Animal as the Solution: Searching for Environmentally Friendly Dairy Cows
Abstract
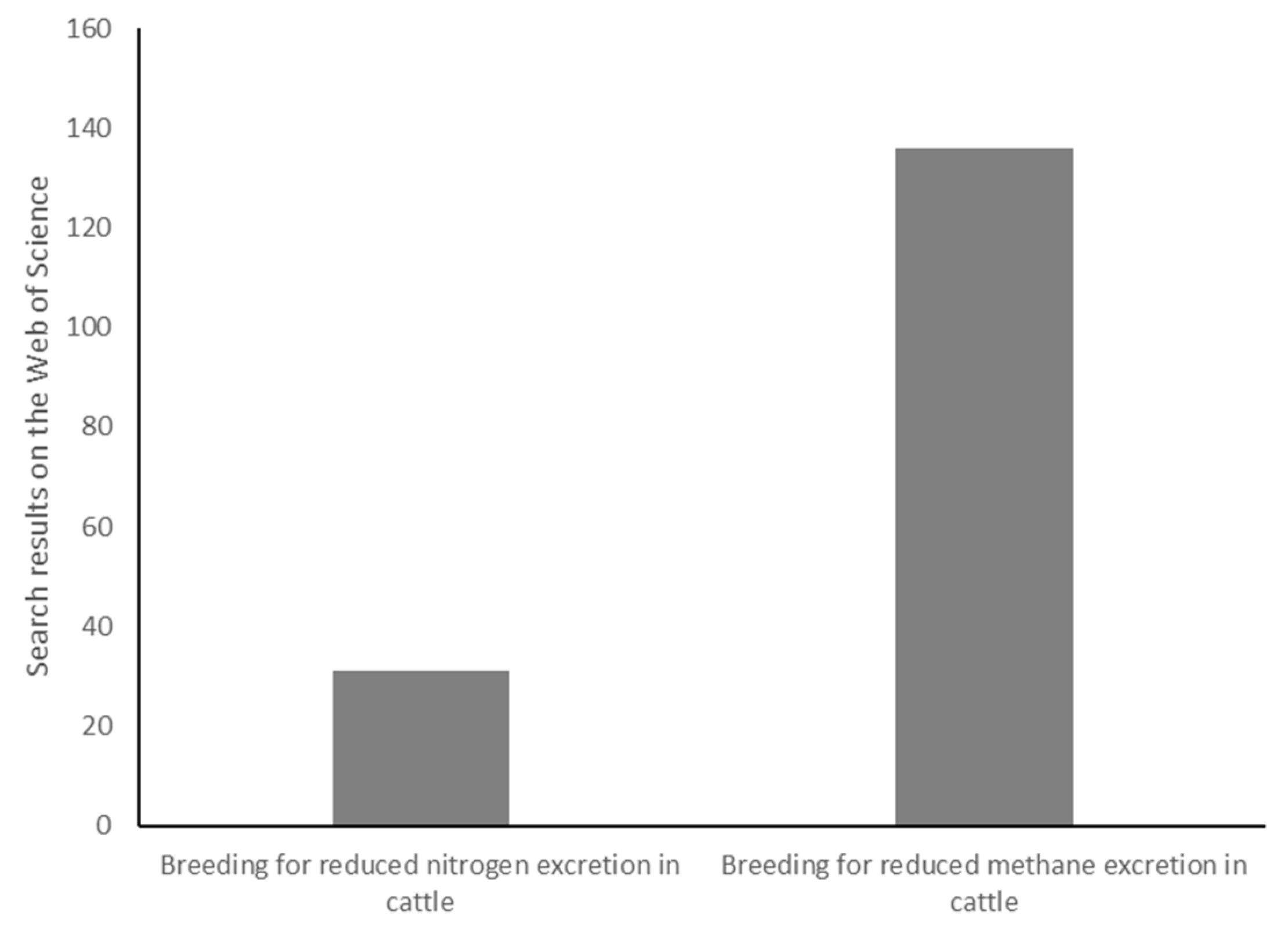
1. Introduction
2. Methodology
3. Discussion
3.1. Breeding for Reduced Milk Urea Nitrogen Content
3.2. Breeding for Lower Methane Production
3.3. Breeding for Increased Nutritional Value
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castillo, A.; Kebreab, E.; Beever, D.; France, J. A review of efficiency of nitrogen utilisation in lactating dairy cows and its relationship with environmental pollution. J. Anim. Feed Sci. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Kebreab, E.; France, J.; Beever, D.E.; Castillo, A.R. Nitrogen pollution by dairy cows and its mitigation by dietary manipulation. Nutr. Cycl. Agroecosystems 2001, 60, 275–285. [Google Scholar] [CrossRef]
- Gregorini, P.; Beukes, P.C.; Dalley, D.; Romera, A.J. Screening for diets that reduce urinary nitrogen excretion and methane emissions while maintaining or increasing production by dairy cows. Sci. Total Environ. 2016, 551–552, 32–41. [Google Scholar] [CrossRef]
- Oudshoorn, F.W.; Kristensen, T.; Nadimi, E.S. Dairy cow defecation and urination frequency and spatial distribution in relation to time-limited grazing. Livest. Sci. 2008, 113, 62–73. [Google Scholar] [CrossRef]
- Clark, C.; Waghorn, G.; Gregorini, P.; Woodward, S.; Clark, D. Diurnal pattern of urinary and faecal nitrogen excretion by dairy cows fed ryegrass pasture twice daily indoors. Adv. Anim. Biosci. 2010, 2, 269. [Google Scholar]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M.A. The Challenge of the Urine Patch for Managing Nitrogen in Grazed Pasture Systems; Advances in Agronomy; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 129. [Google Scholar]
- Eutrophication: Causes, Consequences and Control; Ansari, A., Gill, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar] [CrossRef]
- Day, J.; Yanez-Arancibia, A.; Kemp, W.M.; Crump, B.C. Estuarine Ecology. Estuaries 1990, 1, 1–44. [Google Scholar] [CrossRef]
- Officer, C.B.; Ryther, J.H. The Possible Importance of Silicon in Marine Eutrophication. Mar. Ecol. 1980, 3, 83–91. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Day, J.W.; Gilliam, J.W.; Groffman, P.M.; Hey, D.L.; Randall, G.W.; Wang, N. Reducing nitrogen loading to the gulf of Mexico from the Mississippi River Basin: Strategies to counter a persistent ecological problem. BioScience 2001, 51, 373–388. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Scavia, D. Beyond science into policy: Gulf of Mexico hypoxia and the Mississippi River. BioScience 2002, 52, 129–142. [Google Scholar] [CrossRef]
