Abstract
The lifespan of people with cognitive disabilities (ID) has increased significantly, but the cognitive aspects together with the functional ones comparing normal aging and those with intellectual disabilities had not been previously studied. Objective: This study analyzed the cognitive and functional differences in older adults aging with ID (and with DS), compared with their peers without disabilities, in order to identify the most adapted interventions. Methodology: This study evaluated the outcome variables of MEC, Set-Test, Barthel, Lawton–Brody, and Tinetti with 247 participants: 146 without ID and 101 ID (29 with DS and 72 without DS). Results: At the cognitive level, older people with ID presented lower scores both in MEC (p < 0.01), globally and in each cognitive domain (except in short-term memory), and in verbal fluency (Set-Test) than older people without ID; however, the diagnosis of cognitive impairment and dementia is higher in people without ID. At the functional level, there are no differences in ABDL, but there are in AIDL and Tinetti (p < 0.01), where participants without ID obtain higher scores. The most frequent pathologies in people with ID were obesity and epilepsy. Conclusions: The lower cognitive and functional performance in ID is associated with the disability itself, the low educational level, the neurocognitive underdiagnosis, and the use of poorly adapted assessment tools. The cognitive and functional results indicated the importance of interventions adapted to the characteristics of this population, in their aging process.
1. Introduction
The world is facing a demographic change without precedents [1]. Between 2015 and 2050, the percentage of people over the age of 60 will almost double from 12% to 22% according to the WHO [2]. In Spain, 19.4% of the population in 2019 were above 65 years of age, according to INE [3]. Longer human lives have led to a global burden of late-life disease [4]. The lifespan of people with intellectual disabilities (ID) has increased significantly, so 8.9% of the population with ID in 2018 were above 65 years of age, 1% more than in 2014 [5].
Increases in the life expectancy of people with Intellectual Disability have followed similar trends to those found in the general population. With the exception of people with severe and multiple disabilities or Down syndrome (DS), the life expectancy of this group now closely approximates with that of the general population. Middle and old age, which until 30 years ago were not recognized in this population, are now important parts of the life course of these individuals [6].
IDs are defined as disorders in neuronal development that lead to deficits in intellectual capabilities and adaptive behavior [7]. IDs occur in 1–3% of the general population [8]. The incidence of disorders such as dementia among people with ID is five times greater than among the rest of the population; cognitive problems are generally preceded by behavioral problems [9]. With the early onset of age-related health problems, dementia is more likely to develop by the age of 40 years in individuals with intellectual disability [10].
DS is the most common chromosomal disorder, and the main cause of ID worldwide; its incidence is highly dependent on sociocultural variables [11]. While the life expectancy of the DS population has greatly increased over recent decades, mortality rates are still high and subjects are facing prematurely a phenomenon of atypical and accelerated aging [12]. The genetic material of people with DS makes them more vulnerable to Alzheimer’s disease [13]. Chromosome 21, triplicated in DS, contains several genes that are thought to play a critical role in the development of this neuropathology [14]. All adults with DS demonstrate AD-like brain pathology, including amyloid plaques and neurofibrillary tangles, by age 40, and dementia typically by age 60 [15].
Consequently, older adults with ID form a small but significant and growing proportion of older people in the community. How these persons grow older and how symptoms and complications of the underlying cause of the Intellectual Disability will influence their life expectancy are of the utmost importance [6].
Generally, neurological diagnosis comes when the disorder has already caused substantial deterioration, either because the associated behavioral problems can be taken as features of IDs [13], from the lack of access to appropriate healthcare, or because neuropsychological testing batteries lack specific scales for people with ID [10,16,17].
Initially, neurocognitive changes due to normal aging do not result in the loss of functional abilities [18]. However, people with ID experience different levels of limitations in basic aspects necessary for independent functioning and living. Satisfactory daily functioning is recognized as a major component of health and well-being by the International Classification of Functioning (ICF) of the World Health Organization [19,20,21].
Concerning associated pathologies, people with ID have a health profile more complex than previously known, being more prone to various chronic health conditions than their peers without ID [22]. This includes cardiovascular diseases, high cholesterol, obesity, diabetes, epilepsy, and osteoarticular disorders. Some common diseases in people with DS are hypothyroidism, congenital cardiopathy, and gastrointestinal disorders [11,22]. Recent studies have identified that people with ID have more than twice the number of mental health conditions, visual impairments, hearing impairments, and physical disabilities [23]. However, according to Folch-Mas et al., many health problems associated with senior people with ID remain to be identified [16].
Integrated care has been suggested as a promising solution to the disparities in access and sustained high-quality long-term care emerging in Europe’s ageing population [24]. Recent studies have shown the importance of factors that improve healthy aging, ensure the social inclusion of the elderly, and prolong the time in which they can live independent lives [25]. Improving social sustainability in people with ID would mean improving social inclusion [26].
The literature refers to this gap in our knowledge about aging in people with ID [27]. More research is needed to assess the effects of aging on mental health [28], as well as longitudinal studies that also include people without ID, in order to improve healthcare protocols [29]. Besides that, understanding the possible aging-related mechanisms associated with these clinical manifestations of DS will facilitate therapeutic interventions in mid-to-late adulthood, while at the same time shedding light on basic mechanisms of aging [21].
The joint study of the influence of cognitive and functional factors, comparing people with and without intellectual disabilities in aging, had not been previously studied.
