Morphometric and Structural Properties of a Sustainable Plant Biomass with Water Purification Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Light Microscopy
2.2. Structural and Surface Characteristics
2.3. Water Purification, Wettability, and Sorption Studies
3. Results and Discussion
3.1. Morphometric, Structural, and Surface Properties
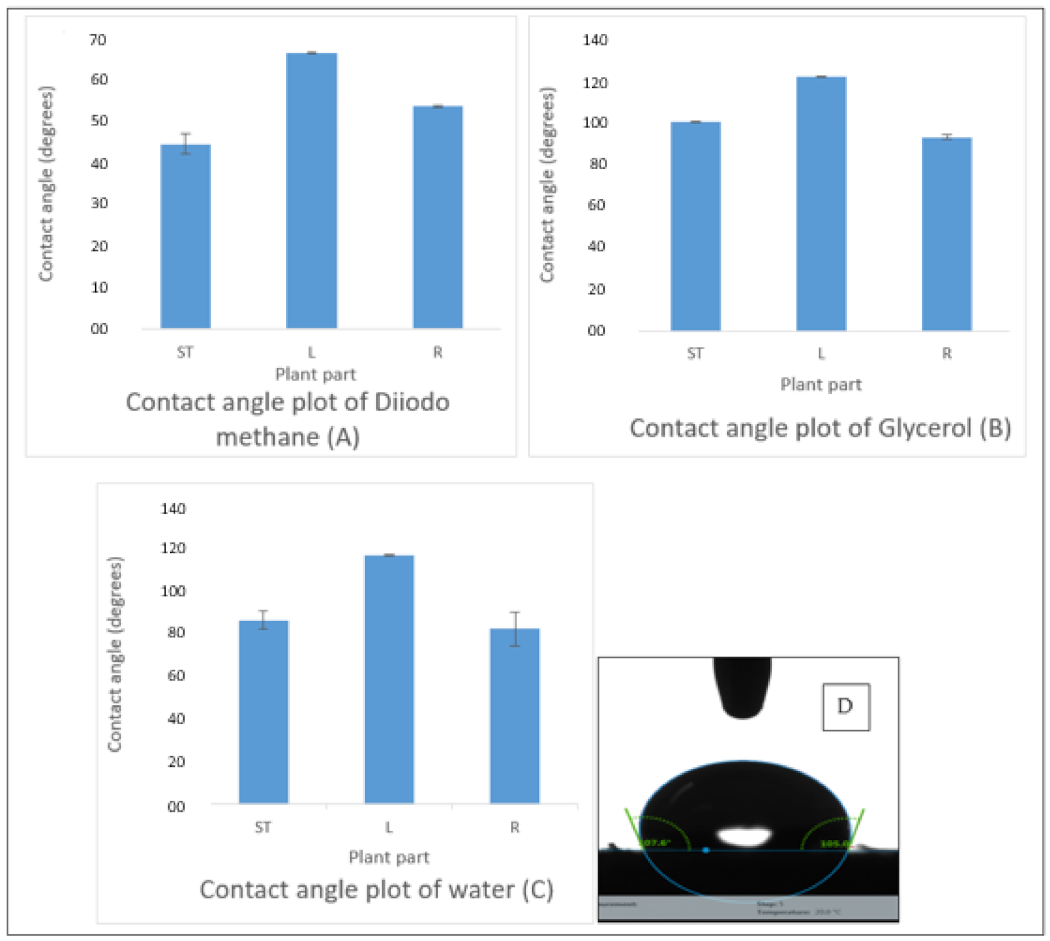
3.2. Drop Shape Analysis and Sorption Properties
3.3. Purification of Methylene Blue Dye in Polluted Water
3.4. Comparative Study Sorption and Functional Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baysal, M.; Bilge, K.; Yılmaz, B.; Papila, M.; Yürüm, Y. Preparation of high surface area activated carbon from waste-biomass of sunflower piths: Kinetics and equilibrium studies on the dye removal. J. Environ. Chem. Eng. 2018, 6, 1702–1713. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; Mosbah, M.B.; Salem, R.B.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Adiguzel, N.; Ersoy, G.; Yilmaz, M.; Arica, M.Y. Removal of Textile Dyes from Aqueous Solution using Amine-Modified Plant Biomass of A. caricum: Equilibrium and Kinetic Studies. Water Air Soil Pollut. 2013, 224, 1640. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Sinha, S.; Gosh, D. Removal of pyridine from aqueous solution using low cost activated carbons derived from agricultural waste materials. Carbon 2004, 42, 2409–2421. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Celik, G.; Arica, M.Y. Biosorption of Reactive Blue 4 dye by native and treated fungus Phanerocheate chrysosporium: Batch and continuous flow system studies. J. Hazard. Mater. 2006, 137, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Arica, M.Y. Preparation of a composite biosorbent using Scenedesmus quadricauda biomass and alginate/polyvinyl alcohol for removal of Cu(II) and Cd(II) ions: Isotherms, kinetics, and thermodynamic studies. Water Air Soil Pollut. 2011, 221, 391–403. [Google Scholar] [CrossRef]
- Trevisan, C.W.; Foletto, E.L.; Meili, L. Removal of tannery dye from aqueous solution using papaya seed as an efficient natural biosorbent. Water Air Soil Pollut. 2013, 224, 1427. [Google Scholar]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.M.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Akar, T.; Ozcan, A.S.; Tunali, S.; Ozcan, A. Biosorption of a textile dye (Acid Blue 40) by cone biomass of Thuja orientalis: Estimation of equilibrium, thermodynamic and kinetic parameters. Bioresour. Technol. 2008, 99, 3057–3065. [Google Scholar] [CrossRef]
- Krishnan, K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef]
- Safa, Y.; Bhatti, H.N. Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination 2011, 272, 313–322. [Google Scholar] [CrossRef]
- Gercel, O.; Gercel, H.F.; Koparal, A.S.; Ogutveren, U.B. Removal of disperse dye from aqueous solution by novel adsorbent prepared from biomass plant material biomass of Thuja orientalis. J. Hazard. Mater. 2008, 160, 668–674. [Google Scholar] [CrossRef]