- Mclaren, R.; Cameron, K. Soil Science: Sustainable Production and Environmental Protection; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Henry, B.; Eckard, R. Greenhouse gas emissions in livestock production systems. Trop. Grassl. 2009, 43, 232–238. [Google Scholar]
- O’Mara, F.P. The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future. Anim. Feed Sci. Technol. 2011, 166–167, 7–15. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassener, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options. Frontiers in Ecology and the Environment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar] [CrossRef]
- McAllister, T.A.; Meale, S.J.; Valle, E.; Guan, L.L.; Zhou, M.; Kelly, W.J.; Henderson, G.; Attwood, G.T.; Janssen, P.H. Ruminant nutrition symposium: Use of genomics and transcriptomics to identify strategies to lower ruminal methanogenesis. J. Anim. Sci. 2015, 93, 1431–1449. [Google Scholar] [CrossRef]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; Royz, A.; Dell, C.; Adesogan, A.; et al. Mitigation of Greenhouse Gas Emissions in Livestock Production A Review of Technical Options for non-CO2 Emissions; Gerber, P.J., Henderson, B., Makkar, H.P.S., Eds.; FAO: Rome, Italy, 2005. [Google Scholar]
- Aumann, H.H.; Ruzmaikin, A.; Teixeira, J. Frequency of severe storms and global warming. Geophys. Res. Lett. 2008, 35, 2–5. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.A.; Lui, H.K.; Hsieh, C.H.; Yanagi, T.; Kosugi, N.; Ishii, M.; Gong, G.C. Deep oceans may acidify faster than anticipated due to global warming. Nat. Clim. Chang. 2017, 7, 890–894. [Google Scholar] [CrossRef]
- Johnson, S.F. Methemoglobinemia: Infants at risk. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Brender, J.D.; Olive, J.M.; Felkner, M.; Suarez, L.; Marckwardt, W.; Hendricks, K.A. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology 2004, 15, 330–336. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals. Available online: https://sustainabledevelopment.un.org/sdgs (accessed on 14 May 2020).