Therefore, the aim of this study was to analyze cognitive and functional differences between senior people with ID (and DS) and their peers without ID, in order to outline the most appropriate attention protocols.
2. Methods
2.1. Participants
An observational study was carried out to detect differences in aging among ID-afflicted and ID-free seniors in two institutions: Fundación La Caridad (ID-free patients from the Day Centre “Los Sitios”) and Atades (patients afflicted with cognitive disorders at Centro Residencial Sonsoles and Centro Ocupacional y Residencial Santo Angel). The data were compiled between June 2018 and December 2019. The minimum sample size for each group (with and without ID) was set at 97, and a difference of 1.5 points was attested in the main variable, with a potency of 80% and a significance level of 5%; it was predicted that up to 35% of the initial participants would drop out. Inclusion criteria were a score in the cognitive mini-test (CMT) [30] of 27–35 for patients aged ≥65 years, and a CAMDEX [31] score of 50–109 for patients with ID aged ≥60 years or patients with DS aged ≥45. The final sample comprised 247 patients, 146 without ID and 101 with ID (29 with DS and 72 without DS).
2.2. Instruments
Data on various types of variables were collected: socio-demographic (age, gender, marital status, support network, family unit, and level of studies), clinical (main diagnosis—DSM-5), and associated pathologies.
2.2.1. The Spanish Version of MMSE (MEC-35)
The primary variable was MEC-35, which is considered one of the most widely used short cognitive tests to study cognitive capacities in primary care. It evaluates eight components: spatiotemporal orientation (10 points), fixation memory (3 points), attention (3 points), calculation (5 points), short-term memory (3 points), and language and praxis (11 points). Its sensitivity is 85–90% and its specificity is 69%. With this questionnaire, global cognition and cognitive functions were evaluated. Classification is based on scores: 30–35 points for people considered to have normal cognitive function; 25–29 points for borderline cognitive deficits; 20–24 points for MCI; 15–19 points for moderate cognitive impairment; ≤14 points for severe cognitive impairment [32]. Unlike the MMSE, MEC-35 includes a three-digit series to repeat two similar items in the reverse order, and subtraction is performed by subtracting three from 30 instead of seven from 100, as in the version of Folstein et al. As the number of items increases, the maximum score in this version reaches 35 points compared to 30 in the original one [33].
We considered using the Spanish version of the MMSE (MEC-35) to assess global cognition and to observe if there was any change in cognitive functions. Other authors warrant further investigation as to whether the overall MMSE assessment reveals areas of concern [34]. Gallego et al. mentioned that the MMSE allows the rapid assessment of cognitive functions and the consideration of the functions of different domains [35]. The validity data of the individual MEC-35 items are also satisfactory (particularly with temporal orientation) [32]. In Spain, it is common to resort to the adaptation of the MMSE proposed by Lobo et al. in 1979 [36] called MEC-35, because some items of the original version of Folstein are difficult for patients with a low cultural level, which affects the scale’s discriminative capacity [33].
Set-test was used to measure the secondary cognitive variable [37], and Barthel [38] and Lawton–Brody [39] were used to measure the secondary functional variable.
2.2.2. Set-Test
Set-test measures the verbal fluency of a categorical type, by asking the subject to cite up to a maximum of ten responses from each of the following categories: colors, animals, fruits, and cities. Information is collected through a hetero-administered questionnaire adapted and validated by Pascual et al. for the Spanish population. Their scores vary between 0 and 40, with 0 being the minimum score and 40 the maximum score. It has been proposed as a diagnostic aid in elderly patients with dementia, with a cut-off point of 27 points for the elderly, with a lower score being indicative of dementia. It has a sensitivity of 87% and a specificity of 67%.
2.2.3. Barthel Index (BI)
The Barthel Index (BI) assesses the level of independence of 10 basic ADL (BADL) [38]. The maximum score for the BI is 100, where scores higher than 60 denote a low dependence with ADL and scores below 20 demonstrate a high dependence with ADL. Internal consistency was 0.90, with an interobserver reliability Kappa Index between 0.47 and 1.00, and the interobserver reliability Kappa Index between 0.84 and 0.97. Cronbach’s alpha was 0.90–0.9228 for the internal consistency evaluation [40]. The SF-BI was confirmed to be a useful evaluation instrument because its test–retest agreement rate, absolute reliability, item internal consistency, and validity were high. Therefore, the short-form BI can be easily used in clinical practice, and both clinicians and researchers can utilize patients’ selective ADL functions with stroke and employ them as useful information [41]. The structural validity, reliability, and interpretability of the BI were considered sufficient for measuring and interpreting changes in geriatric rehabilitation patients’ physical functions [42]. It is a useful tool for measuring disability in health and social care settings in the care continuum [43].
2.2.4. Lawton and Brody Scale (L-B)
The Lawton and Brody scale assesses the degree of autonomy in eight IADL necessary for living independently in the community. These include using the telephone, shopping, preparing food, cleaning, laundry, transportation, taking medications, and managing finances. The assessment is performed according to a score on a scale from 0 to 8 (maximum dependence and independence, respectively [39]:
- Punctuation
- 0–1: Total dependency
- 2–3: Severe dependency
- 4–5: Moderate dependence
- 6–7: Light dependency
- 8: Autonomous
Scores lie between 0 and 8 points. Its sensitivity is 0.57 and its specificity is 0.92 [44]. The minimal important change in the Lawton IAD scale is around half a point. The certainty of this conclusion is reduced by variation across calculation methods [45].
