Abstract
The removal of benzene, toluene and p-xylene (BTX) from water is necessary to avoid various health and environmental concerns. Among various techniques, adsorption is suitable and widely used for the removal of BTX from water. In this study, the adsorption of BTX from water was performed using carbon nanotubes that were impregnated with zinc oxide nanoparticles. The impregnation was performed using the wet impregnation technique. The synthesized materials were characterized using scanning electron microscopy (SEM), energy dispersive X-ray (EDX), X-ray diffraction (XRD) spectroscopy, thermogravimetric analysis (TGA) and nitrogen adsorption–desorption analysis. In batch adsorption experiments, the effect of adsorbent dosage, initial concentration, and contact time were investigated. The percentage removal for a given time and dosage was in the order of p-xylene > toluene > benzene. The kinetics models’ fitting revealed that the pseudo-second-order model fits well the adsorption of benzene, toluene and p-xylene with R2 > 99.4%. The results of adsorption isotherm fitting revealed the best fit with Sips isotherm model (R2 > 99.7%) and the adsorption capacity was p-xylene: 125 mg/g > toluene: 105 mg/g > benzene: 70 mg/g. This behavior is observed probably due to a decrease in solubility and an increase in the molecular weight of BTX.
1. Introduction
The world population is increasing day by day, and industries are also expanding to meet various human needs. Benzene, toluene and p-xylene (BTX) are the most widely used chemicals as a feedstock in petrochemical industries, along with other uses such as solvent, petroleum and gasoline [1,2]. All petrochemical industries use large amounts of water, which can be contaminated with these hazardous compounds. These compounds are also included in water, air and soil due to leakages from storage tanks, pipelines and transportation systems. These compounds are dangerous for humans as well as aquatic life and are classified as hazardous chemicals by the US environmental protection agency (USEPA) and World Health Organization (WHO). Benzene is highly carcinogenic, while toluene and p-xylene can cause some diseases of kidneys, skin and eyes upon longer exposure [3]. BTX are highly volatile in nature, and it is essential to eliminate these toxic materials from the water before draining the water into the environment [4,5,6].
Various methods are studied for the removal of BTX from water, which includes adsorption [7,8,9], advanced oxidation [10], photocatalytic degradation [11] and biological treatments [12,13]. All these methods have some drawbacks (cost, efficiency and durability), but adsorption is mostly used for industrial applications. Adsorption is the cheapest, promising and widely practiced technique because of the possibility to recover the adsorbent and adsorbate [9,14]. The traditional adsorbents, such as activated carbon and fly ash, are lacking in some aspects, such as low adsorption capacity and regeneration difficulties [5,15].
Since its first discovery by Iijima, carbon nanotubes (CNTs) have attracted tremendous attention as a result of their interesting physical, chemical, electrical and structural properties [16]. The high surface area of the CNTs gives them a great advantage in the removal of many contaminants. Carbon nanotubes have been used in many studies for water treatment. Raw and surface modified CNTs provided very good results for the removal of various contaminants especially various organic chemicals [17,18,19,20]. Still, there are challenges associated with the use of CNTs for practical applications due to their hydrophobic nature and higher production cost. The CNTs surface was modified with various functional groups and metal oxide nanoparticles to increase dispersion and hydrophilicity [21,22]. An increase in dispersion of CNTs can expose more surfaces for the π–π stacking of organic molecules with CNTs. An increase in the removal efficiency of various pollutants was observed with the impregnation of CNTs with metal oxides [23,24]. Therefore, in this study, CNTs were impregnated with zinc oxide nanoparticles to improve the deagglomeration of CNTs and enhance the adsorption capacity for the removal of BTX.
The objective of this study was to evaluate the adsorption capacity of zinc oxide impregnated CNTs for the removal of BTX from water. The wet impregnation technique was used to impregnate CNTs with zinc oxide nanoparticles. The CNTs were then characterized using SEM, XRD and TGA. In order to optimize the adsorption parameters and study the kinetics and isotherms modeling of experimental data, batch adsorption experiments were performed.