- Roy, A.; Chakraborty, S.; Kundu, S.P.; Adhikari, B.; Majumder, S.B. Adsorption of anionic-azo dye from aqueous solution by lignocellulose-biomass jute fiber: Equilibrium, kinetics, and thermodynamics study. Ind. Eng. Chem. Res. 2012, 51, 12095–12106. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Freitas, H. Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. Environ. Pollut. 2000, 110, 277–283. [Google Scholar] [CrossRef]
- Luseba, D.; Elgorashe, E.E.; Ntloedibe, D.T.; van-Staden, J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. S. Afr. J. Bot. 2007, 73, 378–383. [Google Scholar] [CrossRef]
- Madiga, M.C.; Cockeran, R.; Mokgotho, M.P.; Anderson, R.; Mampuru, L.J. Dichloromethane extract of Dicerocaryum senecioides leaves exhibits remarkable anti-inflammatory activity in human T-lymphocytes. Nat. Prod. Res. 2009, 23, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- More, G.K.; Tshikalange, T.E.; Lall, N.; Meyer, J.J.M.; Botha, F.S.; Hussein, A.A. Antimicrobial activity of medicinal plants against oral microorganisms. S. Afri. J.Bot. 2007, 73, 332. [Google Scholar] [CrossRef][Green Version]
- Odiyo, J.; Bassey, O.; Ochieng, A.; Chimuka, L. Coagulation efficiency of Dicerocaryum eriocarpum (DE) plant. Water SA 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Msagati, T.A.M.; Popoola, E.O. Removal Efficiency of Faecal Indicator Organisms, Nutrients and Heavy Metals from a Peri-Urban Wastewater Treatment Plant in Thohoyandou, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2015, 12, 7300–7320. [Google Scholar] [CrossRef]
- Bassey, O.J.; Odiyo, J.O.; Chimuka, L.; Aoyi, O.; Bridget, J.B. Application of mucilage from Dicerocaryum eriocarpum plant as biosorption medium in the removal of selected heavy metal ions. J. Environ. Manag. 2016, 177, 365–372. [Google Scholar]
- Odiyo, J.O.; Edokpayi, J.N. Physico-chemical and surface characterisation of a renewable low-cost biosorbent for the uptake of heavy metal ions from aqueous solution. WIT Trans. Ecol. Environ. 2018, 228, 317–327. [Google Scholar]
- Zemnukhova, L.A.; Panasenko, A.E.; Artem’yanov, A.P.; Tsoy, E.A. Dependence of porosity of amorphous silicon dioxide prepared from rice straw on plant variety.Silicon dioxide rice straw. BioResources 2015, 10, 3713–3723. [Google Scholar] [CrossRef]
- Krüss GmbH. DSA1 v 1.9 Drop Shape Analysis for DSA100. 2004. Available online: https://warwick.ac.uk/fac/cross_fac/sciencecity/programmes/internal/themes/am2/booking/dropshapeanalyser/kruss_manual-dsa100.pdf (accessed on 1 July 2021).
