Abstract
Drought and low amounts of mineral nutrients in the soil are the two leading global constraints in arid and semiarid regions. Their detrimental effects on soils and crops can be alleviated by applying controlled release and biodegradable fertilizers to better and sustain the crops. On a global scale, spinach (Spinacia oleracea L.) is an essential leafy green vegetable that is biologically considered a reliable source of essential nutrients and minerals for human health. A comprehensive approach is needed to manage water stress to mitigate the impacts of stress-caused damage and to examine this for better and increased plant production. An experiment was conducted using potassium-nitrate-containing chitosan/montmorillonite microparticles (150 mg) under mild and severe drought stress (MDS: 50% and SDS: 35% FC, respectively). The treatments include control (no KNO3 and 70% FC as normal irrigation (NI)), KNO3 + NI, 50% FC as mild drought stress (MDS), KNO3 + MDS, 35% FC as severe drought stress (SDS) and KNO3 + SDS. Results revealed that drought stress decreased all studied physiological parameters and increased oxidative stress indicators in spinach. Applying KN significantly increased root (122%) and shoot length (4%), shoot fresh weight (32%) and shoot dry weight (71%), chlorophyll a (88%), carotenoids (39%), total soluble proteins (50%), soluble sugars (51%), potassium (80%), and phosphorous (32%) concentrations over No KN at severe drought. While stress indicators, like glycine betaine, malondialdehyde, hydrogen peroxide, electrolyte leakage, peroxidase, superoxide dismutase, and ascorbic acid levels, were increased in stress. Treatment KN was proved efficient and effective in improving spinach physiological status in both MDS and SDS.
Keywords:
osmotic stress; mineral fertilizer; soil mineral; antioxidants; growth attributes; spinach 1. Introduction
Scarcity of good quality water for irrigation creates the majority of lethal stress among all abiotic stresses (i.e., heavy metals, nutrients), called drought [1,2,3,4,5]. Uneven and low rainfall, dry winds, and temperature dynamics are common factors that promote the development of drought conditions [6]. Drought stress play a notorious role in decreasing crops productivity because water shares 80–95% of the fresh biomass of any plant [6]. It is a documented fact that annually, 50% yield of crops is decreased due to water scarcity. This drought-induced yield reduction is being reported in many food crops, including spinach [7]. It has been observed that drought stress can reduce 65% biomass of spinach by decreasing fresh weight [8]. This drastic reduction in fresh biomass is mainly due to the disruption of plant’s photosynthetic capacity by limiting its physiological and biochemical attributes under the water deficit environment [6,9].
Typical symptoms of drought stress are leaf yellowing, leaf rolling, permanent wilting, early leaf senescence, and stunted growth. Extensive damage to root growth is documented in the plants that are subjected to water-deficit conditions [6,10]. Deep, elongated roots with capillary structures help plants to uptake more water from deeper soil layers [8]. Osmotic adjustment through solute accumulation, antioxidative defense system, dynamics of stomatal closure, and especially balance use of potassium (K+) as fertilizer are important strategies of plants to overcome drought stress effects [6]. Potassium, being an essential micronutrient and the most abundant cation in plants, plays an important role in metabolic processes that influence the metabolism and growth of the plant. It is also involved in the defense mechanism against various abiotic and biotic stresses [11]. During drought, stressed plants uptake more potassium to regulate the internal mechanisms and ameliorate water deficiency [6,9,12,13,14,15]. The use of K as a compatible solute can osmo-regulate the cellular environments in water stress conditions. It can decrease electrolyte leakage, maintain the turgor potential of the cell, and improve relative water contents, activation of enzymes, ionic balance, photosynthesis, protein biosynthesis, energy transfer, phloem transport, osmoregulation, and most importantly, stress resistance and nutrient uptake of plants in water stress conditions [11]. Moreover, potassium increases carbohydrate synthesis by stabilizing photosynthetic activity when applied exogenously during water scarcity.
Potassium is well reported to reduce drought stress effects in plants [9]. Plants mostly use Na and K for osmotic adjustments. However, less water uptake resulted in significant decreases of K ions in the spinach leaves [8]. Exogenous application of KNO3 as treatment can increase multiple physiological and biochemical mechanisms [16]. Additional KNO3 increases the photosynthetic pigment contents, potassium content, and enhances plant growth and decreases sodium in moisture-stressed plants. The water deficiency causes lipid peroxidation (measured as MDA) in leaves. Applying KNO3 to plants maintains or even reduces MDA contents, thus suggesting a crucial role of K+ in improving the antioxidant system and ameliorating drought stress conditions [17].