2. Materials and Methods
2.1. Materials
Raw CNTs (purity > 95%, outer diameter: 20–30 nm, length: 10–20 µm) were purchased from Chengdu Chemicals, China. Analytical grade zinc nitrate, ethanol, benzene, toluene and p-xylene were purchased from Sigma-Aldrich and used without any further treatment. The wet impregnation technique was used for the impregnation of the raw carbon nanotubes with zinc oxide nanoparticles, as described elsewhere [1,2].
2.2. Characterizations of Adsorbent
Synthesized materials were characterized using different techniques to obtain information about surface morphology, thermal stability, surface area and pore size analysis.
The analysis of morphology and structure of zinc oxide impregnated CNTs was performed at 20 KV using a Scanning Electron Microscope (SEM Model: TESCAN MIRA 3 FEG-SEM). Quorum sputtering coater (Model: Q150R S) was used to coat the samples with a 5 nm thick layer of platinum before morphological and elemental analysis. The elemental analysis of the adsorbent material was performed using an Energy Dispersive X-ray (EDX). A TA instrument (Model: SDTQ600) was provided to collect the data about thermal degradation and purity of the material and used to perform a thermo-gravimetric analysis (TGA) of the adsorbent. The sample was heated from 30 to 900 °C with a heating rate of 10 °C/min and an airflow rate of 100 mL/min. X-ray diffraction (XRD) measurements of the material that provide the information about the presence of different phases of materials were performed, using an XRD (Model: Bruker D8 Advance) equipped with Cu Kα radiation source (40 kV, 20 mA). A scan rate of 1° min−1 over an angle of 10–80° was used. An automatic volumetric adsorption analyzer (Model: ASAP 2020, Micromeritics, USA) was used to carry out nitrogen adsorption–desorption at 77 K to determine the porosity and the surface area of the materials. Prior to isotherm measurement, degassing of the samples was performed at 300 °C under vacuum conditions. Brunauer–Emmett–Teller (BET) isotherm was used to calculate the surface area (SBET) of the synthesized materials. The Barret–Joyner–Halenda (BJH) model was applied to the adsorption isotherms to determine the total pore volume and pore size distribution of the materials.
2.3. Batch Adsorption Experiments and Concentration of Adsorbate
Adsorption of BTX from the water was conducted using batch adsorption experiments. A stock solution was prepared in deionized water separately for all pollutants. For contact time experiments, an adsorbent of 50 mg was added to glass flasks and filled with a solution of adsorbate and placed on a mechanical shaker for different time intervals of 4–6 h for various components as per preliminary experiments results. In order to observe the effect of adsorbent dosage, glass flasks were filled with different amounts of adsorbent, filled with adsorbate solutions and shaken for 2 h. The initial concentration of benzene was varied from 10 to 100 ppm, for toluene from 10 to 70 ppm and for p-xylene from 10 to 100 ppm. After the shaking was complete, samples were filtered using Whatman filter No 41, and concentration was analyzed using GC-MS and GC-FID.
A headspace autosampler was used for the analysis of benzene below 1 ppm, and the sample was injected in the column at 250 °C and heated from 50 to 200 °C with a heating rate of 35 °C/min. In the GC-FID system sample of 1 µL was injected into the column. Both adsorption capacity and percentage removal were calculated using Equations (1) and (2), respectively,
where: “Co” is the initial concentration (ppm) at time zero, and “Ct” is the concentration at any time “t” (ppm). “V” is the solution volume (L), and “m” represents the amount (g) of the adsorbent dosage.
3. Results
3.1. Characterization of Zinc Oxide Impregnated CNTs
The SEM micrograph and EDX spectrum of zinc oxide impregnated CNTs can be seen in Figure 1. Tubular morphology of CNTs entangled in groups can be observed in SEM images. CNTs are usually agglomerated as a result of the van der Waals forces among nanotubes. It was also revealed that nanoparticles are attached to the surface of CNTs and properly distributed among the whole network, while some aggregation of particles was also noted (shown in large circles). EDX spectra (Figure 1b) revealed the presence of zinc oxide along with the main constituent of carbon and a very small amount of nickel which is already added as a catalyst for the growth of CNTs.