- Beukes, P.C.; Gregorini, P.; Romera, A.J.; Woodward, S.L.; Khaembah, E.N.; Chapman, D.F.; Nobilly, F.; Bryant, R.H.; Edwards, G.R.; Clark, D.A. The potential of diverse pastures to reduce nitrogen leaching on New Zealand dairy farms. Anim. Prod. Sci. 2014, 54, 1971–1979. [Google Scholar] [CrossRef]
- McCaughey, W.P.; Wittenberg, K.; Corrigan, D. Impact of pasture type on methane production by lactating beef cows. Can. J. Anim. Sci. 1999, 79, 221–226. [Google Scholar] [CrossRef]
- Tamminga, S.; Bannink, A.; Dijkstra, J.; Zom, R. Feeding Strategies to Reduce Methane Loss in Cattle; Animal Science Group: Wageningen, The Netherlands, 2007. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Boyle, E. High Steaks Why and How to Eat Less Meat; New Society Publishers, 2012; Available online: https://newsociety.com/books/h/high-steaks (accessed on 20 July 2021).
- Ungar, E.D. Perspectives on the concept of rangeland carrying capacity, and their exploration by means of Noy-Meir’s two-function model. Agric. Syst. 2019, 173, 403–413. [Google Scholar] [CrossRef]
- Dilworth, T.; McGregor, A. Moral Steaks? Ethical Discourses of In Vitro Meat in Academia and Australia. J. Agric. Environ. Ethics 2015, 28, 85–107. [Google Scholar] [CrossRef]
- Tijhuis, M.J.; Ezendam, J.; Westenbrink, S.; van Rossum, C.; Temme, L. Replacement of Meat and Dairy by More Sustainable Protein Sources in the Netherlands: Quality of the Diet; RIVM Letter Report 350123001; 2011; Available online: https://www.rivm.nl/bibliotheek/rapporten/350123001.pdf (accessed on 25 July 2021).
- Leroy, F.; Hite, A.H.; Gregorini, P. Livestock in Evolving Foodscapes and Thoughtscapes. Front. Sustain. Food Syst. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Chalupa-krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. OPEN A metabolomics comparison of plant-based meat and grass—Fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021, 1–13. [Google Scholar] [CrossRef]
- Huntington, G.B.; Archibeque, S.L. Practical aspects of urea and ammonia metabolism in ruminants. J. Anim. Sci. 1999, 77, 1. [Google Scholar] [CrossRef]
- Moharrery, A. Investigation of different levels of RDP in the rations of lactating cows and their effects on MUN, BUN and urinary N excretion. Ital. J. Anim. Sci. 2004, 3, 157–165. [Google Scholar] [CrossRef][Green Version]
- Butler, W.R.; Calaman, J.J.; Beam, S.W. Plasma and Milk Urea Nitrogen in Relation to Pregnancy Rate in Lactating Dairy Cattle. J. Anim. Sci. 1996, 74, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.M.; Rotz, C.A.; Wattiaux, M.A. Potential Use of Milk Urea Nitrogen to Abate Atmospheric Nitrogen Emissions from Wisconsin Dairy Farms. J. Environ. Qual. 2014, 43, 1169–1175. [Google Scholar] [CrossRef]
- Kohn, R. Use of Milk or Blood Urea Nitrogen to Identify Feed Management Inefficiencies and Estimate Nitrogen Excretion by Dairy Cattle and Other Animals. In Florida Ruminant Nutrition Symposium; Gainesville University of Florida: Gainesville, FL, USA, 2007. [Google Scholar]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.S.; Kohn, R.A.; Erdman, R.A. Using Milk Urea Nitrogen to Predict Nitrogen Excretion and Utilization Efficiency in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 2681–2692. [Google Scholar] [CrossRef]