Increasing agricultural sustainability by adding controlled-release fertilizers that are biodegradable and leave no residue is a primary concern these days. These materials improve the water availability to crops and are economical also. The controlled-release coated fertilization aids continuous and regular nutrient delivery, less concentrated fertilizer application, low soil salinization, and lesser nutrient leaching. Chitosan has been extensively used in making the controlled release of microspheres as a coating material. This happens due to its natural biopolymer, non-toxicity, biocompatibility, and biodegradability. In addition, developing new materials by combining inorganic and polymeric materials more than pure polymers are of major concern for the last few years. Enhancing the sorptive capacity of nutrient compounds and water by adding layered silicates, like montmorillonite clay particles, is a promising factor [18]. Clay minerals provide efficient and cost-effective material for this purpose [19].
Spinach (Spinacea oleracea L.), an annual plant from Chenopodiaceae, is the most common leafy green vegetable. It is a good source of minerals and vitamins for humans and is an essential vegetable with good cooking adaptability. Many countries are global commercial producers of spinach, including China, United States, Japan, Indonesia, and Turkey, with the production of 2768 kg·ha−1, 2359 kg·ha−1, 12,471 kg·ha−1, 3424 kg·ha−1, and 9249 kg·ha−1, respectively. However, rapidly changing climate and lesser availability of water limits spinach cultivation [9]. As spinach needs relatively greater water contents, 50% decrease in irrigation can suppress its relative water contents, leaf area, growth, and yield [20].
So far, many scientists have documented the positive role of K against drought stress. Yet, limited work is documented on the use of potassium nitrate containing chitosan/montmorillonite microparticles. Therefore, the present study was conducted to check the potential effects of potassium nitrate containing chitosan/montmorillonite microparticles on spinach under drought stress. It is hypothesized that potassium nitrate (containing chitosan/montmorillonite microparticles) might be effective for better spinach growth under drought stress.
2. Materials and Methods
2.1. Experimental Site, Design, and Treatment Plan
A pot experiment (2019) was performed at Old Botanical Garden, University of Agriculture, Faisalabad. Geographically, Faisalabad is located at 31.4504° N, 73.1350° E at 610 ft above the mean sea level in flat plains of northeast Punjab in a semi-arid temperature zone. Soil (15–20 cm depth) was obtained from university research site and then dried and sieved through a 2-mm sieve. Then, the soil was uniformly packed in pots (25-cm diameter × 18-cm height). Some important soil characteristics include electrical conductivity (EC), 7.82 (dS/m); organic matter (OM), 0.68%, pH, 8.1; total nitrogen (N), 0.034%; available phosphorus (P), 6.13 mg/kg; and extractable potassium (K), 123 mg/kg. The experimental design was a completely randomized block design (CRD) and replicated four times. The treatments include control (no potassium nitrate under 70% FC as normal irrigation (NI)); KNO3 + NI, 50% FC irrigation as mild drought stress (MDS); and KNO3 + MDS, 35% FC irrigation as severe drought stress (SDS), KNO3 + SDS.
2.2. Fertilizer Preparation
Mechano-chemical intercalation of KNO3 and montmorillonite (MM) was done using agate mortar by grinding 75% by weight of KNO3 in distilled water and montmorillonite in 1:3 w/w of MM/KNO3. Then, MM/KNO3 was dried in the oven and crushed into powder. Meanwhile, 4 g chitosan (C) mixed in 100 mL of acetic acid solution (2% v/v) was kept at 25 °C for a day. C/MM-KNO3 was made by mixing 16 g MM-KNO3 with chitosan solution to have final ratio of 1:4 of C/MMt-KNO3. Then, using a mechanical stirrer, the homogenous dispersions were maintained at 12.000 rpm for 1 h. Complete chitosan crosslinking was achieved at two different volumes of sodium tripolyphosphate solution (32 and 64 mL of 1% and 5% w/v, respectively) to C/MM-KNO3 dispersions. After this, the solution was dried using a spray dryer (1.5-mm nozzle cap) to ensure a completely dry material [21]. There were 2 levels of potassium nitrate (KNO3), i.e., control (having no KNO3) and 150 mg KNO3 pot−1. Potassium-nitrate-containing chitosan/montmorillonite microparticles were added to soil at the concentration of 150 mg pot−1 at the time of sowing.
2.3. Plant Material and Seed Sowing
Seeds of Spinach were collected from Ayub Agriculture Research Institute, Faisalabad. The seeds were disinfected using ethanol (95%) for 60 s and then with sodium hypochlorite solution (NaOCl; 70%) for 10 min. All seeds were later washed six times with deionized water.