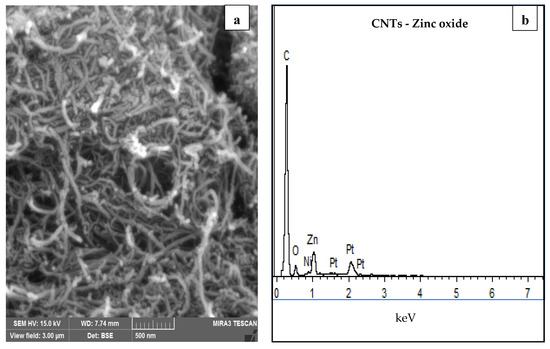
Figure 1.
(a) SEM micrograph (zinc oxide circled in red) and (b) EDX analysis spectra of zinc oxide impregnated CNTs.
Figure 2 indicates the nitrogen adsorption–desorption isotherm of zinc oxide impregnated CNTs. The shape of the isotherm was classified as Type II according to IUPAC classification. After a very small increase in adsorption of N2 up to relative pressure (p/po) of 0.9, isotherm displays a sharp increment after 0.9, indicating the largely mesoporous nature of CNTs. A small closed hysteresis loop was also observed due to capillary condensation inside mesoporous structure and is of type H3. Table 1 summarizes the results of surface area, pore size and pore volume of sample. The inset plot of Figure 2 provides the distribution of pore size, which provides the bimodal distribution of micropore and a large range of mesopores resulting due to aggregation of CNTs.
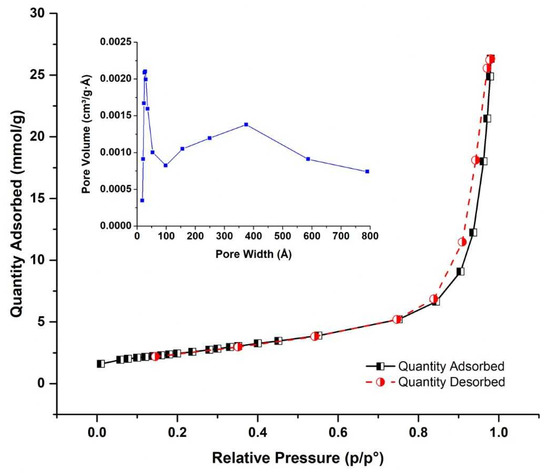
Figure 2.
Nitrogen adsorption–desorption of zinc oxide impregnated CNTs. The inset plot provides the distribution of pore size.

Table 1.
Surface and structural properties of the sample.
Figure 3 represents the XRD diffractogram of the sample. The peaks at a diffraction angle of 26.1 and 43.2 represent the graphitic carbon structure of CNTs. The diffraction peaks located at angles of 32°, 35°, 37°, 57° and 63° are an indication of zinc oxide in the sample [25]. Therefore, it confirms the presence of CNTs and ZnO in the sample with no other significant impurities.
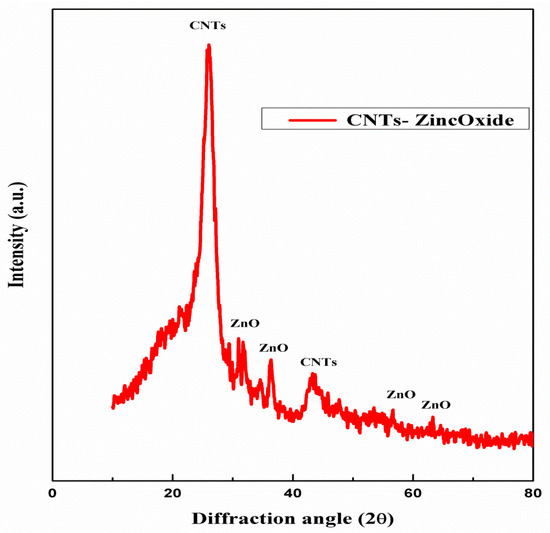
Figure 3.
XRD pattern of zinc oxide impregnated CNTs.
Figure 4 illustrates the TGA curve for zinc oxide impregnated CNTs. By heating the sample initially, around 2–3% loss up to 150 °C is attributed to physically bound water [22]. On further heating, degradation of CNTs started at 450 °C. All CNTs burned in the presence of air up to 610 °C, and at the end of the experiment, the amount of residue left was 7%, which represents the zinc oxide nanoparticles impregnated at CNTs surface.