- Aranberri-Askargorta, I.; Lampke, T.; Bismarck, A. Wetting behavior of flax fibers as reinforcement for polypropylene. J. Colloid. Interface Sci. 2003, 263, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; Brasil, J.L.; da Cunha, B.M.; Simon, N.M.; Cardoso, N.F.; Noreña, C.P.Z. Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr(VI) from aqueous solution. Kinetics and equilibrium study. Biochem. Eng. J. 2008, 42, 67–76. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Odiyo, J.O. Biogenic Synthesis of Silver Nanoparticle From Mushroom Exopolysaccharides and Its Potentials in Water Purification. Open Chem. J. 2018, 5, 64–75. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.; Choi, S.-O.; Kim, J. Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Versieux, L.M.; Medeiros, A.S.M. Leaf anatomical characterization of Guzmania Ruiz & Pav. and Mezobromelia L.B.Sm. (Tillandsioideae, Bromeliaceae). J. Bromel. Soc. 2018, 67, 2018. [Google Scholar]
- Rungsung, W.; Dutta, S.; Mondal, D.N.; Ratha, K.K.; Hazra, J. Pharmacognostical Profiling on the Root of Rauwolfia Serpentina. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 612–616. [Google Scholar]
- Edokpayi, J.N.; Ndlovu, S.S.; Odiyo, J.O. Characterization of pulverized Marula seed husk and its potential for the sequestration of methylene blue from aqueous solution. BMC Chem. 2019, 13, 10. [Google Scholar] [CrossRef]
- Malucellia, L.; Massulob, T.; Magalhãesc, W.; Stofella, N.; Vasconcelosa, E.; Filho, M.; Murakamid, F. Thermal and chemical characterization of Dicksonia sellowiana extract by means of thermal analysis. Rev. Bras. Farmacogn. 2018, 28, 626–630. [Google Scholar] [CrossRef]
- Postai, D.L.; Demarchi, C.A.; Zanatta, F. Adsorption of rhodamine B and methylene blue dyes using waste of seeds of Aleurites Moluccana, a low-cost adsorbent. Alex. Eng. J. 2016, 55, 1713–1723. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ghorbani, G.R.; Khorvash, M.; Emam, M.R.; Karimi, K. Chemical composition, cell wall features and degradability of stem, leaf blade and sheath in untreated and alkali-treated rice straw. Animal 2013, 7, 1106–1112. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Elizalde-Gonzalez, M.P.; Mattusch, J.; Pelaez-Cid, A.A.; Wennrich, R. Characterization of adsorbent materials prepared from avocado kernel seeds: Natural, activated and carbonized forms. J. Anal. Appl. Pyrolysis 2007, 78, 185–193. [Google Scholar] [CrossRef]
- Zvinowanda, C.M.; Okonkwo, J.O.; Agyei, N.M.; Shabalala, P.N. Physicochemical characterization of maize tassel as an adsorbent. I. surface texture, microstructure and thermal stability. J. Appl. Polym. Sci. 2009, 111, 1923–1930. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Ribeiro, L.A.D.; Thim, G.P.; Ferreira, R.R.; Alvarez-Mendez, M.O.; Coutinho, A.D. Activated carbon derived from macadamia nut shells: An effective adsorbent for phenol removal. J. Porous Mater. 2013, 20, 619–627. [Google Scholar] [CrossRef]
- Guettler, B.E.; Moresoli, C.; Simon, L.C. Contact angle and surface energy analysis of soy materials subjected to potassium permanganate and autoclave treatment. Ind. Crops Prod. 2013, 50, 219–226. [Google Scholar] [CrossRef]
