang, Y.; Lim, J.W. Identification of microbial inhibitions and mitigation strategies towards cleaner bioconversions of palm oil mill effluent (POME): A review. J. Clean. Prod. 2020, 280, 124346. [Google Scholar] [CrossRef]
- The Commissioner of Law Revision, M.; Federal Subsidiary Legislation, Division of Environment. Environmental Quality (Prescribed Premises) (Crude Palm Oil) (Amendment) Regulations 1982. Available online: https://www.doe.gov.my/portalv1/wp-content/uploads/2015/01/Environmental_Quality_Prescribed_Premises_Crude_Palm_Oil_Amendment_Regulations_1982_-P.U.A_183-82.pdf (accessed on 13 October 2021).
- Mohapatra, S.; Samantaray, D.P.; Samantaray, S.M.; Mishra, B.B.; Das, S.; Majumdar, S.; Pradhan, S.K.; Rath, S.N.; Rath, C.C.; Akthar, J.; et al. Structural and thermal characterization of PHAs produced by Lysinibacillus sp. through submerged fermentation process. Int. J. Biol. Macromol. 2016, 93, 1161–1167. [Google Scholar] [CrossRef]
- Bala, J.D.; Lalung, J.; Ismail, N. Studies on the reduction of organic load from palm oil mill effluent (POME) by bacterial strains. Int. J. Recycl. Org. Waste Agric. 2015, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]


| Fermentation Time (h) | Group | Weight Reduction (%) | MC (%) | CEV (MJ/kg) |
|---|---|---|---|---|
| 0 | G1 | 95.52 | 1.41 ± 0.01 | 18.04 ± 0.05 d |
| G2 | 95.70 | 2.75 ± 0.04 | 17.78 ± 0.02 | |
| G3 | 95.72 | 2.18 ± 0.04 | 18.10 ± 0.02 | |
| 1 | G1 | 95.50 | 1.54 ± 0.01 | 17.89 ± 0.22 c |
| G2 | 95.69 | 2.42 ± 0.00 | 17.48 ± 0.07 | |
| G3 | 95.60 | 2.10 ± 0.01 | 18.74 ± 0.05 | |
| 2 | G1 | 95.32 | 1.67 ± 0.01 | 19.16 ± 0.10 d |
| G2 | 95.37 | 2.52 ± 0.01 | 18.59 ± 0.33 | |
| G3 | 95.32 | 1.75 ± 0.05 | 18.58 ± 0.04 | |
| 3 | G1 | 95.29 | 1.27 ± 0.01 | 19.36 ± 0.04 c |
| G2 | 95.15 | 2.27 ± 0.01 | 16.85 ± 0.56 | |
| G3 | 95.10 | 1.49 ± 0.03 | 18.13 ± 0.07 | |
| 4 | G1 | 95.55 | 1.42 ± 0.01 | 18.06 ± 0.13 d |
| G2 | 95.57 | 2.03 ± 0.01 | 17.71 ± 0.19 | |
| G3 | 95.02 | 1.76 ± 0.09 | 18.05 ± 0.04 | |
| 24 | G1 | 95.48 | 1.32 ± 0.02 | 18.23 ± 0.29 d |
| G2 | 95.72 | 1.42 ± 0.02 | 18.01 ± 0.46 | |
| G3 | 94.90 | 1.25 ± 0.05 | 17.73 ± 0.06 | |
| 48 | G1 | 94.39 | 1.21 ± 0.01 | 21.25 ± 0.19 a |
| G2 | 94.17 | 1.21 ± 0.01 | 18.86 ± 0.96 | |
| G3 | 94.62 | 1.37 ± 0.05 | 17.64 ± 0.01 | |
| 72 | G1 | 94.42 | 1.08 ± 0.02 | 20.27 ± 0.18 b |
| G2 | 94.60 | 1.05 ± 0.01 | 18.39 ± 0.70 | |
| G3 | 94.42 | 1.39 ± 0.03 | 17.50 ± 0.01 | |
| 96 | G1 | 94.12 | 0.76 ± 0.04 | 19.29 ± 0.02 c |
| G2 | 94.26 | 0.34 ± 0.04 | 18.25 ± 0.78 | |
| G3 | 94.00 | 1.10 ± 0.02 | 17.11 ± 0.04 | |
| 120 | G1 | 94.02 | 0.67 ± 0.01 | 18.69 ± 0.00 c,d |
| G2 | 94.00 | 0.43 ± 0.03 | 20.04 ± 0.02 | |
| G3 | 94.01 | 1.26 ± 0.06 | 17.56 ± 0.42 |
| This Study | Loh 2017 [54] | ||
|---|---|---|---|
| Element (wt, %) | POME 0 h | POME 48 h | POME |
| Carbon, C * | 48.60 | 49.70 | 50.01 |
| Oxygen, O * | 37.80 | 36.30 | 46.90 |
| Potassium, K | 22.67 ± 0.65 | 21.44 ± 0.81 | 1.85 |
| Chlorine, Cl | 2.68 ± 0.08 | 2.43 ± 0.07 | 0.10 |
| Silica, Si | ND | ND | 39.60 |