2.4. Irrigation and Drought Stress
For irrigation purposes, tap water was used. There were three water regimes, i.e., controlled, normal irrigation (70% FC), mild drought stress (50% FC), and severe drought stress (35% FC). All the irrigations levels were maintained in w/w basis. For determination of field capacity (FC), 5 kg of soil was taken in a pot. After that, soil was fully saturated with water. The volume of water that was used for saturation of soil was also weighed on analytical grade balance (y). Finally, the pot was left for 24 h so that gravitational water may leached down. The next day, the weight of leachate water (z) was determined on analytical grade balance. The difference in weight provides the amount of water required for achievement of 100%FC of 5 kg soil. From this, 70 and 50% FC for selected soil was calculated by using unit method equations, i.e.,
- For 100% FC weight of water required = (y − z) = x (g)
- For 1% FC weight of water required = x/100 (g)
- For 70% FC weight of water required = (x/100) × 70 (g)
- For 50% FC weight of water required = (x/100) × 50 (g)
2.5. Harvesting and Data Collection
The harvest was taken after 25 days of sowing. After harvest, all plants were rinsed twice with distilled water. Fresh weights were measured immediately after harvesting using a digital weighing balance at the experimental site. Plant shoot and root lengths were measured using a measuring tape. Fresh plants samples were stored in biomedical freezer at −30 °C. Samples from each treatment were oven dried for 72 h at 65 °C and were used to measure dry weight and ion analysis.
2.6. Determination of Chlorophyll Contents
Chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents were assessed following the standard Arnon protocol [22]. A total of 0.1 g of fresh leaf samples were extracted in 95% of acetone (8 mL) at 4 °C temperature in the dark for 24 h. Then, 646-, 663-, and 450-nm absorbance were recorded by spectrophotometer (xMark Microplate Absorbance Spectrophotometer; Bio-Rad, Hercules, CA, USA).
2.7. Malondialdehyde (MDA)
MDA contents were evaluated to determine lipid peroxidation extent during oxidative stress. A total of 0.1 g fresh leaf samples were homogenized in 25 mL of 50 mM concentrated phosphate buffer (pH 7.8) having 1% polyethene pyrrole at 4 °C and centrifuged for 15 min at 10,000× g. This mixture was boiled at 100 °C for 20 min and cooled immediately in an ice bath. The absorbance of supernatant liquid in solution was recorded by spectrophotometer (xMark Microplate Absorbance Spectrophotometer; Bio-Rad, Hercules, CA, USA) at 532-, 600-, and 450-nm wavelengths. Peroxidation of lipid components was recorded using protocol by Heath and Packer [23].
2.8. Hydrogen Peroxide (H2O2)
H2O2 estimation of leaf and root samples was done using 3 mL of sample extracts in 1 mL of 0.1% titanium sulfate and 20% H2SO4 (v/v) and then centrifuged for 15 min at 6000× g. The color intensity of samples was analyzed by spectrophotometer at a wavelength of 410 nm following the Jana and Choudhuri method [24].
2.9. Electrolyte Leakage (EL)
EL caused by drought stress of flag leaves was estimated following the Dionisio-Sese and Tobita method [25]. The samples were immersed in 8 mL of distilled water in test tubes and incubated for 2 h, and EC 1 (initial electrical conductivity) was measured. The samples extracts were then autoclaved for 20 min at 121 °C temperature and cooled at room temperature, and EC 2 (final electrical conductivity) wula:
EL (%) = (EC 1/EC 2) × 100
2.10. Antioxidant Enzymes
To assess antioxidant activities, 0.5 g fresh leaf samples were homogenized in liquid nitrogen, 0.15 mol NaCl, 50 mmol of sodium phosphate buffer (5 mL, pH 7.0), and 0.5 mmol EDTA solution. This extracted mixture was centrifuged for 10 min at 12,000× g with 4 °C temperature. The supernatant liquid was used for the evaluation of peroxidase and superoxidase dismutase activities in plants. SOD activity was determined by taking 3 mL sample mixtas measured by the following formure having 50 mM concentrated sodium phosphate buffer of pH 7, 56 mM of nitro blue tetrazolium chloride, 1.17 mM of riboflavin, 10 mM methionine, and 100 mL of enzyme extract. The light intensity of the final reaction mixture was estimated by spectrophotometer, following the method of Chen and Pan [26]. Peroxidase (POD) enzyme activity in the spinach leaf sample was analyzed by the Sakharov and Ardila [27] method. For this, guaiacol substrate was used. A 3-mL mixture was prepared, containing 0.1 mL of 4% guaiacol solution, 0.05 mL of enzyme extract, 2.75 mL of phosphate buffer (50 mM, pH 7.0), and 0.1 mL of 1% H2O2. Color intensity was recorded at 470 nm wavelength.
2.11. Soluble Sugars and Non-Enzymatic Antioxidants
To determine the concentrations of osmolytes and non-enzymatic antioxidant constituents, ethanol extracts of spinach plant samples were prepared using 50 mg of dried plant matter that was extracted in 10 mL of 80% ethanol and filtered using a filter paper and re-extracted in 10 mL ethanol. The 20-mL final volume of the solution was maintained by mixing the two extracts. This reaction mixture was used to determine concentrations of the following: total soluble proteins [28], glycine betaine [29], total sugars [30], and ascorbic acid [31] contents in plants.
