Preparation and Characterization of Cattail-Derived Biochar and Its Application for Cadmium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation and Characterization of the Biochar
2.3. Cd Adsorption Isotherm Experiment
2.4. Cd Adsorption Kinetics Experiment
2.5. Effect of Ionic Strength on the Adsorption Experiments
2.6. Leaching Experiments of the Inorganic Components
3. Results and Discussions
3.1. Physical and Chemical Properties of the Biochar
3.1.1. Basic Properties of Cattail Biochar
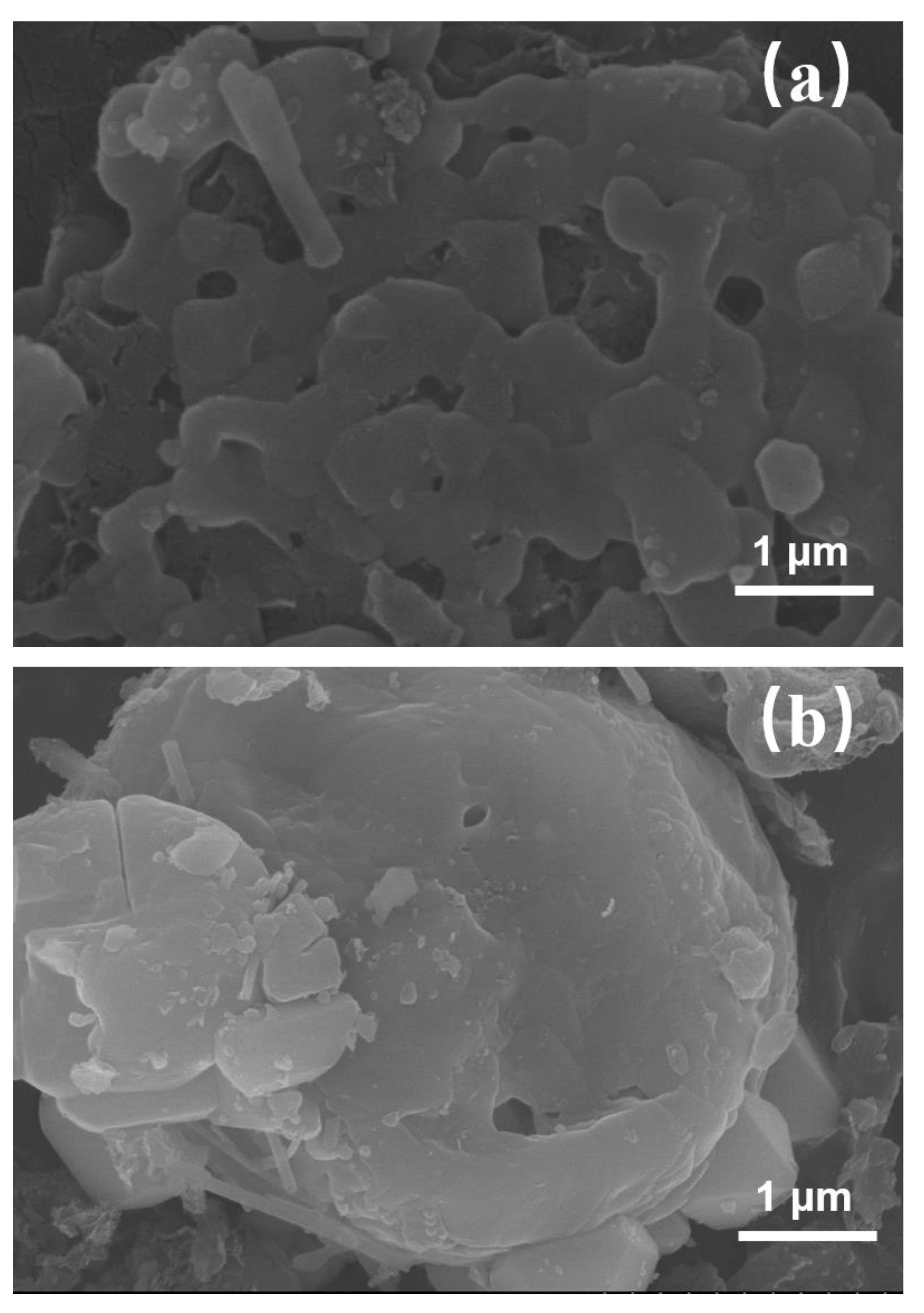
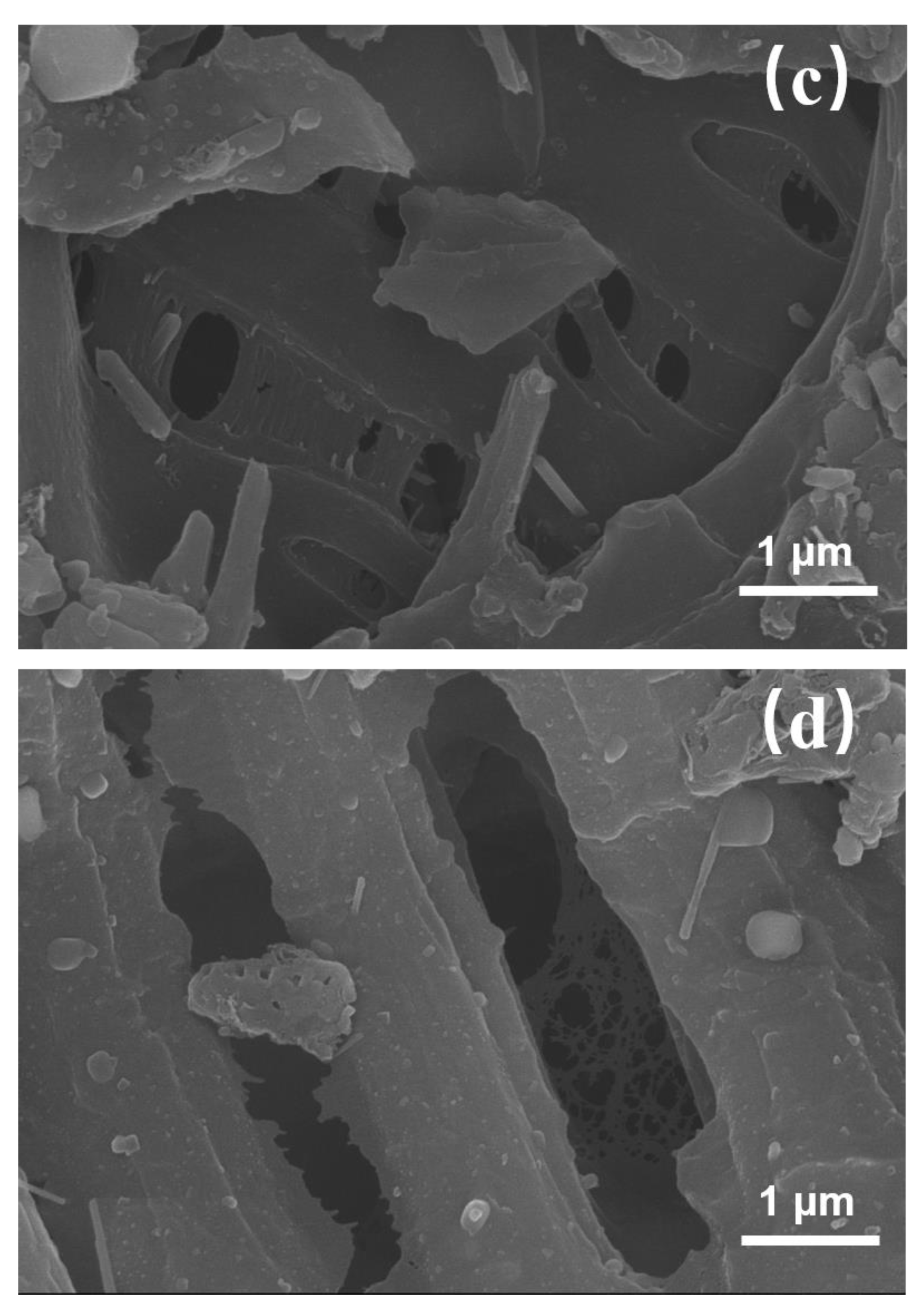
3.1.2. Scanning Electron Microscopy of Cattail Biochar (SEM)
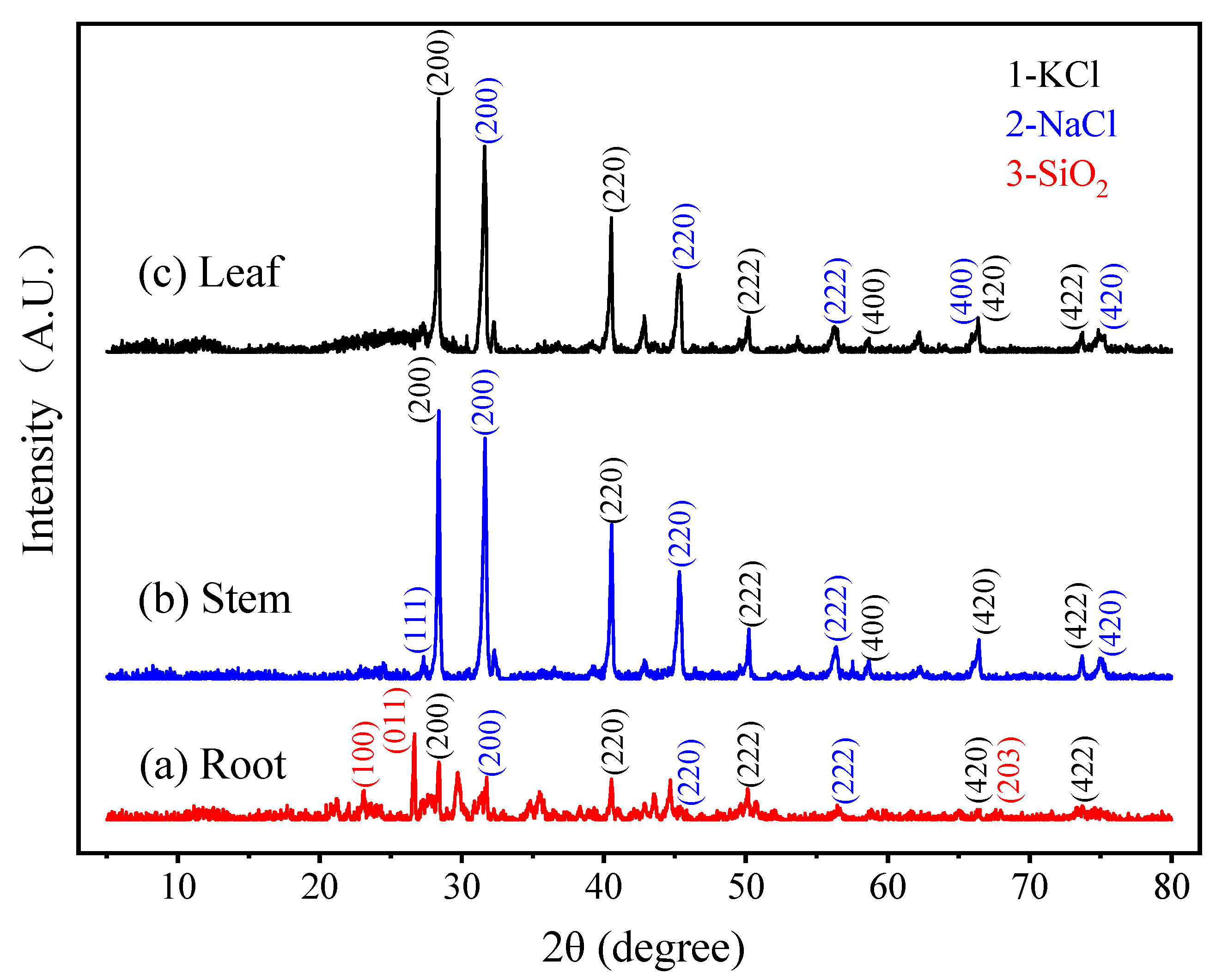
3.1.3. XRD Patterns of Cattail Biochar
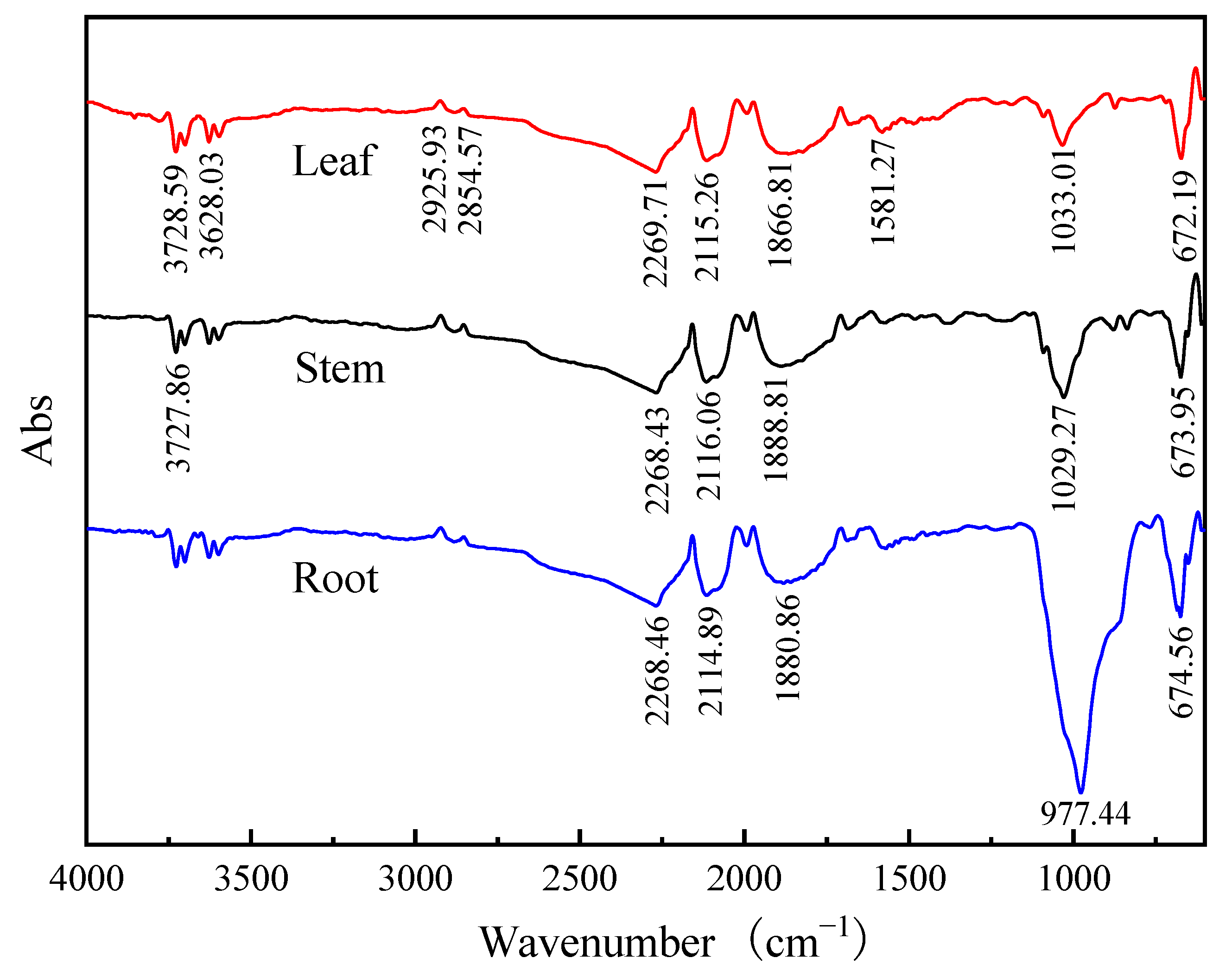
3.1.4. FTIR Spectra of Cattail Biochar
3.2. Study on the Adsorption Behavior of Metal Ions by Biochar
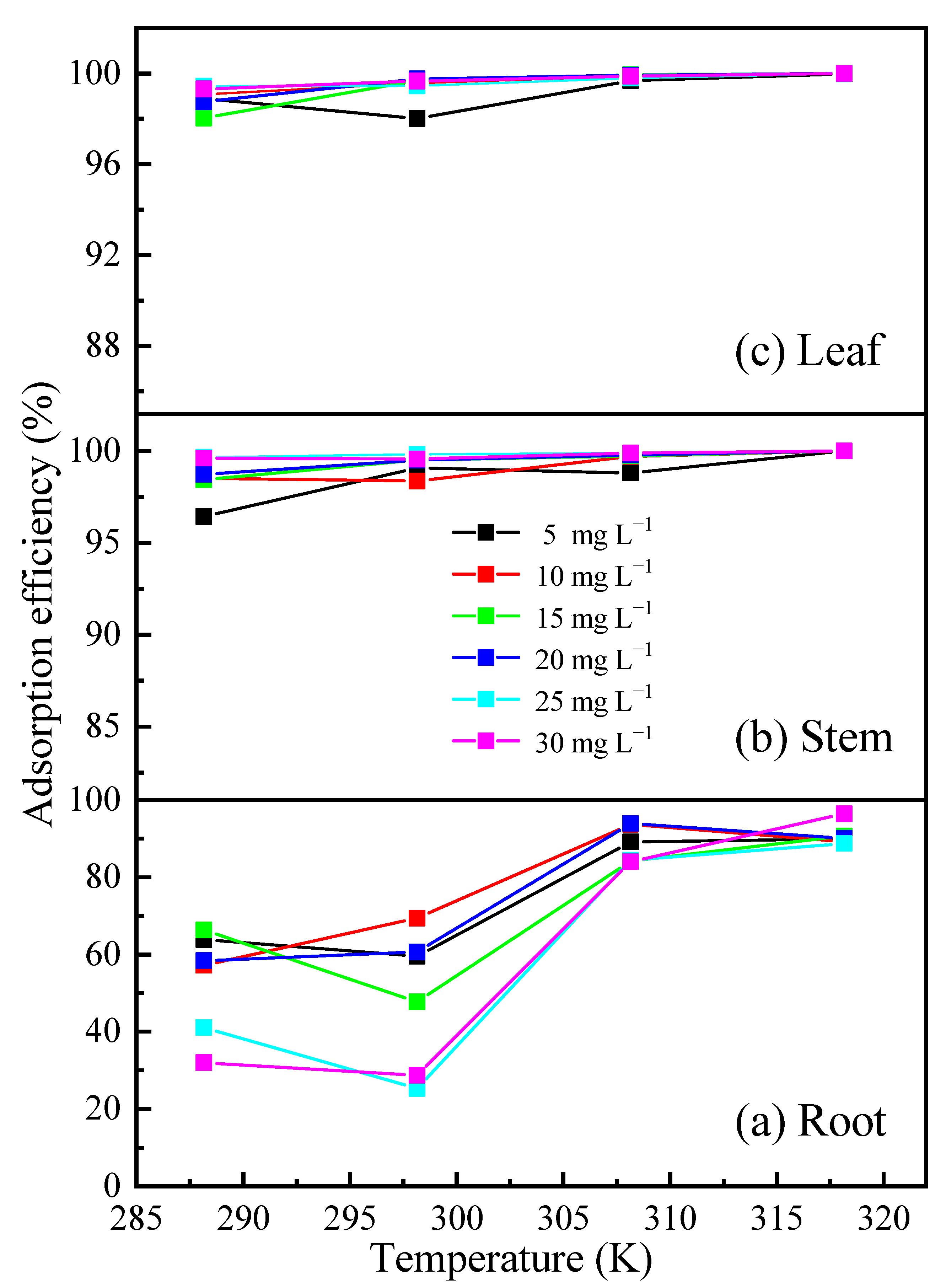
3.2.1. Effects of the Temperature and Initial Concentration on Cd Sorption by Biochar
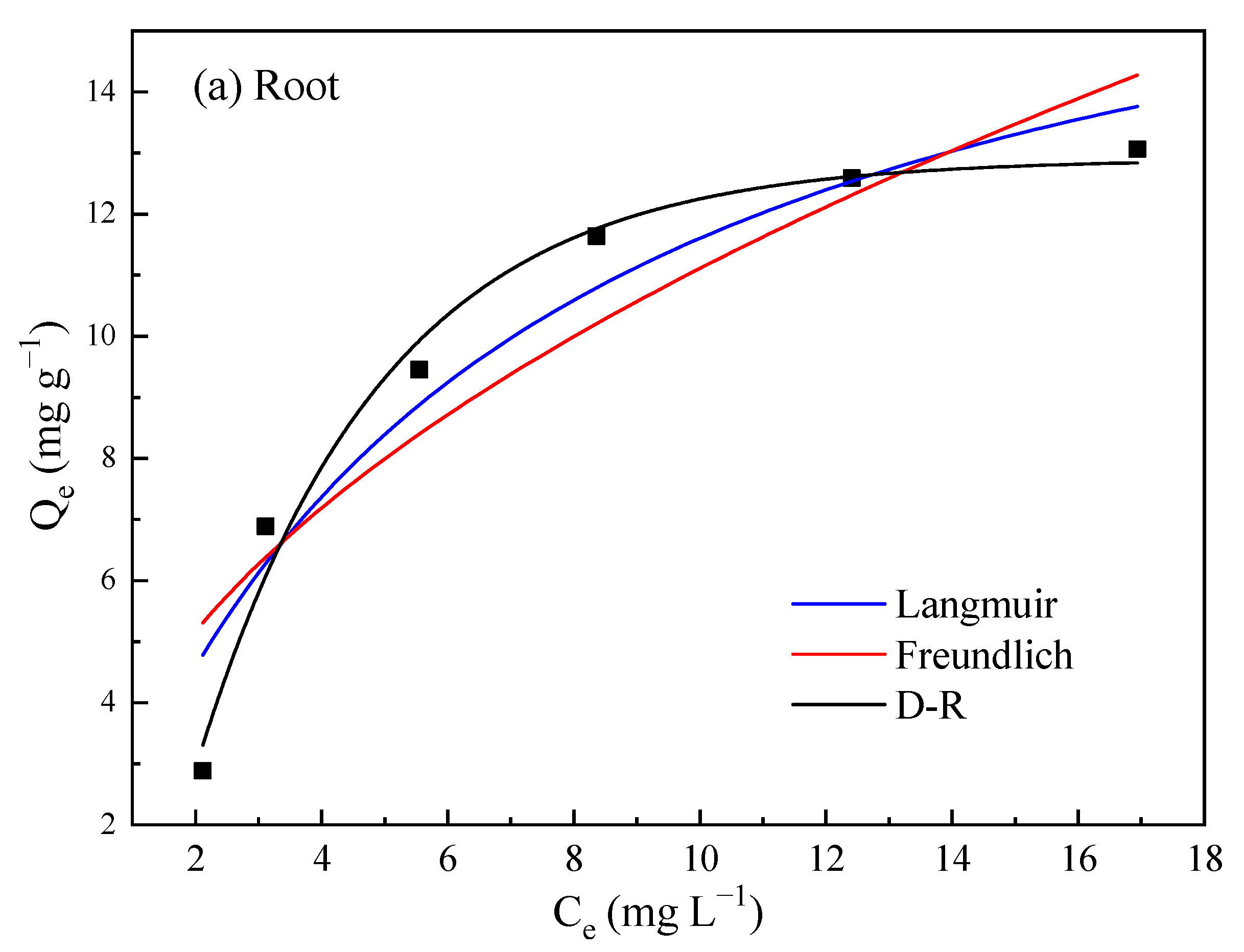
3.2.2. Isothermal Adsorption Characteristics
3.2.3. Dynamic Adsorption Model
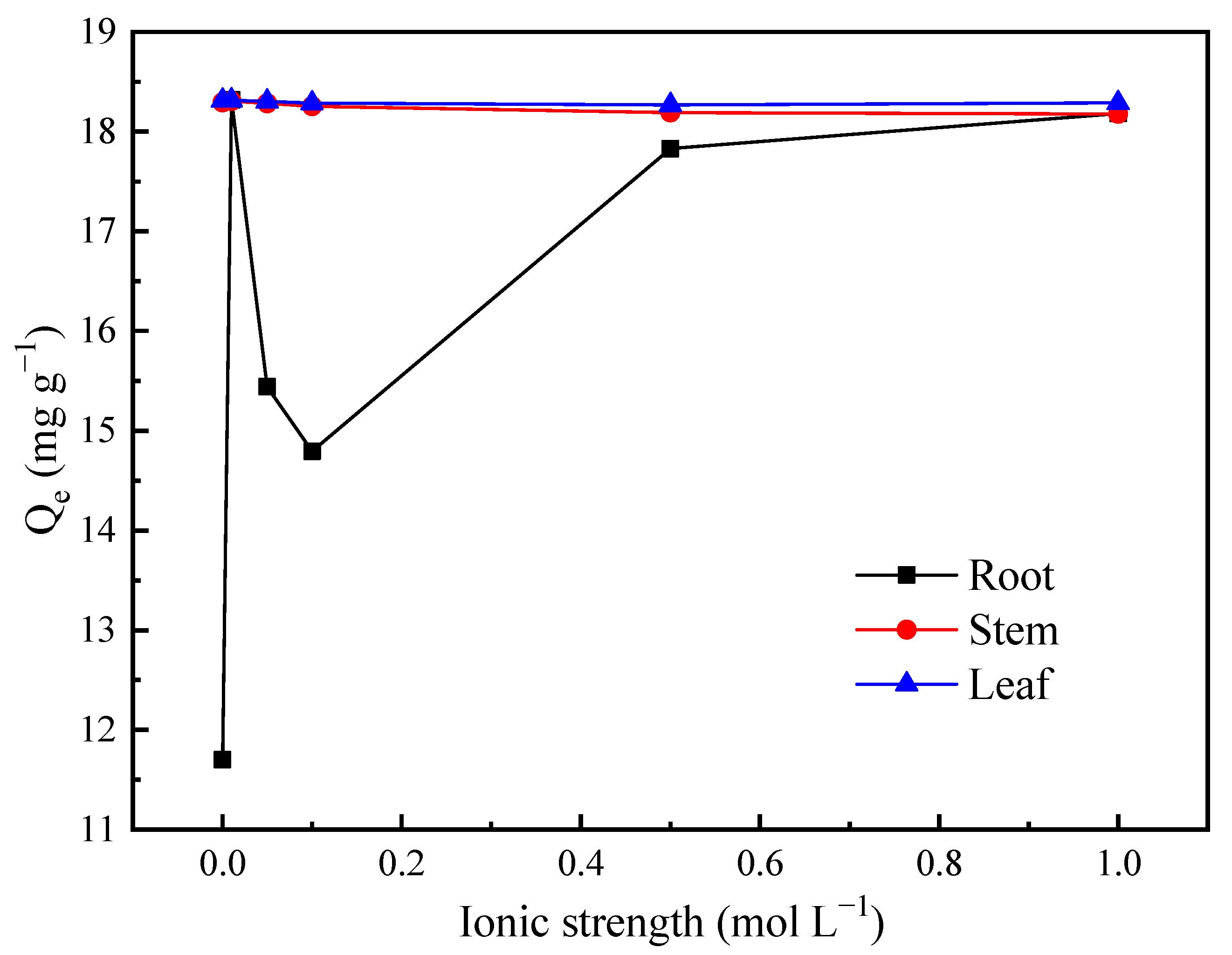
3.2.4. Effects of the Ion Intensity on the Adsorption of Heavy Metal Ions by Three Kinds of Cattail Biochar