It is considered a more appropriate scale for women (many of the activities that the scale measures have been traditionally carried out by them) but its application to men is also recommended, although it is still pending to identify those instrumental activities carried out by them according to the social patterns.
2.2.5. The Tinetti Test
The Tinetti test [46] measures the static and dynamic balance of senior patients. The scale has two domains: gait and balance; its main objective is to detect those elderly at risk of falls, and it has a greater predictive value than muscle examination does [47,48]. The scale is made up of nine balance items and seven gait items. The responses are scored as follows: 0 means the person does not achieve or maintain stability in changes in position or has an inappropriate gait pattern, according to the parameters described on the scale, which is considered abnormal; a score of 1 means they achieve changes in position or gait patterns with postural compensations, this condition being called adaptive; finally, rating 2 means a person without difficulties performing the different tasks on the scale, which is considered normal. The maximum score of balance is 16 and that of gait is 12; from the sum of both, a total score of 28 is obtained, with which the risk of falls is determined. It is considered that between 19 and 24, the risk of falls is minimal, at <19, the risk of falls is high [47]; the test presents a sensitivity of 53% and a specificity of 86% [46].
After the selection of participants, the instruments were used to obtain homogenous cognitive, functional, and physical results. Afterward, the results obtained in both groups were compared.
2.3. Statistical Analysis
The statistical analysis was performed with the IBM SPSS Statistics Package, v.22 (SPSS Inc., Chicago, IL, USA). The descriptive statistics are shown according to the nature of each variable: mean (m) and standard deviation (SD), or by the number of participants in each category (n) and the proportion of patients over the total (%). To study the association between the categorical variables, Pearson’s chi-square test was conducted. Student’s t-test was used for independent samples to determine differences in cognitive, functional, and diagnostic variables between the two groups under study. This was followed by a covariance analysis adjusted to age. The association between cognitive impairment and DS was calculated by binary logistic regression adjusted to age. The cut-off score to establish ‘cognitive impairment’ was ≤23 out of 35 in the CMT. All the statistical calculations were undertaken with the statistical program SPSS 22.0. The level of significance adopted was p < 0.05. Finally, the statistical software AMOS v.24 was used to consider a model of structural equations to validate and quantify the relationships between cognitive and functional aspects. We also performed a multigroup analysis to assess the differences in these constructs between participants with and without ID.
2.4. Ethical Considerations
The project was endorsed by the Ethical Research Committee of Aragón (CEICA, C.P.-C.I.PI18/152). Personal data protection regulations were followed and patients or their legal guardians signed informed consent forms. The study adhered to the guidelines set out in the Declaration of Helsinki (General Assembly, Edinburgh, October 2000) [49], good clinical practices, and current legislation. The relationships with the participants in both institutions are based on their empowerment and self-determination. Their participation in the study does not in any way affect the care they receive in their centers or their relationship with the research team.
3. Results
The sample comprised 247 participants, 146 without ID and 101 with ID (29 withtDS and 72 without DS). Table 1 summarizes the socio-demographic characteristics of the sample. The average age of participants was 70.6 ± 11.5 years (with ID: 60.6 ± 8.3 years; without ID: 77.6 ± 7.6 years).

Table 1.
Socio-demographic characteristics.
Table 2 presents the result of cognitive tests. Participants without ID yielded higher MEC scores, both globally and in each separate dominion (p < 0.01), except STM. All differences were statistically significant after the results were adjusted by the age co-variable, including STM (p = 0.033). Concerning DS, the only significant differences attested concerned the language variable (p = 0.001). Regarding the association between cognitive impairment and ID, the study concluded that 88.1% of participants with ID suffered some form of cognitive impairment (84.7% without DS and 96.6% with DS). Logistic regression showed that, regardless of age, participants with ID were 25 times more likely to be afflicted by cognitive impairment than ID-free participants were (p < 0.05). Concerning verbal fluency, participants without ID yielded higher scores (p < 0.001) than their disabled peers did. The difference remained significant after the results were adjusted by age. No significant differences were attested between DS and non-DS participants in this variable (p = 0.062).

Table 2.
Cognitive differences between groups with and without ID and with and without DS.
Table 3 presents the neurological results. The proportion of participants with mild cognitive impairment (MCI), dementia, and cognitive impairment was higher in the ID-free group of participants (p < 0.001). No significant differences between the groups were attested concerning memory loss and Parkinson’s.

Table 3.
Frequency of neurological diagnoses for patients with and without ID.
Table 4 presents the functional results. No significant differences were attested between ID and non-ID participants in terms of ADLs (p = 0.512), but there were significant differences in terms of IADLs (p < 0.01), with participants without ID yielding higher overall and Tinetti’s static test scores (both p < 0.05). After the results were adjusted for age, only the differences in IADLs remained significant. No significant differences were attested between groups in the functional tests (all p > 0.05). After adjusting the results by age, participants without ID yielded higher values in the Lawton–Brody scale (2.7 vs. 0.9; p = 0.020), Tinetti’s dynamic test (10.6 vs. 8.9; p = 0.005), and overall Tinetti’s test score (25.4 vs. 23.0; p = 0.035).

Table 4.
Functional differences between groups with and without ID and with and without DS.
