Evaluation of the Ecological Benefits of Recycling Multiple Metals from Lithium Battery Saggars Based on Emergy Analysis
Abstract
:1. Introduction
2. Methods and Data
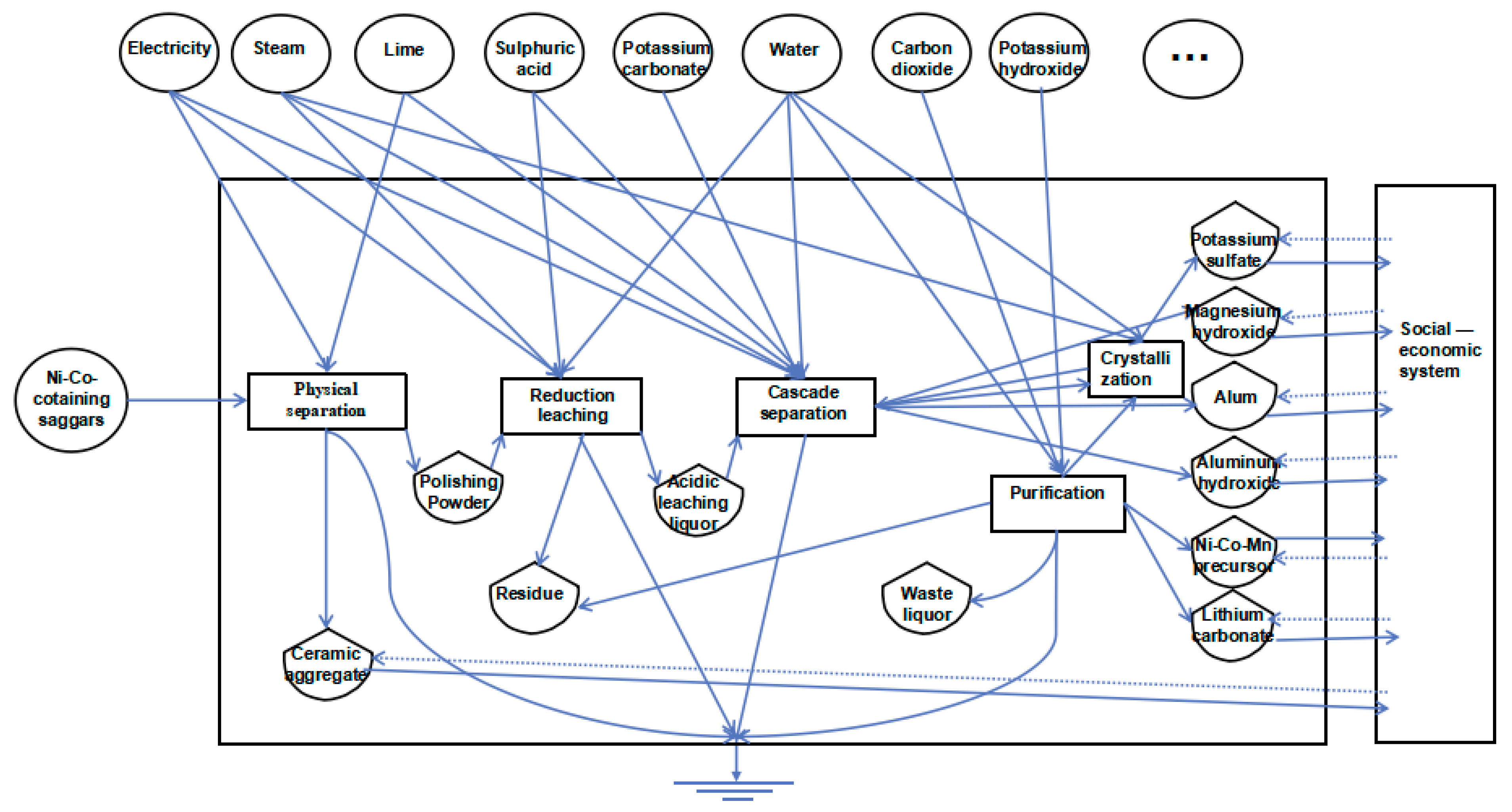
2.1. Overview of the Recycling Process of Ni–Co-Containing Saggars
2.2. Emergy Analysis Method
- (1)
- Data collection and emergy analysis table compilation
- (2)
- Emergy system diagram
- (3)
- Establishment of an emergy indicator system
- (4)
- System development evaluation and strategy analysis
2.3. Data Sources
3. Analysis of the Calculation Results
3.1. Economic Benefit Analysis
3.2. Emergy Analysis Results
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Process | Sort | Material | Standard | Unit | Amount | Emergy Transformity (sej/Unit) | Emergy (sej) |
|---|---|---|---|---|---|---|---|
| Physical separation | Input | Ni-Co-containing saggars | kg | 1000.00 | 1.21 × 1012 | 1.21 × 1015 | |
| Water | kg | 149.20 | 4.56 × 108 | 6.80 × 1010 | |||
| Electricity | kwh | 8.25 | 7.96 × 1011 | 6.57 × 1012 | |||
| Output | Ceramic aggregate | Moisture content 10% | kg | 977.80 | 1.21 × 1012 | 1.18 × 1015 | |
| Polishing powder | Moisture content 30% | kg | 171.40 | 0.00 | |||
| Reduction and leaching | Input | Polishing powder | Moisture content 30% | kg | 171.40 | 0.00 | |
| Sulfuric acid | 98% | kg | 216.00 | 5.28 × 1011 | 1.14 × 1014 | ||
| Leaching residue washing water | kg | 160.00 | 4.56 × 108 | 7.30 × 1010 | |||
| Water | kg | 216.00 | 4.56 × 108 | 9.85 × 1010 | |||
| Electricity | kwh | 4.95 | 7.96 × 1011 | 3.94 × 1012 | |||
| Steam | kg | 10.00 | 2.04 × 107 | 2.04 × 108 | |||
| Output | Acidic leaching liquor | kg | 647.00 | 0.00 | |||
| Emission | Leaching residue | Moisture content 60% | kg | 128.40 | 1.59 × 109 | 2.04 × 1011 | |
| Cascade separation | Input | Acidic leaching liquor | kg | 647.00 | 0.00 | ||
| Potassium hydroxide | Industrial grade | kg | 55.60 | 1.86 × 1012 | 1.03 × 1014 | ||
| Water | 85.60 | 4.56 × 108 | 3.90 × 1010 | ||||
| Potassium sulfate mother liquor | Saturated solution | kg | 59.60 | 0.00 | |||
| Potassium sulfate | kg | 20.00 | 4.44 × 1012 | 8.88 × 1013 | |||
| Electricity | kwh | 3.96 | 7.96 × 1011 | 3.15 × 1012 | |||
| Steam | kg | 12.00 | 2.04 × 107 | 2.45 × 108 | |||
| Output | Alum | Industrial grade | kg | 166.20 | 1.86 × 1012 | 3.09 × 1014 | |
| Ni-Co-Mn precursor | crude product | kg | 5.80 | 0.00 | |||
| Filter liquor | kg | 693.20 | 0.00 | ||||
| Magnesium hydroxide | Industrial grade | kg | 1.40 | 1.86 × 1012 | 2.60 × 1012 | ||
| Aluminum hydroxide | Industrial grade | kg | 1.20 | 1.86 × 1012 | 2.23 × 1012 | ||
| Crystallization | Input | Filter liquor | kg | 693.20 | 0.00 | ||
| Potassium carbonate | Industrial grade | kg | 66.00 | 1.86 × 1012 | 1.23 × 1014 | ||
| Water | Pure water | kg | 96.40 | 4.56 × 108 | 4.40 × 1010 | ||