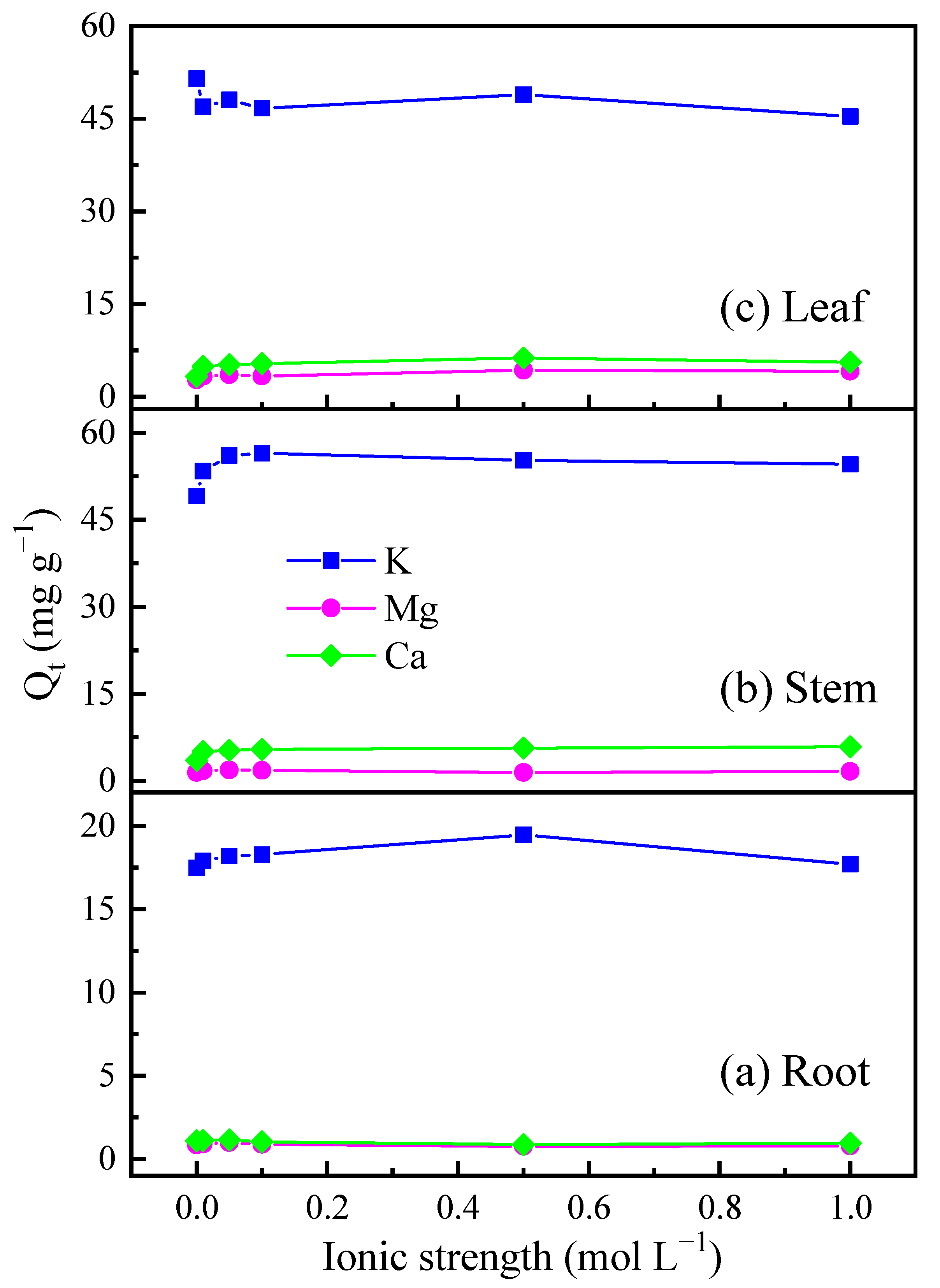
3.3. Alkali and Alkali Soil Metals Affected the Cd2+ Sorption on Biochar
3.4. Mechanisms of Cd(II) Removal by Cattail Biochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Li, J.T.; Qiu, J.W.; Wang, X.W.; Zhong, Y.; Lan, C.Y.; Shu, W.S. Cadmium contamination in orchard soils and fruit trees and its potential health risk in Guangzhou, China. Environ. Pollut. 2006, 143, 159–165. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An overview of carcinogenic pollutants in groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Report on the National General Survey of Soil Contamination [EB/OL]. Available online: http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417_270670.htm (accessed on 17 April 2014).
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiy, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Plant, J.A.; Voulvoulis, N.; Oates, C.J.; Ihlenfeld, C. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.L.; Liu, C.; Wu, H.; Jin, Y.; Hua, M.; Zhu, B.; Chen, K.; Huang, L. Association of soil cadmium contamination with ceramic industry: A case study in a Chinese town. Sci. Total Environ. 