2.12. Nutrient Concentration
Shoots and roots were washed in redistilled water twice for plant nutrient analysis. The plant samples were oven dried for 72 h at 65 °C. The samples were digested in HNO3: HClO4 (7:3 v/v) using wet digestion protocol until colorless samples were obtained. The samples were filtered, followed by dilution in redistilled water. Volume was maintained up to 50 mL. Yoshida et al. [32] proposed the method for determining calcium, sodium, and potassium ions concentration in plants. Their concentrations were estimated by using Atomic Absorption Spectrum using a flame photometer. Concentrations of unknown samples of respective elements were found by constructing standard curves using standard series. The yellow color method was used to determine phosphorous contents at 420 nm absorbance using spectrophotometer [33].
2.13. Statistical Analysis
The data were analyzed with two-way ANOVA (ANOVA), while difference in treatments was computed by least significant difference (p < 0.05) test [34]. For data normalization before analysis, logarithmic transformations were performed where necessary. Pearson’s correlation analysis was carried out using Origin 2021.
3. Results
3.1. Root Length, Shoot Length, Shoot Fresh Weight, and Shoot Dry Weight
Application of potassium nitrate (KN) significantly affected the root length, shoot length, shoot fresh weight, and shoot dry weight of spinach under normal irrigation (NI), mild drought stress (MDS), and severe drought stress (SDS). Increasing drought stress caused a significant reduction in root and shoot length, fresh weight, and dry weight in spinach. In NI, treatment KN plants showed significant enhancement in root length (Figure 1A), shoot length (Figure 1B), and shoot fresh weight (Figure 1C). No significant change was observed in shoot dry weight (Figure 1D) among KN and no KN under NI. Under MDS, application of KN remained significantly better over no KN for root and shoot length, shoot fresh, and shoot dry weight of spinach. However, at SDS, KN differed significantly over no KN for root length, shoot fresh, and dry weights of spinach. Treatment KN and no KN were non-significant for shoot length at SDS.
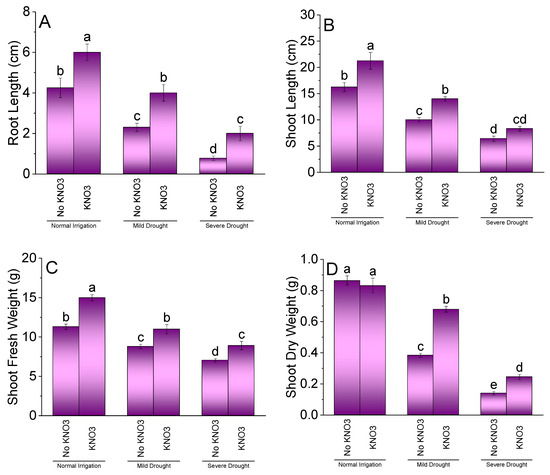
Figure 1.
Effect of different application rates of potassium nitrate (KN) on root length (A), shoot length (B), shoot fresh weight (C), and shoot dry weight (D) of spinach under variable irrigation levels. Bars are means of 4 replicates compared by LSD test. Different letters on bars represent a significant difference at p ≤ 0.05.
3.2. Chlorophyll and Carotenoid Contents
Treatments KN differed significantly for chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids of spinach under NI, MDS, and SDS. Drought stress enhancement significantly decreased all of the photosynthetic pigments of spinach. For NI, addition of KN did not show a significant increase from no KN in chlorophyll a (Figure 2A) and total chlorophyll (Figure 1C). However, KN application remained significantly better for chlorophyll b (Figure 2B) and carotenoids (Figure 2D) of spinach leaves (Figure 2B) compared to no KN. A significant increase in chlorophyll a, chlorophyll b, and total chlorophyll was noted under MDS where KN was applied over no KN. No significant change was observed in carotenoids among KN and no KN under MDS. Under SDS, the application of KN differed significantly better over no KN for chlorophyll pigments and carotenoids of spinach.
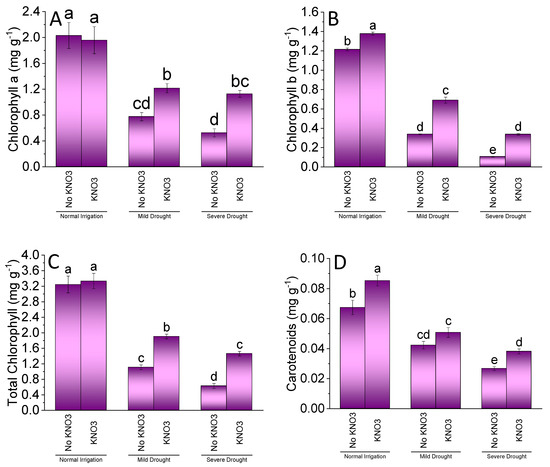
Figure 2.
Effect of different application rates of potassium nitrate (KN) on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoids (D) of spinach under variable irrigation levels. Bars are means of 4 replicates compared by LSD test. Different letters on bars represents significant difference at p ≤ 0.05.