| Electricity | kwh | 16.50 | 7.96 × 1011 | 1.31 × 1013 | |||
| Steam | kg | 198.00 | 2.04 × 107 | 4.04 × 109 | |||
| Output | Potassium sulfate | Industrial grade | kg | 104.00 | 4.44 × 1012 | 4.62 × 1014 | |
| Concentrated mother liquor | kg | 59.60 | 0.00 | ||||
| Condensed water | kg | 656.80 | 4.56 × 108 | 3.00 × 1011 | |||
| Crude lithium carbonate | Crude product | kg | 35.20 | 0.00 | |||
| Purification | Input | Crude Ni-Co-Mn precursor | Crude product | kg | 5.80 | 0.00 | |
| Crude lithium carbonate | Crude product | kg | 35.20 | 0.00 | |||
| Sulfuric acid | 98% | kg | 10.00 | 5.28 × 1011 | 5.28 × 1012 | ||
| Carbon dioxide | kg | 11.00 | 1.42 × 107 | 1.56 × 108 | |||
| Water | Pure water | kg | 16.00 | 4.56 × 108 | 7.30 × 109 | ||
| Ammonia | 25% | kg | 9.00 | 1.86 × 1012 | 1.67 × 1013 | ||
| Sodium hydroxide | kg | 6.00 | 1.86 × 1012 | 1.12 × 1013 | |||
| Electricity | kwh | 2.00 | 7.96 × 1011 | 1.59 × 1012 | |||
| Steam | kg | 10.00 | 2.04 × 107 | 2.04 × 108 | |||
| Output | Ni-Co-Mn precursor | Battery grade | kg | 5.00 | 2.93 × 1013 | 1.47 × 1014 | |
| Lithium carbonate | Battery grade | kg | 28.20 | 4.44 × 1012 | 1.25 × 1014 | ||
| Emission | Calcium Magnesium slag | kg | 1.80 | 1.59 × 109 | 2.86 × 109 | ||
| Wastewater | kg | 58.00 | 9.67 × 106 | 5.61 × 108 |
References
- Yang, Z.; Lu, J.; Bian, D.; Zhang, W.; Yang, X.; Xia, J.; Chen, G.; Gu, H.; Ma, G. Stepwise Co-Precipitation to Synthesize Li Ni1/3Co1/3Mn1/3O2 One-Dimensional Hierarchical Structure for Lithium-Ion Batteries. J. Power Sources 2014, 272, 144–151. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Li, L.; Feng, C. Synthesis and conditions of MnxNiyCozCO3, a precursor of ternary cathode material. Inorg. Salt Ind. 2015, 47, 75–77. [Google Scholar]
- Zhai, P.; Chen, L.; Yin, Y.; Li, S.; Ding, D.; Ye, G. Interactions between mullite saggar refractories and Li-ion battery cathode materials during calcination. J. Eur. Ceram. Soc. 2018, 38, 2145–2151. [Google Scholar] [CrossRef]
- Liu, B. Lithium-Ion Battery Cathode Material Saggars Application Research; Qilu University of Technology: Jinan, China, 2015; pp. 34–35. [Google Scholar]
- Li, J.; Chen, B.; Zhou, H. Exploration on Efficient and Comprehensive Utilization of Retired Power Battery. Battery China Network: The First International Summit on Power Battery Application in 2016. Available online: http://www.cbea.com/content/ff808081582e454601583de1a5a7019a.jhtml (accessed on 10 August 2021).
- Yu, H. Life Cycle Evaluation of Electrolytic Nickel Production; Kunming University of Science and Technology: Kunming, China, 2006; pp. 16–17. [Google Scholar]
- Porvali, A.; Ojanen, S.; Wilson, B.; Serna-Guerrero, R.; Lundström, M. Nickel Metal Hydride Battery Waste: Mechano-hydrometallurgical Experimental Study on Recycling Aspects. J. Sustain. Metall. 2020, 6, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Shi, H. Recovery and Reuse of Li(Co, Ni, Mn)O2 Cathode Material in Waste Lithium Ion Battery; Zhengzhou University: Zhengzhou, China, 2017; pp. 23–25. [Google Scholar]