2015, 514, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Siebecker, M.G.; Gou, W.; Li, L.; Li, W. A review of cadmium sorption mechanisms on soil mineral surfaces revealed from synchrotron-based X-ray absorption fine structure spectroscopy: Implications for soil remediation. Pedosphere 2021, 31, 11–27. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, X.; Zhang, Y.; Hu, Z.; Yang, X.; Chen, C.; Chen, H.; Li, Y.; Wang, J. Removal of cadmium from wastewater by magnetic zeolite synthesized from natural, low-grade molybdenum. Sci. Total Environ. 2021, 772, 145355. [Google Scholar] [CrossRef]
- Gu, Y.; Yeung, A.T. Desorption of cadmium from a natural Shanghai clay using citric acid industrial wastewater. J. Hazard. Mater. 2011, 191, 144–149. [Google Scholar] [CrossRef] [Green Version]
- He, B.-Y.; Yu, D.-P.; Chen, Y.; Shi, J.; Xia, Y. Use of low-calcium cultivars to reduce cadmium uptake and accumulation in edible amaranth (Amaranthus mangostanus L.). Chemosphere 2017, 171, 588–594. [Google Scholar] [CrossRef]
- Ike, A.; Sriprang, R.; Ono, H.; Murooka, Y.; Yamashita, M. Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere 2007, 66, 1670–1676. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Munekage, Y.; Ohnishi, K.; Jana, B.B.; Balcazar, J.L. Isolation and Characterization of Cadmium- and Arsenic-Absorbing Bacteria for Bioremediation. Water Air Soil Pollut. 2014, 225, 2151. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Yong, S.O. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 23–33. [Google Scholar]
- Wang, Y.; Xiao, X.; Xu, Y.; Chen, B. Environmental effects of silicon within biochar (sichar) and carbon–silicon coupling mechanisms: A critical review. Environ. Sci. Technol. 2019, 53, 13570–13582. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; Silva, E.B.D.; De Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochar: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; He, Y.; Li, B.; Dong, Y.; Wang, L. Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour. Technol. 2019, 284, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants—Role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef]
- Caguiat, J.N.; Arpino, G.; Krigstin, S.G.; Kirk, D.W.; Jia, C.Q. Dependence of supercapacitor performance on macro-structure of monolithic biochar electrodes. Biomass Bioenergy 2018, 118, 126–132. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment. Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Zhu, Y.; Li, Y.; Gu, B. Mechanistic investigation of mercury sorption by brazilian pepper biochar of different pyrolytic temperatures based on X-ray photoelectron spectroscopy and flow calorimetry. Environ. Sci. Technol. 2013, 47, 12156–12164. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochar: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Chen, J.; Wen, M.; Li, G.; An, T. Highly efficient visible-light-driven photocatalytic degradation of VOCs by CO2-assisted synthesized mesoporous carbon confined mixed-phase TiO2 nanocomposites derived from MOFs. Appl. Catal. B Environ. 2019, 250, 337–346. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3‘R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochar for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochar. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Mao, J.W.; Jiang, G.M.; Chen, Q.S.; Guan, B.H. Influences of citric acid on the metastability of alpha-calcium sulfate hemihydrate in CaCl2 solution. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 265–271. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, S.; Chen, H.; Huang, L.; Qiu, R. Pb(II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour. Technol. 2013, 147, 545–552. [Google Scholar] [CrossRef]