3.3. MDA, SOD, POD, H2O2, GB, and EL
Results showed that KN addition was significantly different for MDA, SOD, POD, H2O2, GB, and EL of spinach under NI, MDS, and SDS. Treatment KN did not significantly change MDA (Figure 3A) and POD (Figure 3C) from no KN under NI. However, KN application significantly decreased SOD (Figure 3B), H2O2 (Figure 3D), GB (Figure 3E), and EL (Figure 3F) of spinach leaves over no KN at NI. At MDS, a significant decrease was observed in MDA, GB, EL, and POD over no KN. However, KN and no KN remained statistically alike for SOD and H2O2 under MDS. A significant decrease was noted at SDS in MDA, SOD, POD, H2O2, SB, and EL where KN was added compared to no KN.
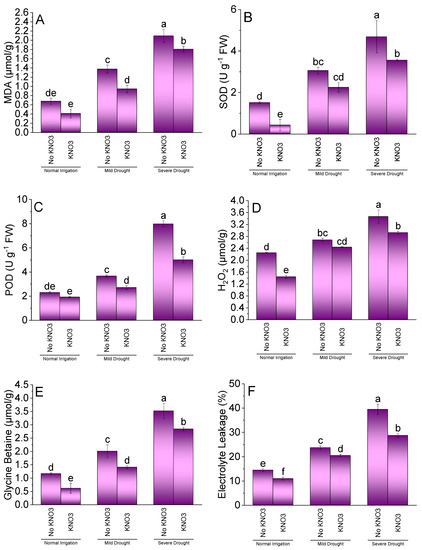
Figure 3.
Effect of different application rates of potassium nitrate (KN) on MDA (A), SOD (B), POD (C), H2O2 (D), GB (E), and EL (F) of spinach under variable irrigation levels. Bars are means of 4 replicates compared by LSD test. Different letters on bars represent a significant difference at p ≤ 0.05.
3.4. K, Na, Ca, and P Concentrations
Effect of KN application was significant for K, Na, Ca, and P of spinach under NI, MDS, and SDS. Results showed that KN showed significant enhancement in K (Figure 4A) and P (Figure 4D) but decreased Na (Figure 4B) and Ca (Figure 4C) compared to no KN in NI. Under MDS, KN differed significantly for K and P enhancement, while Ca decreased over no KN. However, no significant change in Na was noted at MDS among KN and no KN. Treatment KN was significant for increased K and P but a decrease in Ca and Na of spinach under SDS.
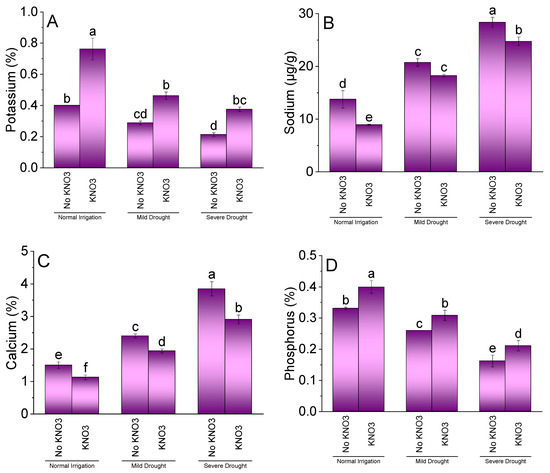
Figure 4.
Effect of different application rates of potassium nitrate (KN) on K (A), Na (B), Ca (C), and P (D) of spinach under variable irrigation levels. Bars are means of 4 replicates compared by LSD test. Different letters on bars represent a significant difference at p ≤ 0.05.
3.5. TSP, SS, and AsA
Application of KN was significantly different for TSP, SS, and AsA of spinach under NI, MDS, and SDS. The addition of KN significantly increased TSP (Figure 5A) and SS (Figure 5B) but remained non-significant for AsA (Figure 5C) over no KN in NI. Under MDS, KN remained significantly better for enhancement in TSP and SS. However, a significant decrease in AsA in KN over no KN was noted at MDS. No significant change in TSP was noted between KN and no KN under SDS. A significant increase in SS was noted at SDS where KN was applied over no KN. Treatment KN was significant for decrease in AsA of spinach compared to no KN under SDS.
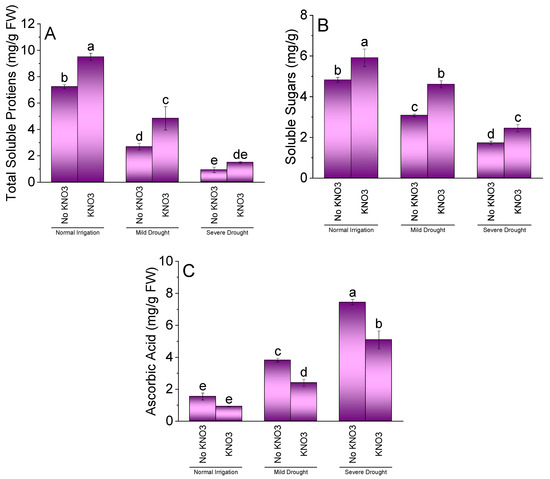
Figure 5.