| Calcium, Ca | 2.99 ± 0.07 | 2.92 ± 0.09 | - |
| Manganese, Mn | 0.10 ± 0.01 | 0.10 ± 0.01 | - |
| Iron, Fe | 2.40 ± 0.06 | 2.38 ± 0.06 | 0.05 |
| Zinc, Zn | 0.04 ± 0.00 | 0.04 ± 0.00 | - |
| Bromine, Br | 0.03 ± 0.00 | 0.04 ± 0.00 | - |
| Strontium, Sr | 0.02 ± 0.00 | 0.02 ± 0.00 | - |
| Ruthenium, Ru | 3.08 ± 0.20 | 3.30 ± 0.14 | - |
| Rhodium, Rh | 4.34 ± 0.18 | 4.64 ± 0.08 | - |
| Palladium, Pd | 3.86 ± 0.16 | 4.05 ± 0.07 | - |
| CEV (MJ/kg) | 18.04 ± 0.05 | 21.25 ± 0.19 | 16.99 ± 0.58 |
| Parameters | G1 | G2 | G3 | |||
|---|---|---|---|---|---|---|
| Fermentation time (hours) | 0 H | 48 H | 0 H | 120 H | 0 H | 1 H |
| Oil and grease content (%) | 13.93 ± 0.03 | 17.95 ± 0.02 | 13.95 ± 0.14 | 12.03 ± 0.02 | 13.90 ± 0.02 | 15.03 ± 0.08 |
| CEV (MJ/kg) | 18.04 ± 0.05 | 21.25 ± 0.19 | 17.78 ± 0.02 | 20.04 ± 0.02 | 18.10 ± 0.02 | 18.74 ± 0.05 |
| DOE | This Study | ||||||
|---|---|---|---|---|---|---|---|
| Parameters (mg/L) | [61] | G1 | G3 | ||||
| Discharge Effluent Limit | Before Treatment | After Treatment | Removal Efficiency (%) | Before Treatment | After Treatment | Removal Efficiency (%) | |
| BOD | 100 | 99.33 | 351.30 | 71.73 | 109.33 | 325.30 | 66.39 |
| COD | - | 24,080 | 11,840 | 50.83 | 25,950 | 20,790 | 19.88 |
| TSS | 400 | 10,620 | 6054 | 42.99 | 10,330 | 6101 | 40.93 |
| Strain | Substrate | Fermentation Condition | Treatment Duration (days) | COD Removal (%) | BOD Removal (%) | TSS Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Lysinibacillus sp. | POME | Batch/Aerobic | 5 | 50.83 | 71.73 | 42.99 | This study |
| L. loumbei sp. | Cassava leaves | Alkaline | 2 | NA | NA | NA | [45] |
| Lysinibacillus sp. 3HHx | NA | Submerged | 30 | NA | NA | NA | [62] |
| B. cereus MF661883 | POME (50%, v/v) | Batch/Aerobic | 6 | 79.35 | 72.65 | 65.91 | [9] |
| B. cereus 103 PB and B. subtilis 106 PB | Raw POME | Batch/Aerobic | 5 | 90.64 | 93.11 | NA | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, S.; Baidurah, S.; Kamimura, N.; Matsuda, S.; Bakar, N.A.S.A.; Muhamad, N.N.I.; Ahmad, A.H.; Dominic, D.; Kobayashi, T. Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel. Sustainability 2021, 13, 11915. https://doi.org/10.3390/su132111915
Mohammad S, Baidurah S, Kamimura N, Matsuda S, Bakar NASA, Muhamad NNI, Ahmad AH, Dominic D, Kobayashi T. Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel. Sustainability. 2021; 13(21):11915. https://doi.org/10.3390/su132111915
Chicago/Turabian StyleMohammad, Sharifah, Siti Baidurah, Naofumi Kamimura, Seitaro Matsuda, Nurul Alia Syufina Abu Bakar, Nik Nur Izzati Muhamad, Aizat Hisham Ahmad, Debbie Dominic, and Takaomi Kobayashi. 2021. "Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel" Sustainability 13, no. 21: 11915. https://doi.org/10.3390/su132111915
APA StyleMohammad, S., Baidurah, S., Kamimura, N., Matsuda, S., Bakar, N. A. S. A., Muhamad, N. N. I., Ahmad, A. H., Dominic, D., & Kobayashi, T. (2021). Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel. Sustainability, 13(21), 11915. https://doi.org/10.3390/su132111915