- Ciszuk, P.; Gebregziabher, T. Milk urea as an estimate of urine nitrogen of dairy cows and goats. Acta Agric. Scand. Anim. Sci. 1994, 44, 87–95. [Google Scholar] [CrossRef]
- Piccione, G.; Grasso, F.; Fazio, F.; Assenza, A.; Caola, G. Influence of different schedules of feeding on daily rhythms of blood urea and ammonia concentration in cows. Biol. Rhythm Res. 2007, 38, 133–139. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use Manag. 2002, 18, 395–403. [Google Scholar] [CrossRef]
- Beatson, P.R.; Meier, S.; Cullen, N.G.; Eding, H. Genetic variation in milk urea nitrogen concentration of dairy cattle and its implications for reducing urinary nitrogen excretion. Animal 2019, 13, 2164–2171. [Google Scholar] [CrossRef]
- Kauffman, A.J.; St-Pierre, N.R. The Relationship of Milk Urea Nitrogen to Urine Nitrogen Excretion in Holstein and Jersey Cows. J. Dairy Sci. 2001, 84, 2284–2294. [Google Scholar] [CrossRef]
- Kohn, R.A.; Kalscheur, K.F.; Russek-Cohen, E. Evaluation of models to estimate urinary nitrogen and expected milk urea nitrogen. J. Dairy Sci. 2002, 85, 227–233. [Google Scholar] [CrossRef]
- Stoop, W.M.; Bovenhuis, H.; van Arendonk, J.A.M. Genetic Parameters for Milk Urea Nitrogen in Relation to Milk Production Traits. J. Dairy Sci. 2007, 90, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.G.; Rogers, G.W.; Dechow, C.D.; Vallimont, J.E.; Cooper, J.B.; Sander-Nielsen, U.; Clay, J.S. Milk urea nitrogen concentration: Heritability and genetic correlations with reproductive performance and disease. J. Dairy Sci. 2005, 88, 4434–4440. [Google Scholar] [CrossRef]
- König, S.; Chang, Y.M.; Borstel, U.U.V.; Gianola, D.; Simianer, H. Genetic and phenotypic relationships among milk urea nitrogen, fertility, and milk yield in Holstein cows. J. Dairy Sci. 2008, 91, 4372–4382. [Google Scholar] [CrossRef]
- Mucha, S.; Strandberg, E. Genetic analysis of milk urea nitrogen and relationships with yield and fertility across lactation. J. Dairy Sci. 2011, 94, 5665–5672. [Google Scholar] [CrossRef]
- Lopez-Villalobos, N.; Correa-Luna, M.; Burke, J.L.; Sneddon, N.; Schutz, M.; Donaghy, D.J.; Kemp, P.D. Genetic parameters for milk urea concentration and milk traits in New Zealand grazing dairy cattle. N. Z. J. Anim. Sci. Prod. 2018, 78, 56–61. [Google Scholar]
- Huhtanen, P.; Cabezas-Garcia, E.H.; Krizsan, S.J.; Shingfield, K.J. Evaluation of between-cow variation in milk urea and rumen ammonia nitrogen concentrations and the association with nitrogen utilization and diet digestibility in lactating cows. J. Dairy Sci. 2015, 98, 3182–3196. [Google Scholar] [CrossRef]
- Kebreab, E.; France, J.; Mills, J.A.N.; Allison, R.; Dijkstra, J. A dynamic model of N metabolism in the lactating dairy cow and an assessment of impact of N excretion on the environment. J. Anim. Sci. 2002, 80, 248–259. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; Bannink, A. Dietary strategies to reducing N excretion from cattle: Implications for methane emissions. Curr. Opin. Environ. Sustain. 2011, 3, 414–422. [Google Scholar] [CrossRef]
- Garrett, K.; Beck, M.R.; Gregorini, P. Strategic feeding management to mitigate enteric methane emissions and urinary nitrogen excretion. N. Z. J. Anim. Sci. Prod. 2019, 79, 20–25. [Google Scholar]
- Freeman, A.E. Animal breeding. Encycl. Br. 2017, 19.