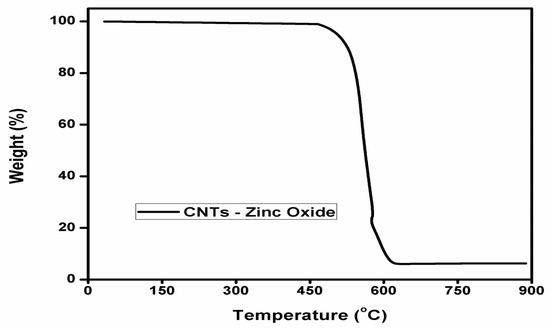
Figure 4.
TGA curve for zinc oxide impregnated CNTs.
3.2. Adsorption Experiments
3.2.1. Effect of Contact Time
Figure 5 depicts the contact times effect on the BTX adsorption as individual components from water using zinc oxide impregnated CNTs. For all adsorbates, different behavior was observed. Initially, adsorption was of the order of p-xylene > toluene > benzene. This is associated with increase in molecular weight (B: 78 g/mol < T:92 g/mol < p-X: 106 g/mol), decrease in water solubility (B: 790 mg/L > T: 530 mg/L > p-X: 150.5 mg/L) and increase in boiling point (B: 80.1 °C < T: 110.7 °C < p-X: 138 °C) [6,26]. A rapid increase in the removal of BTX is initially attributed to the presence of abundant active sites and large surface area of adsorbent, while a further increase in contact time may not conspicuously affect the adsorption rate. Around 14% increase in p-xylene adsorption was observed after 4 h, while for toluene and benzene there was a significant increase. The order to achieve equilibrium was p-xylene > toluene > benzene, explained by the same phenomena of solubility, boiling point and molecular weight.
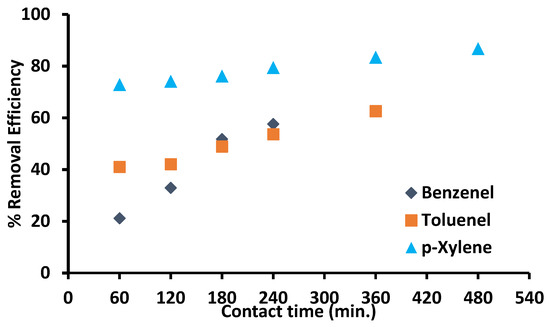
Figure 5.
Effect of contact time on adsorption of BTX (adsorbent dosage: 50 mg/100 mL, initial concentration of benzene: 1 ppm, initial concentration of toluene and p-xylene: 100 ppm each separately, shaking speed: 200 rpm, initial pH: 6).
3.2.2. Effect of Adsorbent Dosage
Figure 6 explains the effect of variation in adsorbent dosage on the adsorption of BTX from water. For benzene and toluene, the removal efficiency increased with increasing adsorbent dosage. This rise with increasing dosage is based on the increase in the number of available active adsorption sites. For p-xylene, increasing dosage does not significantly improve the adsorption, and removal increased only 5% with an increasing amount of adsorbent from 25 mg to 100 mg. On the other hand, with increasing the amount of adsorbent above 25 mg, removal of benzene and toluene increased and reached a maximum of 50% with 100 mg. Benzene removal did not increase significantly (only 7%) after 50 mg because adsorption sites were not occupied due to equilibrium, while for toluene, it increased about (27%). The percentage removal of benzene was higher than toluene initially because the initial concentration of benzene was low (1 ppm) as compared to toluene (100 ppm).
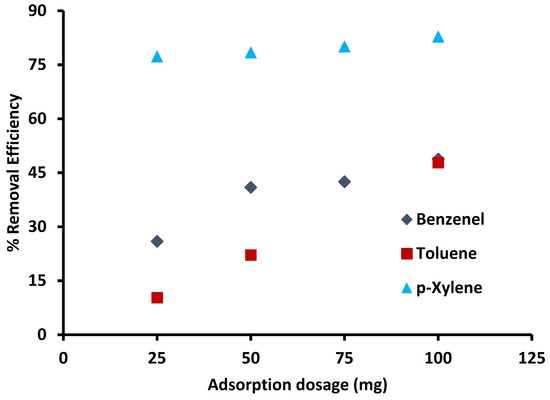
Figure 6.