Effect of different application rates of potassium nitrate (KN) on TSP (A), SS (B), and AsA (C) of spinach under variable irrigation levels. Bars are means of 4 replicates compared by LSD test. Different letters on bars represent a significant difference at p ≤ 0.05.
3.6. Pearson Correlation and Principal Component Analysis
Pearson correlation showed that the studied growth attributes, i.e., plant length, fresh and dry weights, and photosynthetic pigment contents, were significantly negative in correlation with MDA, proline, POD, SOD, Na, Ca, H2O2, GB, and EL. However, plant length, shoot fresh and dry weight, chlorophyll a, chlorophyll b, and total chlorophyll contents were significant positive in correlation with K, P, TSP, and SS (Figure 6). The principal component analysis also showed that K, P, fresh and dry weights of shoot, shoot and root length, TSP, and chlorophyll contents were closely associated with each other. However, AsA, GB, POD, SOD, Ca, Na, H2O2, electrolyte leakage, and proline were opposite and far apart from K, P, vegetative growth characters, TSP, and chlorophyll contents. Catalase was more closely associated with K, P, shoot fresh and dry weight, plant height, TSP, and chlorophyll contents than AsA, GB, POD, SOD, Ca, Na, H2O2 electrolyte leakage, and proline (Figure 7).
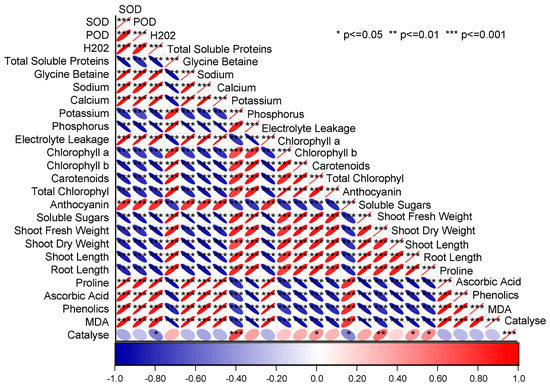
Figure 6.
Pearson correlation for spinach attributes amended with potassium nitrate under NI, MDS, and SDS.
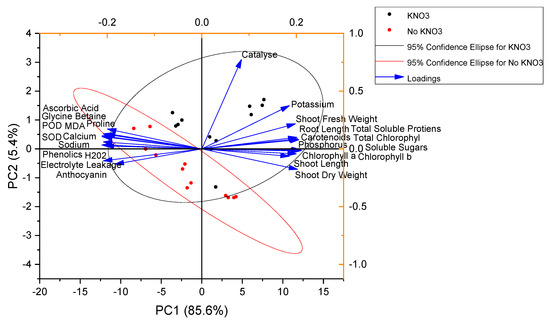
Figure 7.
Principal component analysis for spinach attributes amended with potassium nitrate under NI, MDS, and SDS.
4. Discussion
Spinach is a valuable leafy green vegetable and a good source of nutrients. Like most vegetables, limited water availability badly affects spinach production in semi-arid areas that require proper irrigation systems, but water scarcity is a significant limiting factor. Moreover, the extensive use of synthetic chemical fertilizers has caused great damage to agricultural system. The use of controlled-release fertilizers that are biodegradable to increase yield has recently gained significant momentum for agricultural sustainability [35]. Drought disturbs water relations and impairs normal plant growth. Plants, however, have many biochemical response mechanisms at the cellular and whole-organism level, making drought stress an intricate phenomenon [36].
According to Gargallo-Garriga et al. [37], limited water availability due to drought stress restricted shoot metabolic activities. Lower metabolic rates facilitate plants to conserve water and transfer assimilates from upper parts towards the root. This maintenance of water status and food transfer helps plants to make extensive root systems and absorb more water from the soil. Our study also validates these facts. Elongated roots were observed in spinach plants treated with KN in both water deficit conditions, MDS, and SDS. Extensive cell division is the estimation of improved plant growth. Drought stress changes soil osmotic potential, leading to poor growth and cell division in roots and shoot. Less water uptake due to change in the osmotic potential decreases the fresh weight of root and shoot. It also results in less division and a significant reduction of dry weight [38,39]. Similar results were noticed in the current study. KN treatment effectively increased shoot fresh weight and plant length by conserving more water contents in cells.
Chlorophyll b, a premier light-harvesting pigment, experiences more reduction than chlorophyll a because water deficiency mainly affects photosystem-I, light harvesting systems, antenna complex, and energy-transfer mechanisms. Drought stress significantly reduces chlorophyll b contents in spinach plants even at 80% field capacity [20]. Our results are also in justification of these mechanisms. A significant decrease in photosynthetic pigment contents of spinach was observed under MDS and SDS conditions [40,41]. Application of potassium fertilizer at 150 mg concentrations significantly enhanced chlorophyll b levels. KN also improved plant vigor and photosynthetic activities in drought stress, from which chlorophyll a, total chlorophyll, and carotenoids were also improved.