- Alamin, A.H.; Kaewsichan, L. Adsorption of Zn(II) and Cd(II) ions from aqueous solutions by Bamboo biochar cooperation with hydroxyapatite and calcium sulphate. Int. J. Chemtech Res. 2015, 7, 2159–2170. [Google Scholar]
- Leborans, G.F.; Novillo, A. Toxicity and bioaccumulation of cadmium in Olisthodiscus luteus (Raphidophyceae). Water Res. 1996, 30, 57–62. [Google Scholar] [CrossRef]
- Freundlich, H.M. Concerning the adsorption in solution. Z. Fur Anorg. Und Allg. Chem. 1906, 57, 385–470. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Zhurnal Nevropatol. I Psikhiatrii Im. S.S. Korsakova 1947, 55, 331–333. [Google Scholar]
- Ajayi, A.E.; Horn, R. Modification of chemical and hydrophysical properties of two texturally differentiated soils due to varying magnitudes of added biochar. Soil Tillage Res. 2016, 164, 34–44. [Google Scholar] [CrossRef]
- Park, J.H.; Yong, S.O.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surf. A 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.; Yu, M.; Yakkala, K.; Koduru, J.R.; Yang, J.K. Removal studies of Cd(II) and explosive compounds using buffalo weed biochar-alginate beads. J. Ind. Eng. Chem. 2015, 26, 226–233. [Google Scholar] [CrossRef]