- Marshall, C.J.; Beck, M.R.; Garrett, K.; Barrell, G.K.; Al-Marashdeh, O.; Gregorini, P. Grazing dairy cows with low milk urea nitrogen breeding values excrete less urinary urea nitrogen. Sci. Total Environ. 2020, 739, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J.; Beck, M.R.; Garrett, K.; Barrell, G.K.; Al-Marashdeh, O.; Gregorini, P. Nitrogen balance of dairy cows divergent for milk urea nitrogen breeding values consuming either plantain or perennial ryegrass. Animals 2021, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Ariyarathne, H.B.P.C.; Correa-Luna, M.; Blair, H.; Garrick, D.; Lopez-Villalobos, N. Can nitrogen excretion of dairy cows be reduced by genetic selection for low milk urea nitrogen concentration? Animals 2021, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, P.; Beukes, P.; Romera, A.J.; Clark, C.; Clark, D. A preliminary investigation of individual variation in N excretion by lactating dairy cows. J. Anim. Sci. 2010, 88, 409–410. [Google Scholar]
- Grandl, F.; Furger, M.; Kreuzer, M.; Zehetmeier, M. Impact of longevity on greenhouse gas emissions and profitability of individual dairy cows analysed with different system boundaries. Animal 2019, 13, 198–208. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Saville, W.J.A.; Frazer, G.S.; Wittum, T.E. Association between milk urea nitrogen and fertility in Ohio dairy cows. J. Dairy Sci. 2001, 84, 482–489. [Google Scholar] [CrossRef]
- Hojman, D.; Kroll, O.; Adin, G.; Gips, M.; Hanochi, B.; Ezra, E. Relationships between milk urea and production, nutrition, and fertility traits in Israeli dairy herds. J. Dairy Sci. 2004, 87, 1001–1011. [Google Scholar] [CrossRef]
- Elrod, C.C.; Butler, W.R. Reduction of fertility and alteration of uterine pH in heifers fed excess ruminally degradable protein. J. Anim. Sci. 1993, 71, 694–701. [Google Scholar] [CrossRef]
- Garnsworthy, P.C. The environmental impact of fertility in dairy cows: A modelling approach to predict methane and ammonia emissions. Anim. Feed Sci. Technol. 2004, 112, 211–223. [Google Scholar] [CrossRef]
- Totty, V.K.; Greenwood, S.L.; Bryant, R.H.; Edwards, G.R. Nitrogen partitioning and milk production of dairy cows grazing simple and diverse pastures. J. Dairy Sci. 2013, 96, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Flores, L.; Bionaz, M.; Downing, T.; Sahin, M.; Cheng, L.; Ates, S. Milk production, N partitioning, and methane emissions in dairy cows grazing mixed or spatially separated simple and diverse pastures. Animals 2020, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, S.; Kemp, P.D.; Pain, S.J.; Back, P.J. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2016, 222, 158–167. [Google Scholar] [CrossRef]
- Sneddon, N.W.; Lopez-Villalobos, N.; Davis, S.R.; Hickson, R.E.; Shalloo, L. Genetic parameters for milk components including lactose from test day records in the New Zealand dairy herd. N. Z. J. Agric. Res. 2015, 58, 97–107. [Google Scholar] [CrossRef]
- Christensen, C.L.; Hedley, M.J.; Hanly, J.A.; Horne, D.J. Nitrogen loss mitigation using duration-controlled grazing: Field observations compared to modelled outputs. Proc. N. Z. Grassl. Assoc. 2012, 74, 115–120. [Google Scholar] [CrossRef]
- Aland, A.; Lidfors, L.; Ekesbo, I. Diurnal distribution of dairy cow defecation and urination. Appl. Anim. Behav. Sci. 2002, 78, 43–54. [Google Scholar] [CrossRef]
- Gregorini, P.; Waghorn, G.C.; Kuhn-Sherlock, B.; Romera, A.J.; Macdonald, K.A. Short communication: Grazing pattern of dairy cows that were selected for divergent residual feed intake as calves. J. Dairy Sci. 2015, 98, 6486–6491. [Google Scholar] [CrossRef]
- Marshall, C.J.; Beck, M.R.; Garrett, K.; Fleming, A.E.; Barrell, G.K.; Al-Marashdeh, O.; Gregorini, P. Dairy cows with different milk urea nitrogen breeding values display different grazing behaviours. Appl. Anim. Behav. Sci. 2021, 242. [Google Scholar] [CrossRef]