- Diversified Enterprises. Surface Energy Data for PP: Polypropylene, CAS #s9003-08-0 (Atactic) and 25085-53-4 (Isotactic). 2009, p. 3. Available online: http://www.accudynetest.com/polymer_surface_data/polypropylene.pdf (accessed on 1 July 2021).
- Paul, A.; Joseph, K.; Thomas, S. Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforcedwith short sisal fibers. Compos. Sci. Technol. 1997, 57, 67–79. [Google Scholar] [CrossRef]
- Shaabani, A.; Lee, D. Solvent free permanganate oxidations. Tetrahedron Lett. 2001, 42, 5833–5836. [Google Scholar] [CrossRef]
- Alix, S.; Philippe, E.; Bessadok, A.; Lebrun, L.; Morvan, C.; Marais, S. Effect of chemical treatments on water sorption and mechanical properties of flax fibres. Bioresour. Technol. 2009, 100, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, F.A.; Demirata, B.; Apak, R. Adsorptive removal of methylene blue from simulated dyeing wastewater with melamine-formaldehyde-urea resin. J. Appl. Polym. Sci. 2009, 112, 3442–3448. [Google Scholar] [CrossRef]
- Guo, C.; Kong, Q.; Gao, J.; Ji, Q.; Xia, Y. Removal Of methylene blue dye from simulated wastewater by alginic acid fiber as adsorbent: Equilibrium, kinetic, and thermodynamic studies. Can. J. Chem. Eng. 2011, 89, 1545–1553. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Krishnaiah, D.; Bono, A.; Ooi, L.C. Parametric and adsorption kinetic studies of methylene blue removal from simulated textile water using durian (Durio zibethinus murray) skin. Water Sci. Technol. 2015, 72, 896–907. [Google Scholar] [CrossRef]
- Garg, V.K.; Kumar, R.; Gupta, R. Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: A case study of Prosopis cineraria. Dyes Pigments 2004, 62, 1–10. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; da Cunha, B.M.; Cardoso, N.F.; Brasil, J.L.; Dias, S.L.P. Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solutions. J. Hazard. Mater. 2009, 162, 270–280. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; Cardoso, N.F.; Martins, B.; Calvete, T. Pecan nutshell as biosorbent to remove toxic metals from aqueous solution. Sep. Sci. Technol. 2009, 44, 615–644. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from the Brazilian-pine fruit shell for removal of Procion Red MX 3B from aqueous solution—kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2009, 155, 627–636. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Vaghetti, J.C.P.; Simon, N.M.; Calvete, T.; Veses, R.C. Applications of Brazilian-pine fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions—kinetic and equilibrium study. J. Hazard. Mater. 2009, 164, 1213–1222. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.P.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef]