Figure 1 represents the cognitive and functional differences between groups with and without ID.
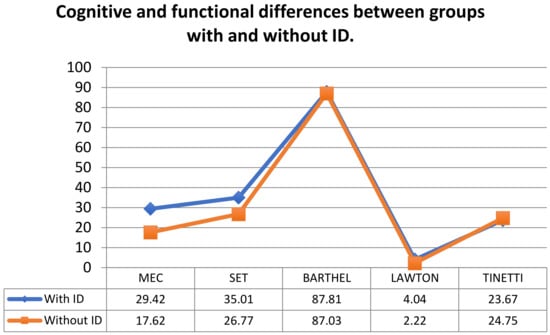
Figure 1.
Cognitive and functional differences between groups with and without ID.
In Figure 1, which represents the cognitive and functional differences between groups with and without ID, it is observed how the group with ID obtained higher scores in the cognitive section, as well as in the Lawton scale. In the rest of the functional tests, hardly any differences were seen between the two groups.
Table 5 presents associated pathologies. The percentage of participants with hypertension, diabetes, cardiopathies, mental health issues, ictus, and technical aid requirements were greater in the group without ID. In contrast, the percentage of participants with obesity and epilepsy were higher in the ID group. No statistically significant differences were attested between groups concerning the other pathologies examined: high cholesterol, tobacco addiction, hearing impairments, vision impairments, and breathing difficulties.

Table 5.
Frequency of associated pathologies in people with and without intellectual disabilities.
Finally, to analyze the relationship between these measures of cognitive and functional variables, a confirmatory factor analysis (CFA) was performed. After performing a first CFA, we found that the fit indices were not appropriate χ2 (4) = 7.046 p < 0.001; χ2/gl = 1.76; CFI = 0.96; NFI = 0.95; TLI = 0.93; RMSEA = 0.075, 95% CI (0.056–0.093).
Finally, an attempt was made to verify the relationship between cognitive and functional aspects and whether it differed between the group of participants with and without ID.
Figure 2 shows the result of the analysis carried out with structural equations by the maximum likelihood method, which confirmed the suitability of the model composed of the constructs contemplated in the present study. The analysis showed a good correlation (r = 0.59) between the cognitive variables and the functional variables.
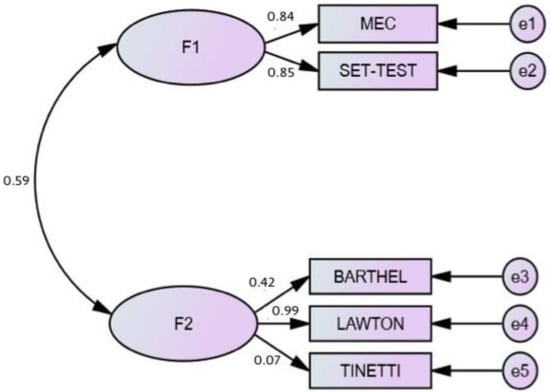
Figure 2.
Model of the structural equations among cognitive and functional variables.
When comparisons of nested models were made (Table 6), and assuming that the model without restrictions is correct, we obtained a comparison made with the measurement weights, which indicated that the model of equal measurement weights showed the same fit as the model without restrictions did. Therefore, the regression weights in the indicated model were the same. Likewise, the models of measurement intersections, structural covariances, and measurement residuals did not fit the data, and the comparison indicated that the model differed statistically and gave a worse fit. These results indicated that it was not necessary to include the presence of ID as a difference in the established model between the cognitive and functional variables.

Table 6.
Multiple-group analysis of cognitive and functional variables in people with and without ID.
4. Discussion
This study examined differences in aging between ID-afflicted and ID-free seniors, taking into consideration cognitive and functional assessments, clinical diagnosis, and pathologies.
In terms of socio-demographic profiles, ID-afflicted seniors are younger, single, institutionalized, and less educated. Age differences are determined by inclusion criteria and premature aging in association with ID [50]. A study by Vancamptort et al. showed that people with intellectual disability have an increased risk of premature mortality [51]. Concerning marital status, the historical segregation of ID-afflicted people by sex has limited the scope of personal relationships during youth [52,53]. Regarding family relationships, early vulnerability and care requirements often lead to their institutionalization [54], although those who live with their relatives have a more positive perspective on their health and welfare [55]. The low educational level presented by ID seniors is confirmed by INE data, according to which 33% of people with ID are illiterate [3]. Historically, students with intellectual disability were not expected to learn to read, and thus were excluded from reading instruction [56].
Concerning cognitive performance, both overall and by dominion, ID-afflicted participants yielded lower scores, except in terms of STM, in which the performance was similar. However, other studies that compared groups of ID and non-ID seniors detected memory issues in all periods of life, except the 19–45 age bracket [57], among people with DS. The ID-related initial cognitive deficits affect executive functions, while in seniors not afflicted by ID, these initially affect episodic memory [58]. Concerning the relationship of ID and language, some studies have argued that language skills are a significant predictor of MCI, that semantic verbal fluency is the most important predictor of dementia, and that the evaluation of linguistic skills can help to detect dementia in ID-afflicted adults [59].
