Abstract
Climate change-related events, such as marine heatwaves, are increasing seawater temperatures, thereby putting pressure on marine biota. The cosmopolitan distribution and significant contribution to marine primary production by the genus Ruppia makes them interesting organisms to study thermal tolerance and local adaptation. In this study, we investigated the photosynthetic responses in Ruppia to the predicted future warming in two contrasting bioregions, temperate Sweden and tropical Thailand. Through DNA barcoding, specimens were determined to Ruppia cirrhosa for Sweden and Ruppia maritima for Thailand. Photosynthetic responses were assessed using pulse amplitude-modulated fluorometry, firstly in short time incubations at 18, 23, 28, and 33 °C in the Swedish set-up and 28, 33, 38, and 43 °C in the Thai set-up. Subsequent experiments were conducted to compare the short time effects to longer, five-day incubations in 28 °C for Swedish plants and 40 °C for Thai plants. Swedish R. cirrhosa displayed minor response, while Thai R. maritima was more sensitive to both direct and prolonged temperature stress with a drastic decrease in the photosynthetic parameters leading to mortality. The results indicate that in predicted warming scenarios, Swedish R. cirrhosa may sustain an efficient photosynthesis and potentially outcompete more heat-sensitive species. However, populations of the similar R. maritima in tropical environments may suffer a decline as their productivity will be highly reduced.
Keywords:
marine heatwaves; PAM fluorometry; seagrass; Fv/Fm; NPQ; Fv/F0; Ruppia cirrhosa; Ruppia maritima 1. Introduction
Global climate change is putting immense pressure on organisms and whole ecosystems [1,2,3,4,5]. Resilience and adaptation to such stress are key factors determining future biodiversity dispersal in terrestrial and marine environments. In the world’s oceans, average mean temperatures are predicted to increase by 1.3 °C before 2065 [1]. Even more alarming may be the acute effects of specific climate change-induced events such as marine heatwaves [2,3], especially influencing shallow coastal waters that get warmed rapidly and where temperatures may fluctuate greatly on a diel basis. Reportedly, such events have caused mass mortality and shifts in ecosystem structure, threatening key habitats in the coastal seascape [2,4,5]. Seagrass meadows are important ecosystem engineers in coastal areas, supporting numerous ecosystem services. These include provision of nursing and living grounds for multiple marine organisms, climate change mitigation due to high CO2 capture and sequestration, and water filtration by particle retention [6,7,8]. Due to increased anthropogenic impact, a worldwide seagrass decline has occurred [9,10], and these natural benefits are therefore under threat. Loss of certain seagrass species may in many areas promote colonization of more tolerant or opportunistic seagrass species or other submerged aquatic vegetation, hence altering the benthic plant community composition [11].
Generally, seagrasses respond with a linear increase in photosynthetic rates with temperature, until a threshold and concomitant tipping point is reached at high temperature [12,13,14,15]. These tipping points are highly species-specific and area dependent; for instance, temperate Zostera marina reaches its limit around 30 °C [12,14,16], Mediterranean Ruppia cirrhosa at approximately 36 °C [17], and tropical species may withhold photosynthetic rates up to 40 °C [15,18,19,20,21]. However, before those photosynthetic limits are reached, biomass loss and tissue degradation can already be encountered [20,22,23]. Negative or detrimental effects of the photosynthetic apparatus at high temperatures are often related to protein degradation, decreased protein synthesis, and loss of membrane stability [24]. Photosystem II (PSII) is the most heat sensitive of the two photosystems [25], where increased temperature may cause direct impairment of the oxygen-evolving complex [26] or indirect damage to the D1 protein caused by lipid peroxidation or accumulation of reactive oxygen species (ROS) [27,28,29]. Moreover, high temperature might stimulate photorespiration resulting in lower productivity, especially in conditions of high oxygen [30,31].
Insights from previous studies revealed diversified responses to warming depending on the plant history and their adaptive capacity to local environments [32,33,34,35,36]. Differing resistance to warming was observed in Zostera marina from northern and southern populations [37,38], whereas several studies shed light on heat stress responses of the Mediterranean seagrasses from different ambient temperatures [32,33,34,35,36]. Results from these investigations suggest higher thermotolerance and greater capability to acclimate to temperature changes in populations inhabiting warmer environments. Nevertheless, this observation is based on studies within the same bioregion. When considering a larger geographic scale, it is hypothesized that tropical marine species will be more sensitive to increasing temperature than temperate species. In addition to their evolutionary history in a relatively constant temperature, it is assumed that the upper temperature threshold of the tropical species are closer to the ambient temperature in their habitat [21,23,39,40,41]. Thus, warming events in tropical areas may push the organisms beyond their operating and survival limits. Studies across bioregions with different temperature regimes conducted using well-designed experimental approaches of ecological relevance are needed to provide supporting evidence for these assumptions. The existing literature thus far is concentrated on animal models, while studies on marine vegetation remain scarce [21,23,39,40,41].
The genus Ruppia comprises a group of aquatic angiosperm species, which are able to sexually reproduce in fresh, brackish, and marine environments [42,43]. This characteristic sets them aside from other seagrasses, which reproduce in marine waters only. Their ability to inhabit fresh and brackish environments suggests a broad range of environmental tolerance [42,43]. They are considered fast-growing pioneer species, which play an important role in shallow-water community dynamics and productivity [44]. In addition, their cosmopolitan distribution across different bioregions makes the genus Ruppia an interesting organism to study how local adaptation contributes to variability in thermal niches of aquatic plants.
The aim of the study was to explore the thermal sensitivity of Ruppia from two different bioregions, including R. cirrhosa from a temperate area and R. maritima from a tropical area. The two Ruppia species are closely related and functionally and morphologically similar [45,46], and each species contribute as an important primary producer in the shallow water of its respective region [44,47,48]. We focused on assessing their photosynthetic responses to increased temperatures within a range occurring in their natural settings encompassing the warming scenarios. The results can provide valuable insights on the future effects of warming on submerged aquatic vegetation at genus and community levels in different bioregions.
2. Materials and Methods
2.1. Study Sites and Plant Material
As this study aims to capture the temperature response of Ruppia from two contrasting temperature regimes, we examined specimens from a temperate (Sweden) and a tropical (Thailand) bioregion (Figure 1A,B). The temperate species was identified as R. cirrhosa and the tropical one was identified as R. maritima. Ruppia cirrhosa is a representative species of the common submerged aquatic vegetation at the Swedish west coast [11], while R. maritima represents the only Ruppia species recorded in Thai waters [45,46]. The mean surface water temperatures (SST) in these regions normally fluctuates between −4 and 24 °C (Sweden) (Figure 1C) and 27 and 33 °C (Thailand) (Figure 1D) on a seasonal basis. However, in shallow coastal areas the temperatures may differ from the average SST. At the time of sampling, ambient temperature was 18 °C at the Swedish site and 28 °C for the Thai site.
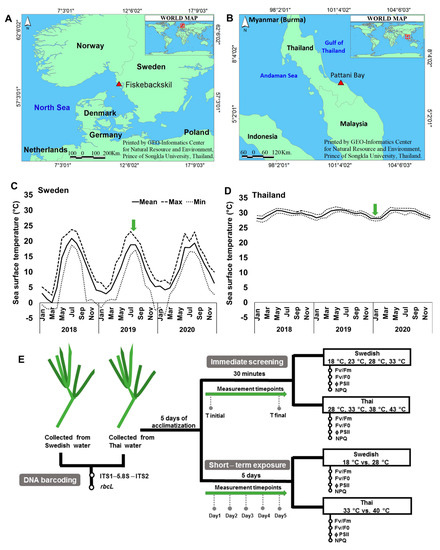
Figure 1.