- Langworthy, A.D.; Verdon, M.; Freeman, M.J.; Corkrey, R.; Hills, J.L.; Rawnsley, R.P. Virtual fencing technology to intensively graze lactating dairy cattle. I: Technology efficacy and pasture utilization. J. Dairy Sci. 2021, 104, 7071–7083. [Google Scholar] [CrossRef] [PubMed]
- Raynor, E.J.; Derner, J.D.; Soder, K.J.; Augustine, D.J. Noseband sensor validation and behavioural indicators for assessing beef cattle grazing on extensive pastures. Appl. Anim. Behav. Sci. 2021, 242, 105402. [Google Scholar] [CrossRef]
- Anderson, D.M. Virtual fencing past, present and future. Rangel. J. 2007, 29, 65–78. [Google Scholar] [CrossRef]
- Verdon, M.; Horton, B.; Rawnsley, R. A Case Study on the Use of Virtual Fencing to Intensively Graze Angus Heifers Using Moving Front and Back-Fences. Front. Anim. Sci. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Giller, K.; Kreuzer, M.; Ulbrich, S.E.; Braun, U.; Schwarm, A. Contribution of ruminal fungi, archaea, protozoa, and bacteria to the methane suppression caused by oilseed supplemented diets. Front. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef]
- Pickering, N.K.; Oddy, V.H.; Basarab, J.; Cammack, K.; Hayes, B.; Hegarty, R.S.; Lassen, J.; McEwan, J.C.; Miller, S.; Pinares-Patino, C.S.; et al. Animal board invited review: Genetic possibilities to reduce enteric methane emissions from ruminants. Animal 2015, 9, 1431–1440. [Google Scholar] [CrossRef]
- Wall, E.; Simm, G.; Moran, D. Developing breeding schemes to assist mitigation of greenhouse gas emissions. Animal 2010, 4, 366–376. [Google Scholar] [CrossRef]
- Cottle, D.J.; Nolan, J.V.; Wiedemann, S.G. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 2011, 51, 491–514. [Google Scholar] [CrossRef]
- Manzanilla-Pech, C.I.V.; Gordo, D.M.; Difford, G.F.; Pryce, J.E.; Schenkel, F.; Wegmann, S.; Miglior, F.; Chud, T.C.; Moate, P.J.; Williams, S.R.O.; et al. Breeding for reduced methane emission and feed-efficient Holstein cows: An international response. J. Dairy Sci. 2021, 104, 8983–9001. [Google Scholar] [CrossRef]
- Lahart, B.; Shalloo, L.; Herron, J.; Brien, D.O.; Fitzgerald, R.; Boland, T.M.; Buckley, F. Greenhouse gas emissions and nitrogen efficiency of dairy cows of divergent economic breeding index under seasonal pasture-based management. J. Dairy Sci. 2021, 104, 8039–8049. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Shalloo, L.; Pierce, K.M.; Buckley, F. Economic assessment of Holstein-Friesian dairy cows of divergent Economic Breeding Index evaluated under seasonal calving pasture-based management. J. Dairy Sci. 2020, 103, 10311–10320. [Google Scholar] [CrossRef]
- Beukes, P.C.; Gregorini, P.; Romera, A.J. Estimating greenhouse gas emissions from New Zealand dairy systems using a mechanistic whole farm model and inventory methodology. Anim. Feed Sci. Technol. 2011, 166–167, 708–720. [Google Scholar] [CrossRef]
- Basarab, J.A.; Beauchemin, K.A.; Baron, V.S.; Ominski, K.H.; Guan, L.L.; Miller, S.P.; Crowley, J.J. Reducing GHG emissions through genetic improvement for feed efficiency: effects on economically important traits and enteric methane production. Anim. Int. J. Anim. Biosci. 2013, 7 (Suppl. S2), 303–315. [Google Scholar] [CrossRef]
- Alemu, A.W.; Vyas, D.; Manafiazar, G.; Basarab, J.A.; Beauchemin, K.A. Enteric methane emissions from low– and high–residual feed intake beef heifers measured using GreenFeed and respiration chamber techniques. J. Anim. Sci. 2017, 95, 3727. [Google Scholar] [CrossRef]
- Dini, Y.; Cajarville, C.; Gere, J.I.; Fernandez, S.; Fraga, M.; Pravia, M.I.; Navajas, E.A.; Ciganda, V.S. Association between residual feed intake and enteric methane emissions in Hereford steers. Transl. Anim. Sci. 2019, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.R.; Hegarty, R.S.; Parnell, P.F.; Cacho, O.J.; Herd, R.M.; Griffith, G.R. The impact of breeding to reduce residual feed intake on enteric methane emissions from the Australian beef industry. Aust. J. Exp. Agric. 2006, 46, 813–820. [Google Scholar] [CrossRef]