In our study, the percentage of participants with MCI and dementia were higher in the non-ID group. However, previous studies have suggested that ID-afflicted people are five times more likely to suffer these conditions [60,61]. On the other hand, it is possible that the underrepresentation of these afflictions among ID participants is caused by the tests used in primary healthcare, which are influenced by educative level [36,60]; although, in our study, we used Set-Test for this very reason [37], the literature demands the use of other tools for the diagnosis of ID patients, such as the Barcelona test [62] or CAMDEX-DS [13], alongside a clinical diagnosis adapted to ID patients [60]. Other studies have stated that cognitive instruments used for the general population are not suitable for people with intellectual disability, because of floor effects [10]. Consensus criteria for both dementia and MCI have been developed for typically developing adults but are of limited applicability for adults with ID, given their pre-existing cognitive impairments [63].
Concerning the functional variables, our study attested no differences in terms of ADLs, although a smaller proportion of ID participants used technical aids, as their static and dynamic balance was better [20,46]. However, another study showed that 40% of adults with ID were completely independent in ADL, but all participants reported activity limitations in at least one IADL. Dynamic balance and MWS, lower-body strength, and manual dexterity showed significant and moderate-to-strong correlations with daily functioning [21]. Regarding IADLs, ID-afflicted people are more dependent, owing to their cognitive deficit [20]. After the age of 50, people with ID suffer a faster process of functional deterioration [64]. The Barthel index and the Lawton–Brody scale are recommended for clinical practice and research with ID patients [20]. The results of a study by Lobete et al. confirmed that supporting the performance of both ADL and AIDL and promoting physical fitness in community care centers for adults with ID improve their functional Independence [21].
The literature consistently reports that balance and gait capacities are affected in persons with ID compared to their age-matched peers. These problems start at a young age and remain present during the entire lifespan of persons with ID, with a relatively early occurrence of age-related decline. The relationship between the cognitive and functional variables was analyzed, finding a link between both, with no effect on whether the person presented ID or not. This review demonstrated that balance and gait are potentially trainable in persons with ID [65].
Regarding associated pathologies, a larger proportion of non-ID participants present hypertension, diabetes, cardiopathies, mental health issues, and ictus. Some studies have argued that these pathologies are more common among ID patients, and that their statistical underrepresentation is due to diagnostic issues, affecting relatives, healthcare professionals, and the patient’s themselves [16]. Other comparative studies between ID and the general population have shown that many conditions occur more frequently among people with ID, while some conditions occur in the same proportion or at a lower rate. This study concluded that the latter associations may reflect an underdiagnosis [22].
Obesity [64] and epilepsy [66,67] are more prevalent among people afflicted by ID, both in the sample and in general.
The review of the existing literature highlights the multiple challenges posed by ID and the need to develop a more collaborative approach involving the institutions caring for ID patients, families, and other stakeholders [68].
Our observational study flagged the different healthcare profiles of people with and without ID. Their comparatively poor cognitive performance is related to the effects of the ID, poor education, cognitive infra-diagnosis, and the use of inadequate measuring tools. Training in adaptive skills, health checks, and education for health programs have led to greater functionality and reduced the incidence of aging-related afflictions in people with ID. These results are clinically relevant insofar as they help prevent cognitive impairment and improve the quality of life of patients, while contributing to a better coordination between primary healthcare and institutions that attend patients afflicted by ID.
The limitations of our study concern the use of cognitive skills-measuring tools that are not adapted to ID but the use of which is widespread in primary healthcare. Another limitation of the study is not having evaluated the lifestyle before and after the diagnosis of ID.
Future research should develop therapeutic activities adapted to patients with ID and carry out comparative studies to assess their effectiveness.
Author Contributions
Conceptualization, O.T.-B., M.S.-P. and E.C.; Data Curation, O.T.-B., M.S.-P., A.G.-C. and E.C.; Formal Analysis, O.T.-B., M.S.-P., A.G.-C., C.S. and E.C.; Funding Acquisition, O.T.-B., M.S.-P. and E.C.; Investigation, O.T.-B., M.S.-P., A.G.-C., P.U. and E.C.; Methodology, O.T.-B., M.S.-P., A.G.-C., C.S. and E.C.; Writing—Original Draft, O.T.-B., M.S.-P., C.S., P.U. and E.C.; Writing—Review and Editing, O.T.-B., M.S.-P., C.S., P.U. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research funds contributed, in equal parts, by ATADES and Fundación La Caridad. Both institutions are non-profit entities.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Research Committee of Aragón (CEICA, C.P.-C.I.PI18/152).
Informed Consent Statement
All respondents were volunteers and signed an informed consent form.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank the geriatric teams in the two institutions in which this study was undertaken. Their help to carry out the measurements was invaluable. Similarly, we wish to thank patients and their families for taking part in this study, which, no doubt, will contribute to improve the quality of life of, and the programs directed at, patients with ID.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parish, A.; Kim, J.; Lewallen, K.M.; Miller, S.; Myers, J.; Panepinto, R.; Maxwell, C.A. Knowledge and perceptions about aging and frailty: An integrative review of the literature. Geriatr. Nurs. 2019, 40, 13–24. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Ginebra: Informe Mundial Sobre el Envejecimiento y la Salud. Informe de un Grupo Científico de la OMS; Organización Mundial de la Salud: Geneve, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/186466/9789240694873_spa.pdf;jsess%20ionid=A18FC62E4436A9287985DD570C9D687F?sequence=1 (accessed on 25 October 2020).