(A,B) Locations of the collection sites, (C,D) sea surface temperature (SST) in Swedish and Thai waters recorded from 2018 to 2020, and (E) experimental design and timeline of the measurements. Sea surface temperatures were retrieved from GHRSST Level 4 MUR Global Foundation Sea Surface Temperature Analysis (v4.1) (https://podaac.jpl.nasa.gov/dataset/MUR-JPL-L4-GLOB-v4.1 accessed on 19 June 2021). The green arrows in C and D indicate the sampling periods in the two sites.
The Swedish part of the study was conducted at Kristineberg Marine Research Station in Fiskebäckskil on the west coast of Sweden in July 2019 and the Thai part at the Coastal Oceanography and Climate Change Research Center at the Prince of Songkla University in January 2020. Specimens of R. cirrhosa were harvested from shallow soft bottoms at approximately 1.2 m depth in Fiskebäckskil, Sweden (58°14′34.8″ N 11°27′59.7″ E, Figure 1A), whereas specimens of R. maritima were collected from shallow areas of Pattani Bay, Thailand (6°53′41.4″ N 101°16′26.7″ E, Figure 1B). Seagrass specimens were instantly transported to the laboratory in buckets of seawater. Upon arrival, the plants were cleaned of sediment and visible epiphytes under running seawater. Specimens were then placed in aerated tanks connected to a seawater flow-through system and left five days for acclimation. Light was provided on a 12 h dark/12 h light scheme with fluorescent light tubes with PAR of approximately 100 µmol photons m−2 s−1 for Swedish R. cirrhosa and 200 µmol photons m−2 s−1 for Thai R. maritima, corresponding to the minimum saturating irradiance (Ek) levels derived from rapid light curves (RLCs, data not shown). The water temperature was maintained at 18 °C for the Swedish design and 28 °C for the Thai set-up. These levels were used as the ambient temperatures at the respective sampling periods. Salinity was 25 practical salinity units (psu) for the Swedish set-up and 30 psu for the Thai set-up (levels recorded at the sampling sites) and pH was 8.1. Please refer to Figure 1E for the experimental design and timeline for the measurements described below.
2.2. DNA Barcoding
2.2.1. Genomic DNA Extraction, Amplification, and Sequencing
As species identification using morphological features has been proven difficult for the genus Ruppia, determination was assisted using DNA barcoding. Plant material was homogenized with mortar and pestle in liquid nitrogen. The total genomic DNA was extracted following the manufacturer’s protocol of DNeasy® Plant Mini Kit (Qiagen, Hilden, Germany). The quality and concentration of genomic DNA samples were determined by agarose gel electrophoresis and using the Thermo NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The amplification and sequence analysis of the internal transcribed spacer (ITS1-5.8S-ITS2) and ribulose-1,5-bisphosphate carboxylase/oxygenase N-methyltransferase (rbcL) regions were undertaken using the primer sets presented in Table 1. The PCR amplification was performed in a 50 μL reaction volume containing 10 μL of 5X PhusionTM HF Buffer, 1 μL of dNTP (10 μM), 1.5 μL of DMSO, 0.5 μL of PhusionTM High-Fidelity DNA Polymerase (Thermo Fisher Scientific, USA) (0.02 U/μL), 1 μL of each primer (10 mM), and 250 ng of template genomic DNA. The thermal cycle was initiated by 30 s of denaturation at 98 °C, followed by 35 cycles of 10 s of denaturation at 98 °C, 30 s of annealing at 55 °C and 20 s of elongation at 72 °C, and a final elongation of 5 min at 72 °C. The PCR products were gel-purified and submitted to sequencing at 1st BASE DNA Sequencing Services (Singapore).

Table 1.
The primer sets used in this study. Primer sequences, melting temperatures (Tm), and corresponding references are indicated.
2.2.2. Phylogenetic Analysis
The ITS (ITS1-5.8S-ITS2) and rbcL sequences were analyzed using BLASTn version BLASTN 2.11.0+ (http://www.ncbi.nlm.nih.gov/blast accessed on 11 May 2021; RID 9JGJJ93C016, RID9JH3TTV0016, RID 9JGHG8NK013 and RID 9JH4N8VC013) and the MEGA software version X. The ITS (ITS1-5.8S-ITS2) and rbcL gene sequences of the Ruppia spp. reference strains; Accession No. AB728749.1 (R. cirrhosa ITS gene), MN958127.1 (R. drepanensis ITS gene), AB728734.1 (R. maritima ITS gene), JQ034337.1 (R. megacarpa ITS gene), JN113279.1 (R. maritima rbcL gene), NC051974.1 (R. brevipedunculata rbcL gene), JN113277.1 (R. cirrhosa rbcL gene), MN233650.1 (R. sinensis rbcL gene), and AB507891.2 (R. megacarpa rbcL gene) were selected from GenBank by subjecting the nucleotide sequences of ITS and rbcL using BLASTn. The datasets were divided into three sets; ITS (ITS1-5.8S-ITS2) dataset, rbcL dataset, and the combination of ITS (ITS1-5.8S-ITS2) and rbcL dataset. Multiple sequence alignment was achieved with ClustalW of MEGA X. Phylogenetic trees were constructed using the maximum likelihood method in MEGA X [51], based on the Kimura 2-parameter model. The maximum parsimony was performed by 1000 bootstrap replications [52].
2.3. Sensitivity of Photosynthesis to Warming
2.3.1. Photosynthetic Responses to a Series of Temperatures
Measurements were conducted in 3 mL airtight experimental chambers (model DWA1, Hansatech, King’s Lynn, UK). In each seawater-filled chamber, seven leaf segments (total width ~0.7 cm) with a length of 3 cm were placed next to each other in a U-shaped manner for effective light harvesting. Light was provided from the side using cold-light sources providing irradiance of approximately 100 µmol photons m−2 s−1 for Swedish R. cirrhosa and 200 µmol photons m−2 s−1 for Thai R. maritima. The temperatures within the chambers were adjusted with a temperature bath (RC20, Lauda, Lauda-Königshofen, Germany, for the Swedish set-up and MP-10C, Shanghai Bluepard Instruments, Shanghai, China, for the Thai set-up) providing water circulation through jackets surrounding the chambers. The temperature treatments used were 18 (ambient water temperature), 23, 28, and 33 °C in the Swedish set-up (n = 9 per treatment) and 28 (ambient water temperature), 33, 38, and 43 °C in the Thai set-up (n = 10 per treatment). The temperature ranges were chosen based on the temperatures occurring in the plants’ natural habitats, encompassing unusually high temperatures recorded in Swedish and Thai water [53,54] and upper thermal thresholds of temperate and tropical seagrasses reported in previous studies [12,14,16,18,19,20,21,23]. The seawater added to the chambers had salinity and pH levels adjusted to ambient field conditions (salinity: ~25/~30 in Sweden/Thailand, respectively; pH: 8.1 for both regions). The water in the chambers was continuously stirred with a magnetic stir bar.
The samples were first incubated in darkness for 15 min. Then the initial maximum photosynthetic efficiency (Fv/Fm) and the PSII potential activity (Fv/F0) were obtained using pulse amplitude-modulated (PAM) fluorometry (Diving-PAM, Walz, Effeltrich, Germany). Values were obtained after dark adaptation where F0 (=minimal fluorescence) and Fm (=maximum fluorescence) were given by the PAM. Fv (=variable fluorescence) was calculated as:
Fv = Fm−F0
After the dark period, the light (100 and 200 µmol photons m−2 s−1 for Swedish and Thai set-up, respectively) was turned on and the effective quantum yield (ΦPSII) of photosystem II and excess energy emission through non-photochemical quenching (NPQ) were measured after 20 min in light. The Fv/Fm and Fv/F0 (Fv/Fm final and Fv/F0 final) were re-assessed after dark adaptation.