Effect of adsorbent dosage on adsorption of BTX (contact time: 2 h, initial concentration of benzene: 1 ppm, initial concentration of toluene and p-xylene: 100 ppm, shaking speed: 200 rpm, initial pH: 6).
3.2.3. Kinetics Models Fitting
Kinetics data obtained for adsorption of BTX on zinc oxide impregnated CNTs is fitted with reaction-based (pseudo-first- and pseudo-second-order models) and diffusion-based (intraparticle diffusion model) kinetics models.
Pseudo-first-order, pseudo-second-order and Weber–Morris intra-particle diffusion models are represented by Equations (3)–(5) respectively,
where qt (mg/g) and qe (mg/g) are the concentrations of contaminants on the surface of adsorbent at time t (min) and at equilibrium, respectively. k1 (min−1) is pseudo-first-order model constant; k2 (g mg−1 min−1) is the second-order-model constant, and kid is the intraparticle diffusion model. C is the intraparticle model constant related to adsorption capacity. Figure 7 represents the fitting of kinetic data, and the results of fitting are tabulated (Table 2).
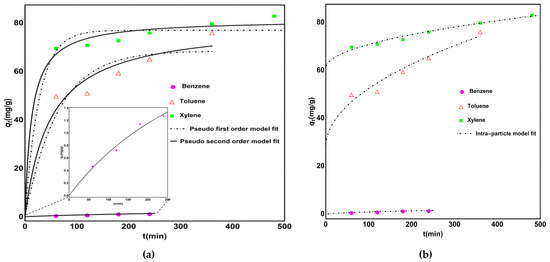
Figure 7.
(a) Kinetics model fitting using pseudo-first-order, pseudo-second-order and (b) intraparticle diffusion model for adsorption of BTX.

Table 2.
Kinetic parameter models fittings for adsorption of benzene, toluene and p-xylene using zinc oxide impregnated CNTs.
The analysis showed that the pseudo-second-order model fits best with the adsorption data of all adsorbates. The values of R2 were found between 99.4% and 99.8%, and the results of “qe” calculated were close to the experimental values using this model.
The intraparticle diffusion model was also suitable to describe the adsorption behavior of all adsorbates, with correlation coefficients varying between 99.7% and 99.9%. This indicates that BTX molecules diffused into the mesoporous matrix of CNTs bundles. Values of constant kid indicate that it is not the sole step for controlling the adsorption.
Overall adsorption kinetics mechanism involves three steps. Initially, adsorption occurs on the external surface. Then, intraparticle diffusion becomes the rate-limiting step where gradual adsorption occurs. In the final and third steps, the system moves to the equilibrium stage as intraparticle diffusion slows down due to decreased adsorbate concentration in the solution.
3.2.4. Isotherm Models Fitting
The analysis of equilibrium data for the adsorption of BTX on zinc oxide impregnated CNTs was performed using Freundlich, Sips and Dubinin-Redlishwang (D-R) models. The following equation represents the non-linear form of these models.
Freundlich isotherm model
Sips isotherm model
D-R isotherm model
where Ce (mg/L) and qe (mg/g) are the concentrations of contaminants in water and on the surface of adsorbent at the adsorption equilibrium, respectively. qm (mg/g) is the maximum adsorption capacity; KF (mg/g)/(mg/L)n Freundlich constants related to the adsorption capacity; KS (L/mg) is the adsorption equilibrium constant of Sips model; while n is the surface heterogeneity of the adsorbent in both Freundlich and Sips isotherm model. The value of “n” close to 1 indicates the homogenous surface. These constants describe the surface properties and adsorption capacity of the adsorbent. The fitting of data with isotherm models can be seen in Figure 8, while Table 3 shows the adsorption parameters and regression data of the models. Values of the regression coefficient (R2) represent that the Sips isotherm model fits best with the adsorption data compared to the Freundlich model for all pollutants. The Sips model is a combination of limited adsorption and nonhomogeneous surface adsorption of the Langmuir and Freundlich isotherm models. Adsorption capacity from the estimated suing Sips model is of the order of p-xylene > toluene > benzene, which can be explained based on the increase in molecular weight and decrease in their solubility in water. It can be noticed from the results that the values of rate constants KS and KF were higher for p-xylene when comparing them to the values for both benzene and toluene. This can be ascribed to low solubility and higher hydrophobicity of p-xylene. The lower solubility of P-xylene in water may be beneficial in providing more attraction towards the surface of CNTs and the fast adsorption rate. In all cases, values of “n” are close to 1, which indicates the suitable and consistent adsorption of benzene, toluene and p-xylene. D-R isotherm model fitting was used to calculate the activation energy “Ea” (kJ/mol). The values of the activation energy were found to be less than 1 kJ/mole. This implies the physical adsorption of BTX molecules on the surface of the adsorbent. This phenomenon can be helpful in the easy regeneration of adsorbents for reuse.