- Li, F.; Shen, K.; Long, X.; Wen, J.; Xie, X.; Zeng, X.; Liang, Y.; Wei, Y.; Lin, Z.; Huang, W.; et al. Preparation and characterization of biochars from Eichornia crassipes for cadmium removal in aqueous solutions. PLoS ONE 2016, 11, e1048132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential mechanisms of cadmium removal from aqueous solution by canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef] [PubMed]


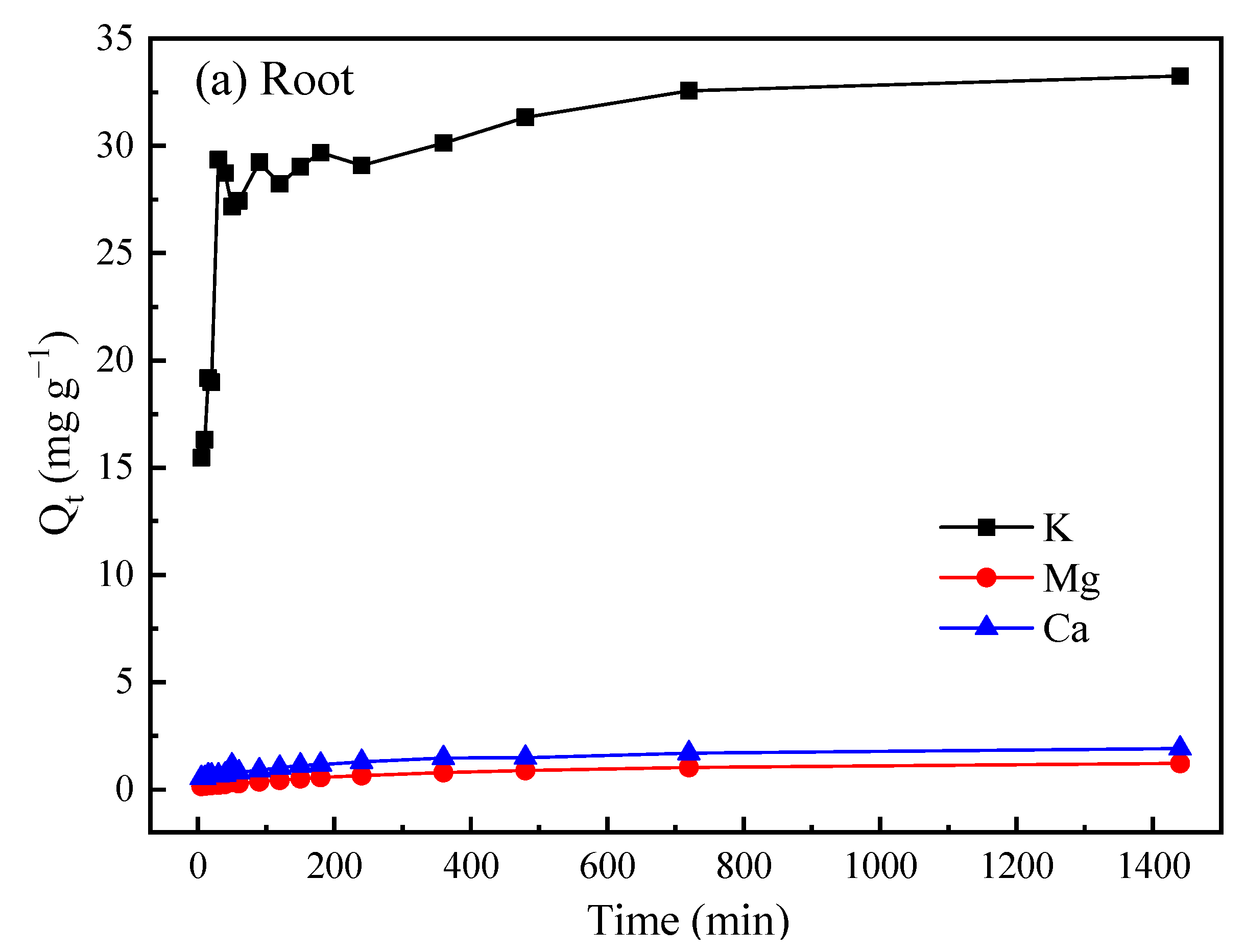
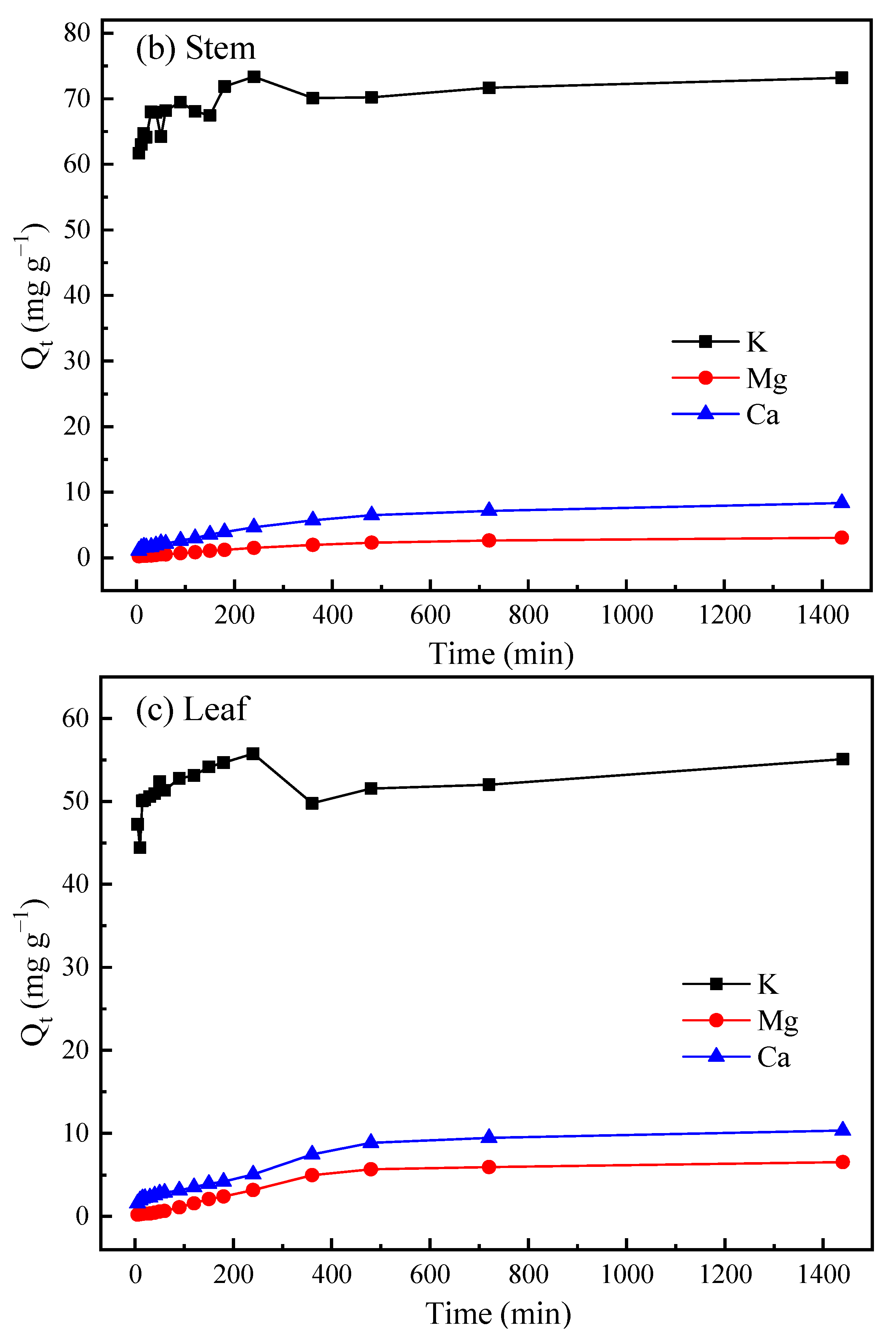
| Materials | Surface Area (m2 g−1) | Pore Volumes (cm3 g−1) | Carbon Yield (%) |
|---|---|---|---|
| CBR | 15.758 | 0.0399 | 36.323 |
| CBS | 7.631 | 0.0331 | 30.622 |
| CBL | 7.243 | 0.0269 | 29.469 |
| Langmiur | Freundlich | Dubinin–Radushkevich | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Qm (mg g−1) | R2 | KF | n | R2 | Qm2 (mg g−1) | Es (KJ mol−1) | |
| CBR | 0.911 | 18.808 | 0.824 | 3.719 | 0.475 | 0.987 | 13.599 | 55.133 |
| CBS | 0.939 | 66.756 | 0.908 | 118.710 | 0.706 | 0.979 | 56.634 | 51.205 |
| CBL | 0.954 | 44.215 | 0.915 | 77.024 | 0.545 | 0.976 | 44.594 | 44.992 |
| Experiment (mg g−1) | Pseudo First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | k1 × 10−3 (min−1) | R2 | Qe (mg g−1) | k2 × 10−3 (g mg−1 min−1) | R2 | ||