- Jurado, M. La Población Mayor de 65 Años Alcanza un Máximo Histórico de 19.4%. Notas de Prensa INE [Internet]; Instituto Nacional de Estadística: Madrid, Spain, 2019; Available online: https://www.65ymas.com/sociedad/la-poblacion-mayor-de-65-anos-alcanza-un-maximo-historico-de-19-4_5206_102.html (accessed on 10 July 2021).
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Base Estatal de Datos de Personas con Valoración del Grado de Discapacidad. Imserso.es. 2018. Available online: https://www.imserso.es/InterPresent1/groups/imserso/documents/binario/bdepID_2018 (accessed on 21 December 2020).
- Coppus, A.M. People with intellectual disability: What do we know about adulthood and life expectancy? Dev. Disabil. Res. Rev. 2013, 18, 6–16. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Yang, P.; Gui, B.H.; Wu, L.Q. Etiology and diagnosis of intellectual disability. Zhongguo Dang Dai Er Ke Za Zhi 2015, 17, 543–548. [Google Scholar] [PubMed]
- Sheehan, R.; Hassiotis, A.; Walters, K.; Osborn, D.; Strydom, A.; Horsfall, L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ 2015, 351, h4326. [Google Scholar] [CrossRef]
- Tse, M.M.; Kwan, R.Y.; Lau, J.L. Ageing in individuals with intellectual disability: Issues and concerns in Hong Kong. Hong Kong Med. J. 2018, 24, 68–72. [Google Scholar] [CrossRef]
- Díaz, S.; Yokoyama, E.; Del Castillo, V. Genómica del síndrome de Down. Acta Pediatr. Méx. 2016, 37, 289–296. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0186-23912016000500289&lng=es&tlng=es (accessed on 25 September 2020). [CrossRef]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Garagnani, P. Down syndrome, accelerated aging and immunosenescence. Semin. Immunopathol. 2020, 42, 635–645. [Google Scholar] [CrossRef]
- Domínguez, J.; Navas, P. Deterioro cognitivo y trastorno neurodegenerativo en personas con discapacidad intelectual. Siglo Cero 2018, 49, 53–67. [Google Scholar] [CrossRef][Green Version]
- Head, E.; Lott, I.T.; Wilcock, D.M.; Lemere, C.A. Aging in Down Syndrome and the Development of Alzheimer’s Disease Neuropathology. Curr. Alzheimer Res. 2016, 13, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Xing, Z.; Chen, Q.D.; Salvi, R.J.; Zhang, X.; Tycko, B.; Mobley, W.C.; Yu, Y.E. Mechanistic Analysis of Age-Related Clinical Manifestations in Down Syndrome. Front. Aging Neurosci. 2021, 13, 700280. [Google Scholar] [CrossRef]
- Folch, A.; Cortés, M.J.; Salvador, L.; Kazah, N.; Irazábal, M.; Muñoz, S. Nuevas consideraciones sobre la salud de las personas con trastornos del desarrollo intelectual. Salud Publica Mex. 2017, 59, 454–461. Available online: https://www.saludpublica.mx/index.php/spm/article/view/8201 (accessed on 25 July 2021). [CrossRef]
- Borrás, C.; Viña, J. Neurofisiología y envejecimiento. Concepto y bases fisiopatológicas del deterioro cognitivo. Rev. Esp. Geriatr. Gerontol. 2016, 51 (Suppl. 1), 3–6. [Google Scholar] [CrossRef]
- Hilgenkamp, T.I.; Wijck, R.; Evenhuis, H.M. (Instrumental) activities of daily living in older adults with intellectual disabilities. Res. Dev. Disabil. 2011, 32, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Cruz, M.R.; Jiménez, M.V. Envejecimiento y discapacidad intelectual. Aproximación a las necesidades de las personas adultas y mayores con discapacidad intelectual y sus familias. Int. J. Educ. Res. Innov. 2017, 7, 76–90. Available online: https://www.upo.es/revistas/index.php/IJERI/article/view/2302/1867 (accessed on 26 July 2021).