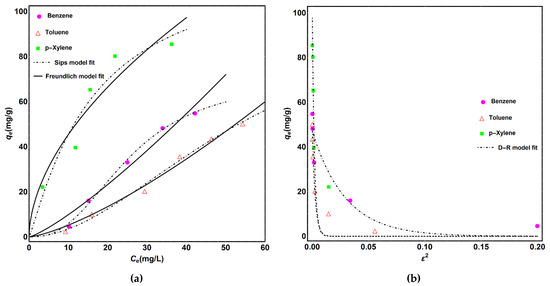
Figure 8.
(a) Isotherm model fitting of BTX adsorption data with Sips, Freundlich and (b) D-R isotherm models.

Table 3.
Isotherm models parameters for toluene and p-xylene adsorption.
4. Conclusions
In this study, the wet impregnation technique was used to impregnate CNTs with zinc oxide nanoparticles. The SEM, EDX, TGA, XRD and nitrogen adsorption techniques were used to characterize materials. The material was used for the removal of BTX from water in batch adsorption experiments. The removal of components was in the order of p-xylene > toluene > benzene after 4 h. The removal of p-xylene was not significantly increased by enhancing by the amount of adsorbent above 25 mg, while toluene and benzene removal increased 27% and 20%, respectively. The pseudo-first-order model was found to fit best for the experimental data of BTX. The Sips isotherm model described the best adsorption data of BTX. The results show that this is a promising method that can be enhanced to be used for the removal of BTX from industrial wastewater.
Funding
This research received no external funding.
Acknowledgments
The author is thankful to the Department of Chemical Engineering, King Fahd University of Petroleum and Minerals, for providing the support to conduct this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbas, A.; Abussaud, B.A.; Ihsanullah, I.; Al-Baghli, N.A.H.; Khraisheh, M.; Atieh, M.A. Benzene Removal by Iron Oxide Nanoparticles Decorated Carbon Nanotubes. J. Nanomater. 2016, 2016, 5654129. [Google Scholar] [CrossRef]
- Abbas, A.; Abussaud, B.A.; Ihsanullah, I.; Al-Baghli, N.A.H.; Redhwi, H.H. Adsorption of Toluene and Paraxylene from Aqueous Solution Using Pure and Iron Oxide Impregnated Carbon Nanotubes: Kinetics and Isotherms Study. Bioinorg. Chem. Appl. 2017, 2017, 2853925. [Google Scholar] [CrossRef]
- WHO. Benzene in Drinking-Water, WHO. 2016. Available online: https://www.who.int/water_sanitation_health/publications/benzene/en/ (accessed on 17 July 2019).
- ATSDR—Toxicological Profile: Benzene, (n.d.). Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=40&tid=14 (accessed on 17 July 2019).