2.3.2. Photosynthetic Responses to Prolonged Warming Treatment
Subsequent prolonged experiments were run for five consecutive days, where seagrass was either placed in aquaria with control (18 °C and 33 °C for Sweden and Thailand, respectively) or with the heated treatment temperatures (28 °C and 40 °C for Sweden and Thailand, respectively). Two temperature levels were chosen from the first experiment. For the Swedish set-up, the ambient temperature (18 °C) at the sampling site was used as control and 28 °C, corresponding to the SST extreme recorded in the Baltic Sea in 2018 [53], was used as the heated treatment. For the Thai set-up, 33 °C was used as control based on no adverse effect on the Fv/Fm and Fv/F0 observed in the first experiment and 40 °C, corresponding to the temperature extreme recorded in a shallow seagrass habitat (unpublished data), was used as the heated treatment. Note that a trial was initially conducted using a 43 °C heated treatment, which led to an immediate mortality within 24 h. The Fv/Fm, Fv/F0, ΦPSII, and NPQ were measured daily at the end of the photoperiod to follow the status of the photosynthetic apparatus of the seagrass over time.
2.4. Statistical Analyses
The statistical analyses were carried out using Statistica version 13. Prior to analysis of variance (ANOVA), homogeneity of variances was tested using Levene’s test [55].
In the first experimental set-up, analysis of covariance (ANCOVA) was used to compare photosynthetic responses in Ruppia cirrhosa and R. maritima to a series of temperatures. Species was used as the categorical factor and temperature (as 0, 5, 10, and 15 °C increase from ambient temperature) was used as the continuous predictor. The Fisher’s least significant difference (LSD) test was used for post-hoc comparisons to determine species-specific effects on the relationships of each photosynthetic parameter with increasing temperatures. In addition, ANOVAs (repeated ANOVA for Fv/Fm and Fv/F0 and two-way ANOVA for ΦPSII and NPQ) were used for comparisons of means of the photosynthetic parameters for main factors and for their interactions. The Fisher’s LSD test was used for pairwise multiple comparisons across species, temperature, and time.
ANCOVA was used to assess the differential effects of the prolonged warming treatment on photosynthesis of R. cirrhosa and R. maritima. Species and treatment were used as categorical factors and treatment duration (day 1–5) was used as the continuous predictor. The Fisher’s LSD test was used for post-hoc comparisons. Repeated-measures ANOVA was used to test for differences of means of the photosynthetic parameters over time. Treatment duration was used as the within group factor and species and temperature treatments were used as the categorical factors. The Fisher’s LSD test was used for pairwise multiple comparisons of means across species, treatments (control and warming treatment), and treatment duration (day after treatment).
3. Results
3.1. Analysis of ITS (ITS1-5.8S-ITS2) and rbcL DNA Sequences
The genes size and accession numbers of the amplified PCR products of ITS sequences containing ITS1-5.8S-ITS2 and rbcL are shown in Table 2. The BLASTn analysis of ITS (ITS1-5.8S-ITS2) and rbcL sequences confirmed that Ruppia sampled in Sweden was close to Ruppia cirrhosa with a 100% and 99.85% sequence identity, respectively, while Ruppia sampled in Thailand was close to R. maritima with a 100% and 99.85% sequence identity, respectively (Table 2). A phylogenetic analysis of the Ruppia specimens based on their ITS (ITS1-5.8S-ITS2) and rbcL regions was performed and a phylogenetic tree was constructed. The comparison between samples collected from Sweden and Thailand and the ITS (ITS1-5.8S-ITS2) and rbcL reference sequences retrieved from GenBank ensured that the identification of Ruppia samples from Sweden were close to R. cirrhosa with the bootstrap supports of 97% (ITS dataset), 50% (rbcL dataset), and 100% (ITS+ rbcL dataset), while Ruppia specimens from Thailand were close to R. maritima with the bootstrap support of 100% (ITS dataset), 50% (rbcL dataset), and 100% (ITS+ rbcL dataset). Ruppia megacarpa was used as an outgoing group (Figure 2).

Table 2.
The BLASTn of partial ITS and rbcL gene.
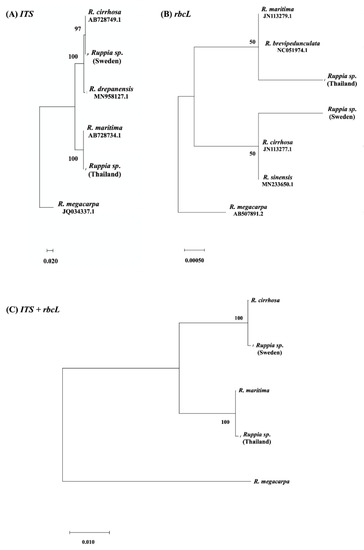
Figure 2.
The phylogenetic tree for species identification of Ruppia samples from Sweden and Thailand in comparison with other Ruppia species using fragments of ITS (ITS1-5.8S-ITS2) and rbcL. The evolutionary history was inferred using the maximum likelihood method, Kimura 2-parameter model, and 1000 bootstrap replications; (A) ITS (ITS1-5.8S-ITS2) dataset, (B) rbcL dataset, and (C) the combined dataset of ITS (ITS1-5.8S-ITS2) and rbcL
3.2. Temperature Effects on the Photosynthetic Efficiency
3.2.1. Photosynthetic Responses to a Series of Temperatures
Ruppia from the two different bioregions were affected by temperature, but to a different extent. A 15 °C increase in temperature (from 18 °C) resulted only in a minor change in the photosynthetic parameters in Ruppia cirrhosa (Swedish), but when R. maritima (Thai) was exposed to 43 °C (increased from 28 °C) it induced a substantial photoinhibition (Figure 3).
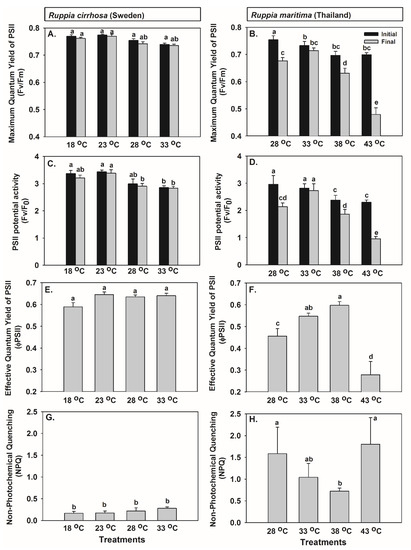
Figure 3.
The effects of experimental temperatures on the photosynthetic parameters, including (A) the maximum quantum yield (Fv/Fm) in Ruppia cirrhosa (Swedish), (B) Fv/Fm in R. maritima (Thai), (C) the PSII potential activity (Fv/F0) in R. cirrhosa (Swedish), (D) Fv/F0 in R. maritima (Thai), (E) the effective quantum yield (φPSII) in R. cirrhosa (Swedish), (F) φPSII in R. maritima (Thai), (G) non-photochemical quenching (NPQ) in R. cirrhosa (Swedish), and (H) NPQ in R. maritima (Thai). Values are shown as mean ± SE (n = 9 for R. cirrhosa (Swedish) and n = 10 for R. maritima (Thai)). Significant differences in mean values across species and treatments are denoted by different letters (Fisher’s LSD test, p < 0.05).
Temperature significantly influenced the maximum quantum yield (Fv/Fm initial and final) and the PSII potential activity (Fv/F0 initial and final). Moreover, significant effects of species on Fv/F0 (initial and final) and interactions between the two factors on Fv/Fm final and Fv/F0 final were encountered (Figure 3A–D, Table 3A). Significant differences between R. cirrhosa and R. maritima were detected in the functional relationships between temperatures and Fv/Fm final, Fv/F0 initial, and Fv/F0 final (Table 3B). For R. cirrhosa (Swedish) (Figure 3A,C), the mean values of Fv/Fm and Fv/F0 at 28 and 33 °C were slightly lower than in the other temperature treatments (Fisher’s LSD test, p < 0.05, supplementary material). For R. maritima (Thai) (Figure 3B,D), exposure to all treatments, except for 33 °C, resulted in a decrease in Fv/Fm and Fv/F0 from the initial values (Fisher’s LSD test, p < 0.05, supplementary material). The percentage reduction in Fv/Fm and Fv/F0 were highest in the 43 °C treatment followed by the treatments of 28 and 38 °C, respectively.