Cellular osmotic adjustment is important to cope with water stress conditions. Plants maintain turgor pressure in cells and regulate several physiological processes to postpone and tolerate the prevailing dehydration conditions. Drought stress denatures protein and enzyme structure, and to protect them, plants use osmo-protectants [42]. Higher sugar levels are maintained in cells to protect denaturalization of proteins in water stress. Similar results were reported by Ahmed et al. [42] and Kishor et al. [43]. Osmoregulation maintains turgor potential in cells and improves physiological processes to cope with prevailing dehydration [6].
ROS cause peroxidation of membranous lipids, and the extent of damage is assessed by malondialdehyde, a lipid peroxidation product. The spinach plant’s amount of malondialdehyde (MDA), ascorbic acid, glycine betaine, and proline increases under water stress conditions. Levels of hydrogen peroxide (H2O2) and antioxidant enzymes (SOD, POD, CAT) remained unaffected even at the levels of 40% field capacity in spinach [20]. The higher synthesis of MDA, phenolics, and H2O2 in the present study also validated the mitigation of severe water stress under KN. H2O2 has various physiological and growth-related roles in plants, thus ameliorating drought resistance in spinach.
According to Coskun et al. [44], MDA contents positively correlated with Na+ ion uptake in stressed plants. Controlled amounts of Na+ ions are very critical to stress tolerance in plants. Limited amounts of ions prevent ion imbalances, leaf metabolic disorders, and tissue desiccation via osmotic stress. To maintain proper cellular functions, intracellular K+ ions must be maintained in homeostatic concentrations. Plants with efficient absorption and utilization of mineral nutrients effectively increase fertilizer capability to affect plant vigor, thus reducing production cost and nutrient loss to the ecosystem. Plants with high nutrient-use efficiency contribute to a sustainable agriculture system and better water, soil, and air qualities [45]. Our results clearly state that KNO3 fertilization increased Na+, Ca2+, and K+ ion contents and their uptake in spinach. Na+ was relatively in greater amount in plants under drought stress.
5. Conclusions
In conclusion, the application of KNO3 containing chitosan/montmorillonite microparticles under drought positively affects spinach’s growth, physiological, and ionic attributes. Plant physiological parameters, like length, fresh weight, dry weight, chlorophyll pigments, carotenoids, total soluble protein contents, soluble sugars, potassium, and phosphorous, were significantly improved with the supplementation of KNO3 in mitigating the effects of drought stress. KN had efficacious roles in alleviating drought stress in spinach plants. There is a need for more investigation at the field levels considering other climatic conditions to validate these results using KNO3 containing chitosan/montmorillonite fertilizer as an effective amendment against drought.
Author Contributions
Conceptualization, S.A.B.H.B., I.L., S.F.A. and N.M.; methodology, S.A.B.H.B., I.L., S.F.A. and N.M.; software, S.A.B.H.B., I.L., S.F.A. and N.M.; validation, S.A.B.H.B., I.L., S.F.A. and N.M.; formal analysis, S.A.B.H.B., I.L., S.F.A. and N.M.; investigation, S.A.B.H.B., I.L., S.F.A. and N.M.; resources, S.A.B.H.B., I.L., S.F.A. and N.M.; data curation, S.A.B.H.B., I.L., S.F.A. and N.M.; writing---original draft preparation, M.N. (Maliha Naz), M.N. (Muhammad Naeem), S.A.B., M.S., S.D., R.D. and S.F.; writing---review and editing, S.D., R.D., S.A.A.; T.D.M. and S.F.; visualization, S.D., R.D. and S.F.; funding acquisition, S.D., R.D. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zafar-ul-hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, M.S.; El-esawi, M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Khan, M.J.; Muhammad, D.; Fahad, S.; Adnan, M.; Wahid, F.; Alamri, S.; Khan, F.; Dawar, K.M.; Irshad, I.; Danish, S.; et al. Phosphorus Nutrient Management through Synchronization of Application Methods and Rates in Wheat and Maize Crops. Plants 2020, 9, 1389. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Shahjahan, A.; Danish, S.; Abid, M.; Qayyum, M.F. Mitigation of cadmium toxicity induced stress in wheat by ACC-deaminase containing PGPR isolated from cadmium polluted wheat rhizosphere. Pak. J. Bot. 2018, 50, 1727–1734. [Google Scholar]
- Danish, S.; Tahir, F.A.; Rasheed, M.K.; Ahmad, N.; Ali, M.A.; Kiran, S.; Younis, U.; Irshad, I.; Butt, B. Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric. 2019, 4, 381–390. [Google Scholar] [CrossRef]