- Hassan, W.; Farooq, U.; Ahmad, M.; Athar, M.; Khan, M.A. Potential biosorbent, Haloxylon recurvum plant stems, for the removal of methylene blue dye. Arab. J. Chem. 2017, 10, S1512–S1522. [Google Scholar] [CrossRef]
- Singh, R.; Singh, T.S.; Odiyo, J.O.; Smith, J.A.; Edokpayi, J.N. Evaluation of Methylene Blue Sorption onto Low-Cost Biosorbents: Equilibrium, Kinetics, and Thermodynamics. J. Chem. 2020, 2020, 8318049. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Krishni, R.R.; Foo, K.Y.; Hameed, B.H. Adsorptive removal of methylene blue using the natural adsorbent-banana leaves. Desalin. Water Treat. 2014, 52, 6104. [Google Scholar] [CrossRef]




| Parameter | Leaf | Stem | Root |
|---|---|---|---|
| BET surface area (m2/g) | 3.340 | 0.300 | 0.270 |
| Pore size (um) | 0.005 | 0.011 | 0.124 |
| Part | Surface Energy (mN/m) | POLAR (mN/m) | DISPERSE (mN/m) |
|---|---|---|---|
| Leaf | 27.24 | 3.71 | 23.53 |
| Stem | 33.32 | 0.86 | 32.45 |
| Root | 31.80 | 0.19 | 31.61 |
| Sample | Absorbance | Dye Conc (mg/L) | Sorption (%) |
|---|---|---|---|
| Leaf | 0.0695 | 0.48 | 95.2 |
| Stem | 0.0171 | 0.20 | 98.0 |
| Root | 0.0150 | 0.19 | 98.1 |
| Control | 1.8526 | 10.0 | - |
| Parts | Seed | Leaf | Stem | Root |
|---|---|---|---|---|
| Physiological function | Food storage | Water guttation, transpiration, and storage | Fluid conduction and mineral transportation | Water absorption and transportation |
| Surface area (m2/g) | 3.200 | 3.30 | 0.30 | 0.30 |
| Pore size (µm) | 0.005 | 0.005 | 0.010 | 0.120 |
| Surface energy (mN/m) | 28.03 | 27.24 | 33.32 | 31.80 |
| Polar composition (mN/m) | 3.57 | 3.71 | 0.86 | 0.19 |
| Fibre composition (%) | 49.0 | 3.0 | 37.0 | 42.0 |
| Dye removal (%) | 97.4 | 95.2 | 98.0 | 98.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeeyo, A.O.; Odiyo, J.O.; Enitan, A.M.; Motsa, M.M.; Msagati, T.A.M.; Mkoyi, H.D.; Makungo, R. Morphometric and Structural Properties of a Sustainable Plant Biomass with Water Purification Potentials. Sustainability 2021, 13, 11075. https://doi.org/10.3390/su131911075
Adeeyo AO, Odiyo JO, Enitan AM, Motsa MM, Msagati TAM, Mkoyi HD, Makungo R. Morphometric and Structural Properties of a Sustainable Plant Biomass with Water Purification Potentials. Sustainability. 2021; 13(19):11075. https://doi.org/10.3390/su131911075
Chicago/Turabian StyleAdeeyo, Adeyemi O., John O. Odiyo, Abimbola M. Enitan, Machawe M. Motsa, Titus A.M. Msagati, Hosana D. Mkoyi, and Rachel Makungo. 2021. "Morphometric and Structural Properties of a Sustainable Plant Biomass with Water Purification Potentials" Sustainability 13, no. 19: 11075. https://doi.org/10.3390/su131911075
APA StyleAdeeyo, A. O., Odiyo, J. O., Enitan, A. M., Motsa, M. M., Msagati, T. A. M., Mkoyi, H. D., & Makungo, R. (2021). Morphometric and Structural Properties of a Sustainable Plant Biomass with Water Purification Potentials. Sustainability, 13(19), 11075. https://doi.org/10.3390/su131911075