Table 3.
Summary of A. analysis of covariance (ANCOVA) of four photosynthetic parameters in Ruppia cirrhosa (Swedish) and R. maritima (Thai) in responses to a series of temperatures and B. the Fisher’s least significant difference (LSD) post-hoc test. Significant values (p < 0.05) are shown in bold.
Significant interactions of species and temperature on the effective quantum yield of PSII (φPSII) and the effects of species on non-photochemical quenching (NPQ) were detected (Figure 3E–H, Table 3A). Furthermore, the functional relationships between temperature and φPSII and NPQ were significantly different when comparing R. cirrhosa and R. maritima (Table 3B). While no difference in the mean values of φPSII was detected in R. cirrhosa (Swedish) (Figure 3E), a significant change was found in R. maritima (Thai) (Figure 3F). An increasing trend in φPSII as temperature increases from 28 to 38 °C and a sharp decline at 43 °C were detected (Fisher’s LSD test, p < 0.05, supplementary material). The NPQ of R. cirrhosa (Swedish) remained unchanged (Figure 3G), whereas large variation in NPQ across temperatures with a significant decrease at 38 °C was observed in R. maritima (Thai) (Figure 3H).
3.2.2. Photosynthetic Responses to Prolonged Warming Treatments
Two levels of temperature were chosen for a longer exposure experiment (18 and 28 °C for Ruppia cirrhosa (Swedish) and 33 and 40 °C for R. maritima (Thai)). Time course responses to warming displayed in Figure 4 show a slight photoinhibition in R. cirrhosa (Swedish) and a deleterious effect in R. maritima (Thai).
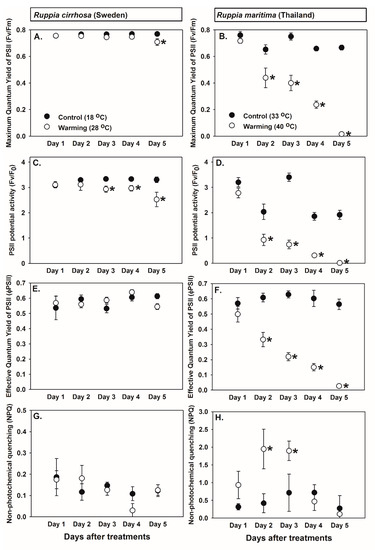
Figure 4.
Time course of the warming effects on the photosynthetic parameters, including (A) the maximum quantum yield (Fv/Fm) in Ruppia cirrhosa (Swedish), (B) Fv/Fm in R. maritima (Thai), (C) the PSII potential activity (Fv/F0) in R. cirrhosa (Swedish), (D) Fv/F0 in R. maritima (Thai), (E) the effective quantum yield (φPSII) in R. cirrhosa (Swedish), (F) φPSII in R. maritima (Thai), (G) non-photochemical quenching (NPQ) in R. cirrhosa (Swedish), and (H) NPQ in R. maritima (Thai). Values are shown as mean ± SE (n = 6 for Ruppia cirrhosa (Swedish) and n = 6 for Ruppia maritima (Thai)). Significant differences in mean values from controls within the same day of measurements are denoted by * (Fisher’s LSD test, p < 0.05).
Analysis of covariance (ANCOVA, Table 4A) showed significant effects of species and treatments and interactions between these factors for time course responses of all photosynthetic parameters (Figure 4). The Fisher’s LSD test showed that the regression slopes of the photosynthetic parameters (Fv/Fm, Fv/F0, φPSII, and NPQ) over time in R. maritima in the warming treatment differed significantly from those derived from the other species and treatments (Table 4B), while no significant difference was found among R. cirrhosa (control), R. cirrhosa (warming treatment), and R. maritima (control).

Table 4.
Summary of A. analysis of covariance (ANCOVA) of four photosynthetic parameters in Ruppia cirrhosa (Swedish) and R. maritima (Thai) in responses to prolonged warming treatments and B. the Fisher’s least significant difference (LSD) post-hoc test comparing R. maritima in warming treatment to R. maritima (control), R. cirrhosa (control), and R. cirrhosa (warming treatment). Significant values (p < 0.05) are shown in bold.
For Ruppia cirrhosa (Swedish), the three parameters measured in controls remained stable throughout the experiment. A significant difference in the maximum quantum yield of PSII (Fv/Fm) (Figure 4A) between controls and the warming treatment was detected on day 5 (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material), whereas a significant difference in the PSII potential activity (Fv/F0) (Figure 4C) between controls and warming treatment was detected from day 3 onwards (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material). No significant difference in mean values of the effective quantum yield (φPSII) (Figure 4E) and non-photochemical quenching (NPQ) (Figure 4H) between controls and the warming treatment was detected.
For Ruppia maritima (Thai), the Fv/Fm (Figure 4B) measured in controls remained stable throughout the experiment, whereas a significant decline in Fv/Fm in the warming treatment, thus causing a significant difference from controls, was detected from day 2 onwards (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material). Further, a decline was observed on day 4 and day 5, reaching almost zero. Significant variations in Fv/F0 (Figure 4D) were observed in the controls (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material); however, the Fv/F0 measured in the warming treatment on day 2 and onwards was significantly lower than the controls (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material). The φPSII (Figure 4F) measured in controls remained stable throughout the experiment, whereas φPSII measured in the warming treatment displayed a steady decline (repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, Supplementary Material). No significant variation in NPQ was detected in controls (Figure 4H). An increase in NPQ was observed in the warming treatment on day 2 and day 3 followed by a notable decrease. This resulted in a significant difference in NPQ between controls and the warming treatment on days 2 and 3 (Figure 4H, repeated-measures ANOVA and Fisher’s LSD test, p < 0.05, supplementary material). It is worth noting that mortality was observed at the end the experiment, which corresponded to the chlorophyll fluorescence parameters that were found approaching zero.
4. Discussion
The results demonstrate distinguished photosynthetic responses to warming above ambient levels between temperate Ruppia cirrhosa and tropical R. maritima, most likely depending on how close to their thermal tipping point the plants are growing in their natural habitat. Temperate R. cirrhosa exhibited high tolerance to increasing temperature, indicating that in this region R. cirrhosa might not suffer from future predicted warming scenarios. On the contrary, tropical R. maritima showed severe sensitivity to increased temperatures, suggesting that in tropical waters the Ruppia productivity and distribution may be reduced if seawater temperatures continue to rise as predicted.
Analysis of ITS (ITS1-5.8S-ITS2) and rbcL DNA sequences indicated that samples of Ruppia from Sweden was close to Ruppia cirrhosa, while samples from Thailand was close to Ruppia maritima. Nevertheless, Ruppia sampled from the two different bioregions shared highly similar sequences of ITS (ITS1-5.8S-ITS2) and rbcL (Figure S2 and S3). Phylogenetic placement of species within the family Ruppiaceae is a debated and difficult task because of their reduced morphology, high intraspecific variability, and hybridization [45,56]. While R. maritima and R. cirrhosa were identified as separate species [45,56,57,58,59,60,61], three to four species and one cosmopolitan species complex (containing several lineages, including R. maritima and R. cirrhosa) have been proposed based on molecular evidence showing hybridization and a variety in polyploidy [57,58,59,60,61]. In addition, typification issues were raised by den Hartog and Triest [56] and Ruppia spiralis L. ex Dumortier was proposed as a more correct name for R. cirrhosa. Our study, however, focused on comparing thermal sensitivity of Ruppia from contrasting temperature regimes, addressing the effects of warming at the genus level.
Ruppia from the two different bioregions had clear differences in their photosynthetic response to increased temperature. Overall, tropical R. maritima from Thailand was more sensitive to the tested temperatures than the temperate Swedish Ruppia cirrhosa, whether looking at direct effects at four different temperatures or over a five-day period of heat exposure in comparison to control levels. It is considered that marine tropical species are more sensitive to increased temperatures than temperate species, as tropical species are in an evolutionary point of view adjusted to environments that are more constant [39,40,41]. Moreover, the ambient temperatures in tropical habitats are assumed to be closer to the upper thermal threshold of the organisms [23,39,40,41]. Thus, warming events in tropical areas may push the organisms beyond their operating and survival limits as demonstrated in our results.