- Furuhashi, T.; Nakamura, T.; Iwase, K. Analysis of Metabolites in Stem Parasitic Plant Interactions: Interaction of Cuscuta--Momordica versus Cassytha--Ipomoea. Plants 2016, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Gavili, E.; Moosavi, A.A.; Moradi, F. Assessing cattle manure biochar potential for ameliorating physical soil features and spinach responses under drought stress conditions. Arch. Agron. Soil Sci. 2018, 64, 1714–1727. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Gilani, M.; Danish, S.; Ahmed, N.; Rahi, A.A.; Akrem, A.; Younis, U.; Irshad, I.; Iqbal, R.K. Mitigation of drought stress in spinach using individual and combined applications of salicylic acid and potassium. Pak. J. Bot. 2020, 52, 1505–1513. [Google Scholar] [CrossRef]
- Majid, M.; Ali, M.; Shahzad, K.; Ahmad, F.; Ikram, R.M.; Ishtiaq, M.; Alaraidh, I.A.; Al-Hashimi, A.; Ali, H.M.; Zarei, T.; et al. Mitigation of osmotic stress in cotton for the improvement in growth and yield through inoculation of rhizobacteria and phosphate solubilizing bacteria coated diammonium phosphate. Sustainability 2020, 12, 10456. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought Stress Alleviation by ACC Deaminase Producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without Timber Waste Biochar in Maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pak. J. Bot. 2020, 52, 49–60. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M. Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton (Buenos Aires) 2020, 89, 217–227. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Zahra, M.B.; Danish, S.; Abbas, M. Multi-strain Inoculation with PGPR Producing ACC Deaminase is More Effective Than Single-strain Inoculation to Improve Wheat (Triticum aestivum) Growth and Yield. Phyton-Int. J. Exp. Bot. 2020, 89, 405–413. [Google Scholar]
- Moaaz Ali, M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of seed priming with potassium nitrate on the performance of tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, B.R.; Bacalhau, F.B.; dos Santos Pereira, T.; Souza, C.F.; Faez, R. Chitosan-montmorillonite microspheres: A sustainable fertilizer delivery system. Carbohydr. Polym. 2015, 127, 340–346. [Google Scholar] [CrossRef]
- Abuchenari, A.; Hardani, K.; Abazari, S.; Naghdi, F.; Keleshteri, M.A.; Jamavari, A.; Chahardehi, A.M. Clay-reinforced nanocomposites for the slow release of chemical fertilizers and water retention. J. Compos. Compd. 2020, 2, 85–91. [Google Scholar] [CrossRef]
- Jabeen, M.; Akram, N.A.; Ashraf, M.; Aziz, A. Assessment of biochemical changes in spinach (Spinacea oleracea L.) subjected to varying water regimes. Sains Malays. 2019, 48, 533–541. [Google Scholar] [CrossRef]
- Messa, L.L.; Souza, C.F.; Faez, R. Spray-dried potassium nitrate-containing chitosan/montmorillonite microparticles as potential enhanced efficiency fertilizer. Polym. Test. 2020, 81, 106196. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Chen, C.-N.; Pan, S.-M. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 1996, 37, 107–111. [Google Scholar]
- Sakharov, I.Y.; Ardila, G.B. Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Holmström, K.-O.; Somersalo, S.; Mandal, A.; Palva, T.E.; Welin, B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000, 51, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Azuma, K.; Nakayama, M.; Koshioka, M.; Ippoushi, K.; Yamaguchi, Y.; Kohata, K.; Yamauchi, Y.; Ito, H.; Higashio, H. Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem. 1999, 47, 3963–3966. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice; CAB International: Los Banos, Philippines, 1971. [Google Scholar]
- Montañés, L.; Heras, L.; Abad’\ia, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J. Plant Nutr. 1993, 16, 1289–1308. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997; p. 246. [Google Scholar]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. (Amsterdam) 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmawan, D. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2019; Volume 53, ISBN 9788578110796. [Google Scholar]
- Paul, S.; Aggarwal, C.; Manjunatha, B.; Rathi, M.S. Characterization of osmotolerant rhizobacteria for plant growth promoting activities in vitro and during plant-microbe association under osmotic stress. Indian J. Exp. Biol. 2018, 56, 582–589. [Google Scholar]
- Zafar-Ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Danish, S.; Zafar-ul-Hye, M.; Hussain, M.; Shaaban, M.; Núñez-delgado, A. Rhizobacteria with ACC-Deaminase Activity Improve Nutrient Uptake, Chlorophyll Contents and Early Seedling Growth of Wheat under PEG- Induced Osmotic Stress. Int. J. Agric. Biol. 2019, 21, 1212–1220. [Google Scholar] [CrossRef]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Rajesh, K.; Reddy, P.S.; Seiler, C.; Sreenivasulu, N. Drought stress tolerance mechanisms in barley and its relevance to cereals. In Biotechnology in Agriculture and Forestry; Kumlehn, J., Stein, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 69, pp. 161–179. [Google Scholar]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).