- Wang, L. Research on the Recovery and Recycling of Nickel from Waste Nickel-Hydrogen Batteries; Kunming University of Science and Technology: Kunming, China, 2017; pp. 12–14. [Google Scholar]
- Yu, M. Study on Synthesizing Waste Nickel, Cobalt and Manganese Ternary Materials and Related Fine Chemicals by High Value of Cyclic Leaching Process; Beijing University of Chemical Technology: Beijing, China, 2018; pp. 27–29. [Google Scholar]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Unterreiner, L.; Juelch, V.; Reith, S. Recycling of Battery Technologies–Ecological Impact Analysis Using Life Cycle Assessment. Energy Procedia 2016, 99, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Raugei, M.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Richa, K.; Babbitt, C.W.; Gaustad, G. Eco-Efficiency Analysis of a Lithium-Ion Battery Waste Hierarchy Inspired by Circular Economy. J. Ind. Ecol. 2017, 21, 715–730. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Y.; Yu, Y.; Huang, K.; Wang, L. Superior “green” electrode materials for secondary batteries: Through the footprint family indicators to analyze their environmental friendliness. Environ. Sci. Pollut. Res. 2019, 26, 36538–36557. [Google Scholar] [CrossRef]
- Ali, U.; Malik, R.N.; Syed, J.H.; Mehmood, C.T.; Sanchez-Garcia, L.; Khalid, A.; Chaudhry, M.J.I. Mass burden and estimated flux of heavy metals in Pakistan coast: Sedimentary pollution and eco-toxicological concerns. Environ. Sci. Pollut. Res. 2015, 22, 4316–4326. [Google Scholar] [CrossRef]
- El-Alfy, M.A.; El-Amier, Y.A.; El-Eraky, T.E. Land use/cover and eco-toxicity indices for identifying metal contamination in sediments of drains, Manzala Lake, Egypt. Heliyon 2020, 6, e03177. [Google Scholar] [CrossRef] [Green Version]
- Ulgiati, S.; Brown, M.T. Quantifying the environmental support for dilution and abatement of process emissions: The case of electricity production. J. Clean. Prod. 2002, 10, 335–348. [Google Scholar] [CrossRef]
- Giannetti, B.F.; Bonilla, S.H.; Silva, I.; Almeida, C.M.V.B. Cleaner production practices in a medium size gold-plated jewelry company in Brazil: When little changes make the difference. J. Clean. Prod. 2008, 16, 1106–1117. [Google Scholar] [CrossRef]
- Pereira, C.L.; Ortega, E. Sustainability assessment of large-scale ethanol production from sugarcane. J. Clean. Prod. 2010, 18, 77–82. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Ulgiati, S.; Sarkis, J. Emergy analysis of an industrial park: The case of Dalian, China. Sci. Total. Environ. 2010, 408, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Ni, W. Emergy evaluation of polygeneration systems. Front. Energy Power Eng. China 2007, 1, 223–227. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Feng, M.; Zuo, X.; Hao, X.; Xu, Z.; Ni, W. Emergy Evaluation of Double Gas Polygeneration Systems. J. Chin. Soc. Power Eng. 2010, 30, 798–803. [Google Scholar]