A few data are available on thermal biology of R. cirrhosa from Sweden and the surrounding areas, while populations from the Mediterranean have been extensively investigated [17,46]. In the Mediterranean, R. cirrhosa has been shown to survive temperatures up to 38 °C [17,46], however, with a clear decline in photosynthetic performance at 36 °C [17] and thermal optima of between 20 and 30 °C [62]. As the Swedish R. cirrhosa was quite unaffected by temperature increase, it seems that the individuals can sustain their photosynthesis within the general temperature acceptance of the species reported in the Mediterranean populations and above the temperature range occurring in their habitats [17,46]. It needs to be emphasized that some of the tested temperatures (28–33 °C) are not commonly encountered in Swedish water. During summer months, the highest temperature recorded in shallow bays at the Swedish west coast, environments often inhabited with Ruppia, was 22–23 °C [63,64]. Swedish SST generally does not exceed 24 °C; however, in extreme years, such as 2018, a SST of 28 °C was measured in the Baltic Sea [53]. In addition, predicted future scenarios indicate that the temperature in the Västra Götaland County on the Swedish west coast is expected to increase by almost 3 °C by the end of the century according to RCP4.5 and close to 5 °C according to RCP8.5. RCP8.5 also shows an increase in the number of very warm days, with an annual average of 18 consecutive days with daily average temperatures above 20 °C at the end of the century [65]. Even so, the predicted future temperature scenarios may not be as detrimental for the productivity and performance of the Swedish R. cirrhosa as for the Thai species.
Direct or indirect damage of the photosynthetic machinery at temperatures above the thermal tolerance of a plant may be related to protein degradation, hampering of protein synthesis, or decreased membrane stability [24]. Lowered Fv/Fm and Fm and increased F0 during heat stress have previously been attributed to loss of membrane integrity [66], and hence it may be a plausible explanation to the lowered Fv/Fm and Fv/F0 values encountered in especially the Thai R. maritima of this study. Nevertheless, even though the Fv/Fm was lowered with higher temperature, the effective quantum yield (φPSII) was highest at 38 °C, indicating that the light-driven photochemistry is still working at this temperature. However, the remaining φPSII may be sustained by potential alternative electron transports, such as photorespiration and the Mehler reaction [67]. Fv/Fm is a conventional methodological approach when assessing maximum quantum yield of PSII [68,69,70]. However, the less commonly used variable chlorophyll fluorescence ratio Fv/F0 (indicating PSII potential activity) could be considered a more sensitive parameter detecting stress upon the photosynthetic apparatus at an earlier stage and with a stronger signal [19,71,72]. This was specifically clear in the response of the Swedish R. cirrhosa in prolonged temperature exposure, and it might be discussed that this ratio is preferable in future measurements of PSII activity, in order to capture plant stress on a more detailed level. The φPSII, indicating the working capacity of the photosynthetic apparatus, did not reveal any great differences in the Swedish R. cirrhosa, even though the optimum temperature seemed to be at 23 °C. The optimum temperature of φPSII at 38 °C for the Thai R. maritima was followed by a great decline at 43 °C. This may indicate that at high temperature, captured energy is not used for photochemistry, but dissipated through non-photochemical quenching. There were no clear signs of NPQ contribution upon immediate temperature stress; however, after prolonged exposure a brief increase in NPQ was detected in the Thai R. maritima suggesting photoprotective efforts of the plants. In other seagrass species, e.g., Posidonia oceanica and Zostera muelleri, an increase in NPQ with temperature has been attributed to the activation of the xanthophyll cycle as a protective measure to discard excess energy [73,74]. However, when reaching a thermal threshold, NPQ was lowered [74], suggesting that also NPQ in seagrass is sensitive to extreme temperatures. As NPQ formation requires a transmembrane proton gradient, compromised integrity of biological membranes caused by heat stress may contribute to a decline in NPQ at high temperature [75]. Heating events may thus affect seagrass physiology in several negative ways, not just impaired photosynthesis but also a decrease in protective functions and direct biomass loss [20,23]. Moreover, with increased temperature, there might be an overall lowering of primary productivity due to photorespiration. This is particularly important in conditions of high oxygen and high temperature [64], often encountered in shallow vegetated areas [76]. The Thai R. maritima seems more resistant to high temperature than the specimens from the Mediterranean and Tampa Bay, Florida [17,46]. It remains to be elucidated whether local adaptation to the tropical climate may have shifted their thermal threshold. Nevertheless, the thermotolerance range of the Thai R. maritima might not allow this species to cope with extreme warming events in the tropical latitudes. While the prediction of future increase in SST in Thailand is not yet available, exceptionally warm temperatures exceeding 40 °C have been recorded in tropical intertidal seagrass meadows [20,23]. This implies that the Thai R. maritima may suffer as temperature rises, especially when more frequent extreme weather events and larger numbers of warm days are expected as a result of a changing climate [75].
Photosynthesis of the Swedish R. cirrhosa seemed intact with direct and prolonged exposure to increased temperature. Hence, a predicted future temperature increase may not affect Swedish R. cirrhosa in the same negative way as Z. marina, a seagrass species that in the same geographic area showed photoinhibition at lower temperatures [19]. Although community dynamics resulted from complex interactions among species and environmental drivers, our short-term data may provide supporting evidence for some ecological observations reported earlier. The authors of previous field investigations have discussed potential replacement of more slow growing climax species such as Zostera marina in favor for the fast-growing colonizing opportunist R. maritima in high temperature scenarios [11,77,78]. In North American waters, shifts from Z. marina to R. maritima-dominated systems have been reported as R. maritima colonizes areas that are opened up by Z. marina die-backs, most likely due to the higher tolerance of Ruppia to temperature and salinity change [11,77,78]. From an ecological point of view, a dominance shift from Zostera to Ruppia had no effects on canopy invertebrate assemblages, suggesting that the two seagrasses could harbor similar function for marine fauna. However, Ruppia had a lower sediment retention due to coarsening of the bottom substrate [79]. Hence, some ecosystem services provided by one seagrass species may not be lost due to a shift in dominance, while some may vanish. Moreover, in experiments simulating nitrate enrichment as a form of eutrophication, shoot production of R. maritima was increased by more than 300%, while Z. marina showed a reduction in number of shoots [80]. Ruppia has different growth cycles than other seagrasses, where they start developing shoots in winter months when most other species are dormant. In addition, their die-back in fall is earlier than most other species. This different timing might pose a colonization advantage [43]. These different examples highlight the broad adaptive capability of this genus over many other seagrass species to environmental change, something that according to our photosynthetic response to temperature results could be especially important in Swedish waters, where Ruppia seems to have higher tolerance threshold than the co-occurring species [12,19].
In tropical seas, including Thai waters, other seagrass species tolerate similar and higher temperatures [15,19,20] than the R. maritima of this study, indicating that R. maritima may be outcompeted in tropical environments. However, the life history of opportunistic traits combined with their environmental sturdiness may allow them to recover from short-term heat stress. Furthermore, Ruppia is able to withstand salinities from 0 to 70, whereas other seagrasses are restricted to 5–45 [81]. Moreover, there is accumulating evidence suggesting that thermal sensitivity, measured as short-term physiological responses, is not necessarily translated to the acclimation capacity in various organisms [82,83,84]. In a longer term, organisms may be able to acclimate to a warming environment [82,83,84]. Additionally, acclimation capacity may differ significantly among species and populations. Long-term studies exploring species’ acclimation capacity as well as complex biotic interactions and multiple environmental drivers are necessary to improve the prediction of the fate of Ruppia populations in the face of climate change.