- Delgado, L.; Montes, R.; Freire, C.; Ferradás, M.D.M. Performance of (Instrumental) Activities of Daily Living and Physical Capacity in Spanish Adults with Intellectual Disabilities: A Cross-Sectional Pilot Study. Healthcare 2021, 9, 435. [Google Scholar] [CrossRef]
- Liao, P.; Vajdic, C.; Trollor, J.; Reppermund, S. Prevalence and incidence of physical health conditions in people with intellectual disability—A systematic review. PLoS ONE 2021, 16, e0256294. [Google Scholar] [CrossRef]
- Dunn, K.; Rydzewska, E.; Fleming, M.; Cooper, S.A. Prevalence of mental health conditions, sensory impairments and physical disability in people with co-occurring intellectual disabilities and autism compared with other people: A cross-sectional total population study in Scotland. BMJ Open 2020, 10, e035280. [Google Scholar] [CrossRef] [PubMed]
- Holterman, S.; Lahr, M.; Wynia, K.; Hettinga, M.; Buskens, E. Integrated Care for Older Adults: A Struggle for Sustained Implementation in Northern Netherlands. Int. J. Integr. Care 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Morato, J.; Sanchez, S.; Iglesias, A.; Campillo, A.; Fernández, C. Sustainable Technologies for Older Adults. Sustainability 2021, 13, 8465. [Google Scholar] [CrossRef]
- Carnemolla, P.; Kelly, J.; Donnelley, C.; Healy, A.; Taylor, M. “If I Was the Boss of My Local Government”: Perspectives of People with Intellectual Disabilities on Improving Inclusion. Sustainability 2021, 13, 9075. [Google Scholar] [CrossRef]
- Torr, J.; Davis, R. Ageing and mental health problems in people with intellectual disability. Curr. Opin Psychiatry 2007, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- McCarron, M.; Cleary, E.; McCallion, P. Health and Health-Care Utilization of the Older Population of Ireland: Comparing the Intellectual Disability Population and the General Population. Res. Aging 2017, 39, 693–718. [Google Scholar] [CrossRef]
- Lobo, A.; Gómez, F.; Escolar, V.; Seva, A. El Mini-Examen Cognoscitivo en pacientes geriátricos. Folia Neuropsiquiátr. (Granada) 1979, 14, 244–251. [Google Scholar]
- González, M.J.; Escrivà, R.; Vinyoles, E.; Espel, C.; Davins, J.; Borrell, M. Estimaciones de la frecuencia de déficit cognitivo según el test empleado. Rev. Aten. Primaria 1997, 20, 173–179. [Google Scholar]
- Olazarán, J. ¿Puede diagnosticarse la demencia en la Atención Primaria? Aten. Primaria 2011, 43, 377–384. [Google Scholar] [CrossRef][Green Version]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; Cámara, C.; de la Ventura, T.; Aznar, S. Revalidación y normalización del Mini-Examen Cognoscitivo (primera versión en castellano del Mini-Mental Status Examination) en la población general geriátrica. Med. Clin. 1999, 112, 767–774. [Google Scholar]
- Calero, M.D.; Navarro, E.; Robles, P.; García, T.M. Estudio de validez del Mini-Examen Cognoscitivo de Lobo et al para la detección del deterioro cognitivo asociado a demencias. Neurología 2000, 15, 337–342. [Google Scholar] [PubMed]
- Norris, D.; Clark, M.S.; Shipley, S. The Mental Status Examination. Am. Fam. Physician 2016, 94, 635–641. [Google Scholar] [PubMed]
- Gómez, M.; Gómez, J. Musicoterapia en la enfermedad de Alzheimer: Efectos cognitivos, psicológicos y conductuales. Neurología 2017, 32, 300–308. [Google Scholar] [CrossRef]
- Lobo, A.; Escobar, V.; Ezquerra, J.; Seva, A. El Mini-Examen Cognoscitivo: Un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes psiquiátricos. Actas Luso-Esp. Neurol. Psiquiatr. 1979, 3, 189–202. Available online: https://psycnet.apa.org/record/1982-24794-001 (accessed on 20 July 2021).
- Pascual, L.F.; Martínez, J.V.; Modrego, P.; Mostacero, E.; López del Val, J.; Morales, F. El Set-test en el diagnóstico de la demencia. Neurología 1990, 5, 82–85. [Google Scholar]
- Cid, J.; Damián, J. Valoración de la discapacidad física: El índice de Barthel. Rev. Esp. Salud Pública 1997, 71, 127–137. [Google Scholar]
- Larrión, J.L. Valoración geriátrica integral (iii): Valoración de la capacidad funcional del paciente anciano. An. Sist. Sanit. Navar. 1999, 22, 71–84. [Google Scholar]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Park, C.S. The test-retest reliability and minimal detectable change of the short-form Barthel Index (5 items) and its associations with chronic stroke-specific impairments. J. Phys. Ther. Sci. 2018, 30, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, H.; Smit, E.B.; Wattel, E.M.; Van derWouden, J.; Hertogh, C.M.; Terluin, B.; Terwee, C.B. Measurement Properties of the Barthel Index in Geriatric Rehabilitation. J. Am. Med. Dir. Assoc. 2019, 20, 420–425.e1. [Google Scholar] [CrossRef]
- Castiglia, S.F. The culturally adapted Italian version of the Barthel Index (IcaBI): Assessment of structural validity, inter-rater reliability and responsiveness to clinically relevant improvements in patients admitted to inpatient rehabilitation centers. Funct. Neurol. 2017, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.I.; Kurosaki, M.T.T.; Harrah, J.C.H.; Chance, J.M.; Filos, R.S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Suijker, J.J.; Van Rijn, M.; Ter, G.; Van Charante, E.P.M.; De Rooij, S.E.; Buurman, B.M. Minimal important change andminimal detectable change in activities of daily living in community-living older people. J. Nutr. Health Aging 2016, 21, 165–172. [Google Scholar] [CrossRef]
- Guevara, C.; Lugo, L. Validez y confiabilidad de la Escala de Tinetti para población colombiana. Rev. CR 2012, 19, 218–233. [Google Scholar] [CrossRef]
- Sánchez-Barrera, E.; Vázquez-Chacón, V. Resultados de valoración del equilibrio y riesgo de caídas en población adulta femenina mexicana. Rev. Fisioter. Tecnol. Médica 2020, 4, 13–19. Available online: https://www.ecorfan.org/taiwan/research_journals/Fisioterapia/vol4num12/Revista_de_Fisioterapia_y_Tecnologia_Medica_V4_N12_3.pdf (accessed on 5 September 2021).