- Li, T.; Song, Y.; Shen, J. Clean Power Dispatching of Coal-Fired Power Generation in China Based on the Production Cleanliness Evaluation Method. Sustainability 2019, 11, 6778. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Feng, X. Emergy analysis and comparison of methanol synthesis processes using different feedstocks. Chem. Ind. Eng. Prog. 2006, 1461–1466, 1483. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yang, J.; Jiang, Z.; Zhang, H.; Wang, Y. Emergy based sustainability evaluation of spent lead acid batteries recycling. J. Clean. Prod. 2020, 250, 1167–1194. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; LI, Y. A Research on the Sustainable Development Level of Kashgar Based on Energy Analysis. Environ. Sustain. Dev. 2014, 39, 161–164. [Google Scholar] [CrossRef]
- Odum, H. Blisset M E C. Ecology and Economy: Emergy Analysis and Publicly in Texas; University of Texas—School of Public Affairs and Texas Dept of Agriculture: Austin, TX, USA, 1987; pp. 22–24. [Google Scholar]
- Odum, H. Environmental Accounting Emergy and Environmental Decision Making; John Wiley and Sons: New York, NY, USA, 1996; pp. 17–19. [Google Scholar]
- Lan, S.; Qin, P.; Lu, H. Emergy Analysis of Eco Economic System; Chemical Industry Press: Beijing, China, 2002; pp. 27–42. [Google Scholar]
- Lan, S.; Qin, P. Emergy analysis of ecosystems. Chin. J. Appl. Ecol. 2001, 12, 129–131. [Google Scholar]
- Li, H.; Liao, Y.; Yan, M. Emergy Analysis on the Ecological -economic System of Jiangxi Province. Acta Agric. Univ. Jiangxiensis 2003, 25, 93–98. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z. Emergy Analysis Theory and Practice: Ecological Economic Accounting and Urban Green Management; Science Press: Beijing, China, 2018; pp. 362–372. [Google Scholar]
- Yan, M.; Odum, T. New Visual Angle to View Eco-Economic System—Emergy Evaluation Case studies of Chinese Regional Eco-Economic System; China Zhigong Publishing House: Beijing, China, 2001; pp. 56–61. [Google Scholar]

| Sort | Indicator | Unit | Calculation Formula | Note |
|---|---|---|---|---|
| Flow indicator | Input emergy (IMP) | sej | IMP | The sum of the emergy of each material input |
| Renewable resource emergy input Ri | sej | Ri | The sum of the emergy of each renewable material input. | |
| Nonrenewable resource emergy input Ni | sej | Ni | The sum of the emergy of each nonrenewable material input. | |
| Waste emergy emission (WEM) | sej | WEM | The total emergy of each waste emission. | |
| Export emergy (EXP) | sej | EXP | The total emergy value of all products. | |