5. Conclusions
This study clearly demonstrates that Ruppia from two different bioregions have very different responses in photosynthetic capacity to predicted future water temperature increases. The Swedish temperate R. cirrhosa was able to maintain photosynthetic efficiency at temperatures way above their ambient conditions, indicating that they will likely withstand a future increase of ocean temperature and warming events. As the other dominant seagrass in this geographic area, Zostera marina, is less tolerant to temperature increase, climate change may lead to a shift towards a Ruppia-dominant system in Swedish waters. On the contrary, Thai R. maritima were negatively affected by an increase in temperature, directly and over time, indicating that individuals are close to their thermal threshold and more sensitive to warming scenarios. Hence, in Thai waters, climate change effects may more likely trigger a shift in the coastal community towards a more negative outcome for Ruppia abundance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13169433/s1, Figure S1: Experimental design and timeline of the measurements. Figure S2: The multiple sequence alignment result of ITS gene sequences of the Ruppia cirrhosa from Sweden (Ruppia_sp_SW) and R. maritima from Thailand (Ruppia_sp_TH) with the reference strains; R. maritima (Accession No. AB728734.1) and R. cirrhosa (Accession No. AB728749.1) using MUSCLE (MUltiple Sequence Comparison by Log Expectation). Figure S3: The multiple sequence alignment result of rbcL gene sequences of the R. cirrhosa from Sweden (Ruppia_sp_SW) and R. maritima from Thailand (Ruppia_sp_TH) with the reference strains; R. maritima (Accession No.JN113279.1) and R. cirrhosa (Accession No. JN113277.1) using MUSCLE (MUltiple Sequence Comparison by Log Expectation).
Author Contributions
Conceptualization, L.M.R., A.N.-o., M.B. and P.B.; Data curation, L.M.R., A.N.-o., T.W. and P.B.; Formal analysis, L.M.R., A.N.-o. and P.B.; Funding acquisition, L.M.R. and P.B.; Investigation, L.M.R., A.N.-o., T.W., M.B., M.G. and P.B.; Methodology, L.M.R., A.N.-o., M.B. and P.B.; Project administration, P.B.; Resources, L.M.R., A.N.-o., M.B., M.G. and P.B.; Software, A.N.-o. and P.B.; Supervision, P.B.; Validation, L.M.R., A.N.-o. and P.B.; Visualization, A.N.-o. and P.B.; Writing—Original draft, L.M.R., A.N.-o., M.B., M.G. and P.B.; Writing—Review and editing, L.M.R., A.N.-o., M.B., M.G. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant Number: ENV6405083M) and KVA fund for scientific renewal and internationalization at the Sven Lovén Centre 2019 for P.B. and mobility grant from the Swedish research council FORMAS, grant number 2017-00363 for L.M.R. The APC was funded by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant Number: ENV6405083M).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data supporting the findings of this study are available in GenBank with the following accession codes, AB728749.1 (R. cirrhosa ITS gene), MN958127.1 (R. drepanensis ITS gene), AB728734.1 (R. maritima ITS gene), JQ034337.1 (R. megacarpa ITS gene), JN113279.1 (R. maritima rbcL gene), NC051974.1 (R. brevipedunculata rbcL gene), JN113277.1 (R. cirrhosa rbcL gene), MN233650.1 (R. sinensis rbcL gene), and AB507891.2 (R. megacarpa rbcL gene), MZ453015 (R. maritima ITS gene (Thailand)), MZ466377 (R. maritima rbcL gene (Thailand)), MZ474644 (R. cirrhosa ITS gene(Sweden)), and MZ466378 (R. cirrhosa rbcL gene (Sweden)).
Acknowledgments
We wish to thank Somsak Buatip for his help with sample collection from Pattani Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O.; Roberts, D.C.; Masson-Delmotte, V.; Zhai, P.; Tignor, M.; Poloczanska, E.; Mintenbeck, K.; Nicolai, M.; Okem, A.; Petzold, J.; et al. (Eds.) IPCC Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; Volume 1. [Google Scholar]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine Heatwaves Threaten Global Biodiversity and the Provision of Ecosystem Services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and More Frequent Marine Heatwaves over the Past Century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; de Bettignies, T.; Bennett, S.; Rousseaux, C.S. An Extreme Climatic Event Alters Marine Ecosystem Structure in a Global Biodiversity Hotspot. Nat. Clim. Chang. 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Duarte, B.; Martins, I.; Rosa, R.; Matos, A.R.; Roleda, M.Y.; Reusch, T.B.H.; Engelen, A.H.; Serrão, E.A.; Pearson, G.A.; Marques, J.C.; et al. Climate Change Impacts on Seagrass Meadows and Macroalgal Forests: An Integrative Perspective on Acclimation and Adaptation Potential. Front. Mar. Sci. 2018, 5, 190. [Google Scholar] [CrossRef] [Green Version]
- Koch, E.W.; Verduin, J.J.; Katwijk, V. Measurements of physical parameters in seagrass habitats. In Global Seagrass Research Methods; Elsevier: Amsterdam, The Netherlands, 2001; pp. 325–344. ISBN 978-0-444-50891-1. [Google Scholar]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass Ecosystems as a Globally Significant Carbon Stock. Nature Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Cullen-Unsworth, L.; Unsworth, R. Seagrass Meadows, Ecosystem Services and Sustainability. Environment 2013, 55, 14–28. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987. [Google Scholar] [CrossRef] [Green Version]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, E.C.; Parrish, D.; Moore, K. Short-Term Temperature Stress Results in Seagrass Community Shift in a Temperate Estuary. Estuaries Coasts 2019, 42, 755–764. [Google Scholar] [CrossRef]
- Rasmusson, L.M.; Gullström, M.; Gunnarsson, P.C.B.; George, R.; Björk, M. Estimation of a Whole Plant Q10 to Assess Seagrass Productivity during Temperature Shifts. Sci. Rep. 2019, 9, 12667. [Google Scholar] [CrossRef] [Green Version]
- Drew, E.A. Physiological Aspects of Primary Production in Seagrasses. Aquat. Bot. 1979, 7, 139–150. [Google Scholar] [CrossRef]
- Marsh, J.A.; Dennison, W.C.; Alberte, R.S. Effects of Temperature on Photosynthesis and Respiration in Eelgrass (Zostera marina L.). J. Exp. Mar. Bio. Ecol. 1986, 101, 257–267. [Google Scholar] [CrossRef]
- Collier, C.J.; Ow, Y.X.; Langlois, L.; Uthicke, S.; Johansson, C.L.; O’Brien, K.R.; Hrebien, V.; Adams, M.P. Optimum Temperatures for Net Primary Productivity of Three Tropical Seagrass Species. Front. Plant Sci. 2017, 8, 1446. [Google Scholar] [CrossRef] [Green Version]
- Hammer, K.J.; Borum, J.; Hasler-Sheetal, H.; Shields, E.C.; Sand-Jensen, K.; Moore, K.A. High Temperatures Cause Reduced Growth, Plant Death and Metabolic Changes in Eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 2018, 604, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Tsioli, S.; Orfanidis, S.; Papathanasiou, V.; Katsaros, C.; Exadactylos, A. Effects of Salinity and Temperature on the Performance of Cymodocea Nodosa and Ruppia cirrhosa: A Medium-Term Laboratory Study. Bot. Mar. 2019, 62, 97–108. [Google Scholar] [CrossRef]
- Campbell, S.J.; McKenzie, L.J.; Kerville, S.P. Photosynthetic Responses of Seven Tropical Seagrasses to Elevated Seawater Temperature. J. Exp. Mar. Bio. Ecol. 2006, 330, 455–468. [Google Scholar] [CrossRef]