- Nkrumah, J.D.; Nkrumah, J.D.; Okine, E.K.; Okine, E.K.; Mathison, G.W.; Mathison, G.W.; Schmid, K.; Schmid, K.; Li, C.; Li, C.; et al. Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J. Anim. Sci. 2006, 84, 145–153. [Google Scholar] [CrossRef]
- Gregorini, P.; Villalba, J.; Chilibroste, P.; Provenza, F. Grazing management: setting the table, designing the menu and influencing the diner. Anim. Prod. Sci. 2017, 57, 1248. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Goopy, J.P.; Donaldson, A.; Hegarty, R.; Vercoe, P.E.; Haynes, F.; Barnett, M.; Oddy, V.H. Low-methane yield sheep have smaller rumens and shorter rumen retention time. Br. J. Nutr. 2013, 111, 578–585. [Google Scholar] [CrossRef]
- McDonnell, R.P.; Hart, K.J.; Boland, T.M.; Kelly, A.K.; McGee, M.; Kenny, D.A. Effect of divergence in phenotypic residual feed intake on methane emissions, ruminal fermentation, and apparent whole-tract digestibility of beef heifers across three contrasting diets. J. Anim. Sci. 2016, 94, 1179–1193. [Google Scholar] [CrossRef]
- Flay, H.E.; Kuhn-Sherlock, B.; Macdonald, K.A.; Camara, M.; Lopez-Villalobos, N.; Donaghy, D.J.; Roche, J.R. Hot topic: Selecting cattle for low residual feed intake did not affect daily methane production but increased methane yield. J. Dairy Sci. 2019, 102, 2708–2713. [Google Scholar] [CrossRef]
- Pinares-Patiño, C.S.; Hickey, S.M.; Young, E.A.; Dodds, K.G.; MacLean, S.; Molano, G.; Sandoval, E.; Kjestrup, H.; Harland, R.; Hunt, C.; et al. Heritability estimates of methane emissions from sheep. Anim. Int. J. Anim. Biosci. 2013, 7 (Suppl. S2), 316–321. [Google Scholar] [CrossRef]
- Donoghue, K.A.; Herd, R.M.; Bird, S.H.; Arthur, P.F.; Hegarty, R.F. Preliminary genetic parameters for methane production in Australian beef cattle. Proc. Assoc. Advmt. Anim. Breed Genet 2013, 20, 290–293. [Google Scholar]
- United Nations. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables; 2015; Available online: https://population.un.org/wpp/Publications/Files/Key_Findings_WPP_2015.pdf (accessed on 15 July 2021).
- Newell-McGloughlin, M. Nutritionally improved agricultural crops. Plant Physiol. 2008, 147, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Primary Industries Omega Lamb. 2020. Available online: https://www.mpi.govt.nz/funding-rural-support/primary-growth-partnerships-pgps/current-pgp-programmes/omega-lamb/?start=16 (accessed on 20 July 2021).
- Hermesch, S.; Jones, R.M. Genetic parameters for haemoglobin levels in pigs and iron content in pork. Animal 2012, 6, 1904–1912. [Google Scholar] [CrossRef]
- Sakuma, H.; Saito, K.; Kohira, K.; Ohhashi, F.; Shoji, N.; Uemoto, Y. Estimates of genetic parameters for chemical traits of meat quality in Japanese black cattle. Anim. Sci. J. 2017, 88, 203–212. [Google Scholar] [CrossRef] [PubMed]
- De Smet, S.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- McAllister, T.A.; Okine, E.K.; Mathison, G.W.; Cheng, K.J. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, C.J.; Gregorini, P. Animal as the Solution: Searching for Environmentally Friendly Dairy Cows. Sustainability 2021, 13, 10451. https://doi.org/10.3390/su131810451
Marshall CJ, Gregorini P. Animal as the Solution: Searching for Environmentally Friendly Dairy Cows. Sustainability. 2021; 13(18):10451. https://doi.org/10.3390/su131810451
Chicago/Turabian StyleMarshall, Cameron J., and Pablo Gregorini. 2021. "Animal as the Solution: Searching for Environmentally Friendly Dairy Cows" Sustainability 13, no. 18: 10451. https://doi.org/10.3390/su131810451
APA StyleMarshall, C. J., & Gregorini, P. (2021). Animal as the Solution: Searching for Environmentally Friendly Dairy Cows. Sustainability, 13(18), 10451. https://doi.org/10.3390/su131810451