| Efficiency indicators | Emergy yield rate (EYR) | - | EXP/IMP | The ratio of product emergy to input emergy. A higher emergy yield rate indicates that the output is higher with the same input. |
| Waste emergy emission rate (WEMR) | - | WEM/EXP | The ratio of dissipated emergy to total emergy output. A higher system waste emission rate indicates that the environmental cost of the system is higher. | |
| Environmental load rate (ELR) | - | IMP/Ri | The ratio of the total energy value entered to the renewable resources. The larger the value is, the greater the environmental load. | |
| Emergy sustainable development index(ESI) | - | EYR/ELR | If ESI < 1, the system is a consumer system and is unsustainable internally. When 1 < ESI < 10, the system has high sustainability. When ESI > 10, the system has weak ability to use emergy and the development level is relatively simple. |
| Item | Eco-Economic Benefit (CNY/t) |
|---|---|
| Resource input | 777.82 |
| Environmental cost | 206.15 |
| Other input | 0 |
| Value of products | 3116 |
| Profit | 2132.03 |
| Yield rate | 3.16 |
| IMP | Ri | Ni | EXP | WEM | |
|---|---|---|---|---|---|
| Physical separation | 1.22 × 1015 | 1.21 × 1015 | 6.57 × 1012 | 1.18 × 1015 | 0 |
| Reduction and leaching | 1.18 × 1014 | 1.71 × 1011 | 1.18 × 1014 | 0 | 20.4 |
| Cascade separation | 1.95 × 1014 | 3.90 × 1010 | 1.95 × 1014 | 3.14 × 1014 | 0 |
| Crystallization | 1.36 × 1014 | 4.40 × 1010 | 1.36 × 1014 | 4.62 × 1014 | 0 |
| Purification | 3.48 × 1013 | 7.45 × 109 | 3.48 × 1013 | 2.72 × 1014 | 3.42 × 109 |
| Total | 1.70 × 1015 | 1.21×1015 | 4.90 × 1014 | 2.23 × 1015 | 2.08 × 1011 |
| Indicator | Value |
|---|---|
| EYR | 1.31 |
| WEMR | 9.31 × 10−5 |
| ELR | 0.45 |
| ESI | 3.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Z.; Li, S.; Dong, S.; Xia, B.; Wang, C. Evaluation of the Ecological Benefits of Recycling Multiple Metals from Lithium Battery Saggars Based on Emergy Analysis. Sustainability 2021, 13, 10745. https://doi.org/10.3390/su131910745
Zhang W, Li Z, Li S, Dong S, Xia B, Wang C. Evaluation of the Ecological Benefits of Recycling Multiple Metals from Lithium Battery Saggars Based on Emergy Analysis. Sustainability. 2021; 13(19):10745. https://doi.org/10.3390/su131910745
Chicago/Turabian StyleZhang, Wenbiao, Zehong Li, Shaopeng Li, Suocheng Dong, Bing Xia, and Chunying Wang. 2021. "Evaluation of the Ecological Benefits of Recycling Multiple Metals from Lithium Battery Saggars Based on Emergy Analysis" Sustainability 13, no. 19: 10745. https://doi.org/10.3390/su131910745
APA StyleZhang, W., Li, Z., Li, S., Dong, S., Xia, B., & Wang, C. (2021). Evaluation of the Ecological Benefits of Recycling Multiple Metals from Lithium Battery Saggars Based on Emergy Analysis. Sustainability, 13(19), 10745. https://doi.org/10.3390/su131910745