- Rasmusson, L.M.; Buapet, P.; George, R.; Gullström, M.; Gunnarsson, P.C.B.; Björk, M. Effects of Temperature and Hypoxia on Respiration, Photorespiration, and Photosynthesis of Seagrass Leaves from Contrasting Temperature Regimes. ICES J. Mar. Sci. 2020, 77, 2056–2065. [Google Scholar] [CrossRef]
- George, R.; Gullström, M.; Mangora, M.M.; Mtolera, M.S.P.; Björk, M. High Midday Temperature Stress Has Stronger Effects on Biomass than on Photosynthesis: A Mesocosm Experiment on Four Tropical Seagrass Species. Ecol. Evol. 2018, 8, 4508–4517. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Borum, J.; Zavala-Perez, A.; Kendrick, G.A. Heat Stress of Two Tropical Seagrass Species during Low Tides—Impact on Underwater Net Photosynthesis, Dark Respiration and Diel in Situ Internal Aeration. New Phytol. 2016, 210, 1207–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.A.; Jarvis, J.C. Environmental Factors Affecting Recent Summertime Eelgrass Diebacks in the Lower Chesapeake Bay: Implications for Long-Term Persistence. J. Coast. Res. 2008, 10055, 135–147. [Google Scholar] [CrossRef]
- Collier, C.J.; Waycott, M. Temperature Extremes Reduce Seagrass Growth and Induce Mortality. Mar. Pollut. Bull. 2014, 83, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant. Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat Stress: An Overview of Molecular Responses in Photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Shimizu, Y.; Pospíšil, P.; Nijo, N.; Fujiwara, A.; Taninaka, Y.; Ishikawa, T.; Hori, H.; Nanba, D.; Imai, A.; et al. Quality Control of Photosystem II: Lipid Peroxidation Accelerates Photoinhibition under Excessive Illumination. PLoS ONE 2012, 7, e52100. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y. Quality Control of Photosystem II: The Mechanisms for Avoidance and Tolerance of Light and Heat Stresses Are Closely Linked to Membrane Fluidity of the Thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein Synthesis Is the Primary Target of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Physiol. Plant. 2011, 142, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ogren, W.L. Photorespiration: Pathways, Regulation, and Modification. Annu. Rev. Plant. Physiol. 1984, 35, 415–442. [Google Scholar] [CrossRef]
- Björkman, O. The Effect of Oxygen Concentration on Photosynthesis in Higher Plants. Physiol. Plant. 1966, 19, 618–633. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Ruiz, J.M.; Dattolo, E.; Garcia-Munoz, R.; Procaccini, G. Physiological and Molecular Evidence of Differential Short-Term Heat Tolerance in Mediterranean Seagrasses. Sci. Rep. 2016, 6, 28615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Guirao, L.; Bernardeau-Esteller, J.; García-Muñoz, R.; Ramos, A.; Ontoria, Y.; Romero, J.; Pérez, M.; Ruiz, J.M.; Procaccini, G. Carbon Economy of Mediterranean Seagrasses in Response to Thermal Stress. Mar. Pollut. Bull. 2018, 135, 617–629. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Entrambasaguas, L.; Dattolo, E.; Ruiz, J.M.; Procaccini, G. Molecular Mechanisms behind the Physiological Resistance to Intense Transient Warming in an Iconic Marine Plant. Front. Plant Sci. 2017, 8, 1142. [Google Scholar] [CrossRef] [Green Version]
- Tutar, O.; Marín-Guirao, L.; Ruiz, J.M.; Procaccini, G. Antioxidant Response to Heat Stress in Seagrasses. A Gene Expression Study. Mar. Environ. Res. 2017, 132, 94–102. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Guihéneuf, F.; Marín-Guirao, L.; Bernardeau-Esteller, J.; García-Muñoz, R.; Stengel, D.B.; Ruiz, J.M. Effects of an Experimental Heat Wave on Fatty Acid Composition in Two Mediterranean Seagrass Species. Mar. Pollut. Bull. 2018, 134, 27–37. [Google Scholar] [CrossRef]
- Franssen, S.U.; Gu, J.; Bergmann, N.; Winters, G.; Klostermeier, U.C.; Rosenstiel, P.; Bornberg-Bauer, E.; Reusch, T.B.H. Transcriptomic Resilience to Global Warming in the Seagrass Zostera marina, a Marine Foundation Species. Proc. Natl. Acad. Sci. USA 2011, 108, 19276–19281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winters, G.; Nelle, P.; Fricke, B.; Rauch, G.; Reusch, T. Effects of a Simulated Heat Wave on Photophysiology and Gene Expression of High- and Low-Latitude Populations of Zostera marina. Mar. Ecol. Prog. Ser. 2011, 435, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Thermal Tolerance and the Global Redistribution of Animals. Nat. Clim. Chang. 2012, 2, 686–690. [Google Scholar] [CrossRef]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.M.; Jess, M.; Williams, S.E. Predicting Organismal Vulnerability to Climate Warming: Roles of Behaviour, Physiology and Adaptation. Phil. Trans. R. Soc. B 2012, 367, 1665–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Ecology: Putting the Heat on Tropical Animals. Science 2008, 320, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; McRoy, C.P. Seagrass Productivity: The Effect of Light on Carbon Uptake. Aquat. Bot. 1982, 12, 321–344. [Google Scholar] [CrossRef]
- Lazar, A.C.; Dawes, C.J. A Seasonal Study of the Seagrass Ruppia maritima L. in Tampa Bay, Florida. Organic Constitutents and Tolerances to Salinity and Temperature. Bot. Mar. 1991, 34, 265–269. [Google Scholar] [CrossRef]
- Blomqvist, B.; Wikström, S.A.; Carstensen, J.; Qvarfordt, S.; Krause-Jensen, D. Response of Coastal Macrophytes to Pressures; WATERS Report no. 2014:2; Havsmiljöinstitutet: Gothenburg, Sweden, 2014. [Google Scholar]
- Martínez-Garrido, J.; Serrão, E.A.; Engelen, A.H.; Cox, C.J.; García-Murillo, P.; González-Wangüemert, M. Multilocus Genetic Analyses Provide Insight into Speciation and Hybridization in Aquatic Grasses, Genus Ruppia. Biol. J. Linn. Soc. 2016, 117, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Comín, F.A.; Menéndez, M.; Lucena, J.R. Proposals for macrophyte restoration in eutrophic coastal lagoons. In Biomanipulation Tool for Water Management; Gulati, R.D., Lammens, E.H.R.R., Meijer, M.-L., van Donk, E., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 427–436. ISBN 978-90-481-4074-9. [Google Scholar]
- Flora of Thailand, Volume 11, Part 4: Campanulaceae, Elatinaceae, Lythraceae, Onagraceae, Ruppiaceae, Sapotaceae & Staphyleaceae; Santisuk, T.; Balslev, H.; Newman, M.; Chayamarit, K. (Eds.) The Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2014; ISBN 978-616-316-174-1. [Google Scholar]
- Moksnes, P.-O.; Gipperth, L.; Eriander, L.; Laas, K.; Cole, S.; Infantes, E. Förvaltning Och Restaurering Av Ålgräs I Sverige: Ekologisk, juridisk Och Ekonomisk Bakgrund; Rapport 2016:8; Havs-och vattenmyndigheten: Gothenburg, Sweden, 2016. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Ed.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 978-0-08-088671-8. [Google Scholar]
- Baldwin, B.G. Phylogenetic Utility of the Internal Transcribed Spacers of Nuclear Ribosomal DNA in Plants: An Example from the Compositae. Mol. Phylogenet. Evol. 1992, 1, 3–16. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Siegel, H.; Gerth, M. Sea Surface Temperature in the Baltic Sea in 2018; HELCOM Baltic Sea Environment Fact Sheets; Baltic Marine Environment Protection Commission (Helsinki Commission-HELCOM): Helsinki, Finland, 2019. [Google Scholar]