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process. Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Impact of surface modification on adsorptive removal of BTX onto activated carbon. J. Mol. Liq. 2019, 280, 238–251. [Google Scholar] [CrossRef]
- Tournus, F.; Charlier, J.-C. Ab initiostudy of benzene adsorption on carbon nanotubes. Phys. Rev. B 2005, 71, 165421. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Hu, S. Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 353, 83–91. [Google Scholar] [CrossRef]
- Diao, R.; Zhang, H.; Zhao, D.; Li, S. Adsorption and structure of benzene, toluene, and p-xylene in carbon slit pores: A Monte Carlo simulation study. Chem. Eng. Sci. 2018, 197, 120–134. [Google Scholar] [CrossRef]
- Abussaud, B.A.; Ulkem, N.; Berk, D.; Kubes, G.J. Wet Air Oxidation of Benzene. Ind. Eng. Chem. Res. 2008, 47, 4325–4331. [Google Scholar] [CrossRef][Green Version]
- Bahmani, M.; Bitarafhaghighi, V.; Badr, K.; Keshavarz, P.; Mowla, D. The photocatalytic degradation and kinetic analysis of BTEX components in polluted wastewater by UV/H2O2-based advanced oxidation. Desalination Water Treat. 2013, 52, 3054–3062. [Google Scholar] [CrossRef]
- Xiong, W.; Mathies, C.; Bradshaw, K.; Carlson, T.; Tang, K.; Wang, Y. Benzene removal by a novel modification of enhanced anaerobic biostimulation. Water Res. 2012, 46, 4721–4731. [Google Scholar] [CrossRef]
- Shim, H.; Ma, W.; Lin, A.; Chan, K. Bio-removal of mixture of benzene, toluene, ethylbenzene, and xylenes/total petroleum hydrocarbons/trichloroethylene from contaminated water. J. Environ. Sci. 2009, 21, 758–763. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Chen, X.; Zheng, J.; Wang, G.; Liang, G. Insights into the adsorption of simple benzene derivatives on carbon nanotubes. RSC Adv. 2014, 4, 58036–58046. [Google Scholar] [CrossRef]
- Gauden, P.; Terzyk, A.; Rychlicki, G.; Kowalczyk, P.; Lota, K.; Raymundo-Pinero, E.; Frackowiak, E.; Beguin, F. Thermodynamic properties of benzene adsorbed in activated carbons and multi-walled carbon nanotubes. Chem. Phys. Lett. 2006, 421, 409–414. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Al Amer, A.M.; Laoui, T.; Abbas, A.; Al-Aqeeli, N.; Patel, F.; Khraisheh, M.; Atieh, M.A.; Hilal, N. Fabrication and antifouling behaviour of a carbon nanotube membrane. Mater. Des. 2016, 89, 549–558. [Google Scholar] [CrossRef]
- Ihsanullah; Laoui, T.; Al-Amer, A.M.; Khalil, A.B.; Abbas, A.; Khraisheh, M.; Atieh, M.A. Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane. Desalination 2015, 376, 82–93. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Asmaly, H.A.; Saleh, T.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Zarin, S.; Aslam, Z.; Zahir, A.; Kamal, M.S.; Rana, A.G.; Ahmad, W.; Ahmed, S. Synthesis of bimetallic/carbon nanocomposite and its application for phenol removal. J. Iran. Chem. Soc. 2018, 15, 2689–2701. [Google Scholar] [CrossRef]
- Aslam, Z.; Qaiser, M.; Ali, R.; Abbas, A.; Ihsanullah, I.; Zarin, S. Al2O3/MnO2/CNTs nanocomposite: Synthesis, characterization and phenol adsorption. Full-Nanotub. Carbon Nanostructures 2019, 27, 591–600. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Patel, F.; Al-Baghli, N.; Abussaud, B.; Tawabini, B.S.; Laoui, T. A Comparative Study of Raw and Metal Oxide Impregnated Carbon Nanotubes for the Adsorption of Hexavalent Chromium from Aqueous Solution. Bioinorg. Chem. Appl. 2017, 2017, 1624243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Saleh, T.A. Syntheses of Carbon Nanotube-Metal Oxides Composites, Adsorption and Photo-degradation; Bianco, D.S., Ed.; InTech: London, UK, 2011; pp. 295–312. Available online: https://www.intechopen.com/chapters/16834 (accessed on 17 July 2019).
- Saravanan, R.; Thirumal, E.; Gupta, V.; Narayanan, V.; Stephen, A. The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. J. Mol. Liq. 2012, 177, 394–401. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Appusamy, A.; Gnanasundaram, N.; Thanabalan, M. Surface modification and characterization of carbonaceous adsorbents for the efficient removal of oil pollutants. J. Hazard. Mater. 2019, 379, 120673. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).