- Köpke, S.; Meyer, G. The Tinetti test: Babylon in geriatric assessment. Z. Gerontol. Geriatr. 2006, 39, 288–291. [Google Scholar] [CrossRef]
- Declaración de Helsinki de la, A.M.M. Principios Éticos Para las Investigaciones Médicas en Seres Humanos; Asociación Médica Mundial: Ferney-Voltaire, France, 2015; Available online: http://www.wma.net/es/30publications/10policies/b3/ (accessed on 9 March 2018).
- Dieckmann, F.; Giovis, C.; Offergeld, J. The Life Expectancy of People with Intellectual Disabilities in Germany. J. Appl. Res. Intellect. Disabil. 2015, 28, 373–382. [Google Scholar] [CrossRef]
- Vancampfort, D.; Schuch, F.; Van Damme, T.; Firth, J.; Suetani, S.; Stubbs, B.; Van Biesen, D. Metabolic syndrome and its components in people with intellectual disability: A meta-analysis. J. Intellect. Disabil. Res. 2020, 64, 804–815. [Google Scholar] [CrossRef]
- Bates, C.; Terry, L.; Popple, K. Partner Selection for People with Intellectual Disabilities. J. Appl. Res. Intellect. Disabil. 2017, 30, 602–611. [Google Scholar] [CrossRef]
- Grey, J.M.; Totsika, V.; Hastings, R.P. Living with family: Perceptions of health and subjective well-being of adults with an intellectual disability. J. Intellect. Disabil. Res. 2018, 62, 474–485. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Ouellette, H.; Martin, L. Frailty as a Predictor of Institutionalization Among Adults With Intellectual and Developmental Disabilities. Intellect. Dev. Disabil. 2016, 54, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Palomino, E.; López, J.M.; Botella, J.; Sotillo, M. Impairment of cognitive memory inhibition in individuals with intellectual disability: A meta-analysis. Psicothema 2019, 31, 384–392. [Google Scholar] [PubMed]
- Reichow, B.; Lemons, C.J.; Maggin, D.M.; Hill, D.R. Beginning reading interventions for children and adolescents with intellectual disability. Cochrane Database Syst. Rev. 2019, 12, ID011359. [Google Scholar] [CrossRef] [PubMed]
- Ptomey, L.T.; Szabo, A.N.; Martin, L.E.; Mayo, M.S.; Washburn, R.A.; Gorczyca, A.M. The promotion of physical activity for the prevention of Alzheimer’s disease in adults with Down Syndrome: Rationale and design for a 12 Month randomized trial. Contemp. Clin. Trials Commun. 2020, 19, 100607. [Google Scholar] [CrossRef] [PubMed]
- Pulsifer, M.B.; Evans, C.; Hom, C.; Krinsky, S.J.; Silverman, W.; Lai, F.; Lott, I.; Schupf, N.; Wen, J.; Rosas, H.D. Language skills as a predictor of cognitive decline in adults with Down syndrome. Alzheimer’s Dement. (Amst.) 2020, 12, e12080. [Google Scholar]
- Strydom, A.; Chan, T.; King, M.; Hassiotis, A.; Livingston, G. Incidence of dementia in older adults with intellectual disabilities. Res. Dev. Disabil. 2013, 34, 1881–1885. [Google Scholar] [CrossRef]
- Axmon, A.; Karlsson, B.; Ahlström, G. Health care utilisation among older persons with intellectual disability and dementia: A registry study. J. Intellect. Disabil. Res. 2016, 60, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Esteba, S.; Pena, J.; Garcia, J.; Castellanos, M.A.; Torrents, D.; Rodriguez, E. Test Barcelona para discapacidad intelectual: Un nuevo instrumento para la valoracion neuropsicologica clinica de adultos con discapacidad intelectual. Rev. Neurol. 2017, 64, 433–444. [Google Scholar]
- Farriols Danés, C. Aspectos específicos del envejecimiento en el síndrome de Down. Rev Med Int Sindr Down. 2012, 16, 3–10. [Google Scholar] [CrossRef]
- Krinsky, S.J.; Silverman, W. Dementia and mild cognitive impairment in adults with intellectual disability: Issues of diagnosis. Dev. Disabil. Res. Rev. 2013, 18, 31–42. [Google Scholar] [CrossRef]
- Ranjan, S.; Nasser, J.A.; Fisher, K. Prevalence and potential factors associated with overweight and obesity status in adults with intellectual developmental disorders. J. Appl. Res. Intellect. Disabil. 2018, 31 (Suppl. 1), 29–38. [Google Scholar] [CrossRef]
- Enkelaar, L.; Smulders, E.; van Schrojenstein, H.; Geurts, A.C.; Weerdesteyn, V. A review of balance and gait capacities in relation to falls in persons with intellectual disability. Res. Dev. Disabil. 2012, 33, 291–306. [Google Scholar] [CrossRef]
- Robertson, J.; Baines, S.; Emerson, E.; Hatton, C. Service Responses to People with Intellectual Disabilities and Epilepsy: A Systematic Review. J. Appl. Res. Intellect. Disabil. 2017, 30, 1–32. [Google Scholar] [CrossRef]
- Robertson, J.; Hatton, C.; Emerson, E.; Baines, S. Prevalence of epilepsy among people with intellectual disabilities: A systematic review. Seizure 2015, 29, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Helm, D.T.; Woodman, A.C. Unique and universal barriers: Hospice care for aging adults with intellectual disability. Am. J. Intellect. Dev. Disabil. 2012, 117, 509–532. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).