- Vichkovitten, T.; Intarachart, A.; Khaodon, K.; Rermdumri, S. Transplantation of Tropical Seagrass Enhalus Acoroides (Lf) in Thai Coastal Water: Implication for Habitat Restoration. Greater Mekong Subreg. Acad. Res. Netw. Int. J. 2016, 10, 113–120. [Google Scholar]
- Levene, H. Robust tests for equality of variances. In Contribution to Probability and Statistics; Olkin, I., Ghurye, S., Hoeffding, W., Madow, W., Mann, H., Eds.; Stanford University Press: Stanford, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Den Hartog, C.; Triest, L. A Profound View and Discourse on the Typification and Status of Three Confused Taxa: Ruppia maritima, R. Spiralis and R. Cirrhosa. Bot. Mar. 2020, 63, 229–239. [Google Scholar] [CrossRef]
- Triest, L.; Beirinckx, L.; Sierens, T. Lagoons and Saltwater Wetlands Getting More Diversity: A Molecular Approach Reveals Cryptic Lineages of a Euryhaline Submerged Macrophyte (Ruppia). Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 370–382. [Google Scholar] [CrossRef]
- Beirinckx, L.; Vanschoenwinkel, B.; Triest, L. Hidden Hybridization and Habitat Differentiation in a Mediterranean Macrophyte, the Euryhaline Genus Ruppia. Front. Plant Sci. 2020, 11, 830. [Google Scholar] [CrossRef]
- Ito, Y.; Ohi-Toma, T.; Murata, J.; Tanaka, N. Hybridization and Polyploidy of an Aquatic Plant, Ruppia (Ruppiaceae), Inferred from Plastid and Nuclear DNA Phylogenies. Am. J. Bot. 2010, 97, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Ohi-Toma, T.; Murata, J.; Tanaka, N. Comprehensive Phylogenetic Analyses of the Ruppia maritima Complex Focusing on Taxa from the Mediterranean. J. Plant Res. 2013, 126, 753–762. [Google Scholar] [CrossRef]
- Ito, Y.; Ohi-Toma, T.; Tanaka, N.; Murata, J.; Muasya, A.M. Phylogeny of Ruppia (Ruppiaceae) Revisited: Molecular and Morphological Evidence for a New Species from Western Cape, South Africa. Syst. Bot. 2016, 40, 942–949. [Google Scholar] [CrossRef]
- Mannino, A.M.; Menéndez, M.; Obrador, B.; Sfriso, A.; Triest, L. The Genus Ruppia L. (Ruppiaceae) in the Mediterranean Region: An Overview. Aquat. Bot. 2015, 124, 1–9. [Google Scholar] [CrossRef]
- Baden, S.P.; Pihl, L. Abundance, Biomass and Production of Mobile Epibenthic Fauna in Zostera Marina (L.) Meadows, Western Sweden. Ophelia 1984, 23, 65–90. [Google Scholar] [CrossRef]
- Buapet, P.; Gullström, M.; Björk, M. Photosynthetic Activity of Seagrasses and Macroalgae in Temperate Shallow Waters Can Alter Seawater PH and Total Inorganic Carbon Content at the Scale of a Coastal Embayment. Mar. Freshw. Res. 2013, 64, 1040. [Google Scholar] [CrossRef]
- Persson, G.; Asp, M.; Berggreen-Clausen, S.; Berglöv, G.; Björck, E.; Axén Mårtensson, J.; Ohlsson, A.; Persson, H.; Sjökvist, E. Framtidsklimat I Hallands Län—Enligt RCP-Scenarier; SMHI: Norrköping, Swedia, 2015. [Google Scholar]
- Srinivasan, A.; Takeda, H.; Senboku, T. Heat Tolerance in Food Legumes as Evaluated by Cell Membrane Thermostability and Chlorophyll Fluorescence Techniques. Euphytica 1996, 88, 35–45. [Google Scholar] [CrossRef]
- Buapet, P.; Björk, M. The Role of O2 as an Electron Acceptor Alternative to CO2 in Photosynthesis of the Common Marine Angiosperm Zostera marina L. Photosynth. Res. 2016, 129, 59–69. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Beer, S.; Björk, M.; Beardall, J. Photosynthesis in the Marine Environment; Wiley Blackwell: Ames, IA, USA, 2014; ISBN 978-1-119-97958-6. [Google Scholar]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin, Heidelberg, 1995; pp. 49–70. ISBN 978-3-540-58571-8. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to Correctly Determine the Different Chlorophyll Fluorescence Parameters and the Chlorophyll Fluorescence Decrease Ratio RFd of Leaves with the PAM Fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Babani, F.; Lichtenthaler, H.K. Light-Induced and Age-Dependent Development OfChloroplasts in Etiolated Barley Leaves as Visualized by Determination of Photosynthetic Pigments, C02 Assimilation Rates and Different Kinds of Chlorophyll Fluorescence Ratios. J. Plant Physiol. 1996, 148, 555–566. [Google Scholar] [CrossRef]
- York, P.H.; Gruber, R.K.; Hill, R.; Ralph, P.J.; Booth, D.J.; Macreadie, P.I. Physiological and Morphological Responses of the Temperate Seagrass Zostera Muelleri to Multiple Stressors: Investigating the Interactive Effects of Light and Temperature. PLoS ONE 2013, 8, e76377. [Google Scholar] [CrossRef] [PubMed]
- Ontoria, Y.; Cuesta-Gracia, A.; Ruiz, J.M.; Romero, J.; Pérez, M. The Negative Effects of Short-Term Extreme Thermal Events on the Seagrass Posidonia Oceanica Are Exacerbated by Ammonium Additions. PLoS ONE 2019, 14, e0222798. [Google Scholar] [CrossRef] [PubMed]
- Sinsawat, V.; Leipner, J.; Stamp, P.; Fracheboud, Y. Effect of Heat Stress on the Photosynthetic Apparatus in Maize (Zea Mays L.) Grown at Control or High Temperature. Environ. Exp. Bot. 2004, 52, 123–129. [Google Scholar] [CrossRef]
- Buapet, P.; Rasmusson, L.M.; Gullström, M.; Björk, M. Photorespiration and Carbon Limitation Determine Productivity in Temperate Seagrasses. PLoS ONE 2013, 8, e83804. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.A.; Shields, E.C.; Parrish, D.B. Impacts of Varying Estuarine Temperature and Light Conditions on Zostera Marina (Eelgrass) and Its Interactions With Ruppia Maritima (Widgeongrass). Estuaries Coasts 2014, 37, 20–30. [Google Scholar] [CrossRef]
- Johnson, M.R.; Williams, S.L.; Lieberman, C.H.; Solbak, A. Changes in the Abundance of the Seagrasses Zostera Marina L. (Eelgrass) and Ruppia maritima L. (Widgeongrass) in San Diego, California, Following and El Niño Event. Estuaries 2003, 26, 106–115. [Google Scholar] [CrossRef]
- French, E.; Moore, K. Canopy Functions of R. Maritima and Z. Marina in the Chesapeake Bay. Front. Mar. Sci. 2018, 5, 461. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Glasgow, H.B.; Cooke, J.E. Comparative Effects of Water-Column Nitrate Enrichment on Eelgrass Zostera marina, Shoalgrass Halodule Wrightii, and Widgeongrass Ruppia Maritima. Mar. Ecol. Prog. Ser. 1994, 105, 121–138. [Google Scholar] [CrossRef]
- Touchette, B.W. Seagrass-Salinity Interactions: Physiological Mechanisms Used by Submersed Marine Angiosperms for a Life at Sea. J. Exp. Mar. Bio. Ecol. 2007, 350, 194–215. [Google Scholar] [CrossRef]
- Donelson, J.M.; Munday, P.L. Thermal Sensitivity Does Not Determine Acclimation Capacity for a Tropical Reef Fish. J. Anim. Ecol. 2012, 81, 1126–1131. [Google Scholar] [CrossRef]
- Pallarés, S.; Colado, R.; Pérez-Fernández, T.; Wesener, T.; Ribera, I.; Sánchez-Fernández, D. Heat Tolerance and Acclimation Capacity in Subterranean Arthropods Living under Common and Stable Thermal Conditions. Ecol. Evol. 2019, 9, 13731–13739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid Responses of Plants to Temperature Changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).