| CBR | 12.640 | 11.623 | 0.635 | 0.706 | 12.616 | 12.378 | 0.985 |
| CBS | 19.905 | 7.430 | 7.160 | 0.870 | 18.650 | 6.120 | 0.999 |
| CBL | 19.955 | 18.984 | 11.020 | 0.987 | 22.957 | 0.660 | 0.937 |
| Feedstock | Pyrolytic Conditions | Qm (mg g−1) | Reference |
|---|---|---|---|
| S. hermaphrodita | 973.15 K (240 min) | 35.71 | [45] |
| Pine bark waste | 1223.15 K (120 min) | 17.793 | [46] |
| Hickory wood | 873.15 K (60 min) | 28.100 | [47] |
| Buffalo weed | 973.15 K (240 min) | 13.369 | [48] |
| E. crassipes | 773.15 K (120 min) | 36.899 | [49] |
| Cattail-root | 873.15 K (50 min) | 18.808 | This paper |
| Cattail-stem | 873.15 K (50 min) | 66.756 | This paper |
| Cattail-leaf | 873.15 K (50 min) | 44.215 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yan, Z.; Song, L.; Wang, Y.; Zhu, J.; Xu, N.; Wang, J.; Chang, M.; Wang, L. Preparation and Characterization of Cattail-Derived Biochar and Its Application for Cadmium Removal. Sustainability 2021, 13, 9307. https://doi.org/10.3390/su13169307
Wang X, Yan Z, Song L, Wang Y, Zhu J, Xu N, Wang J, Chang M, Wang L. Preparation and Characterization of Cattail-Derived Biochar and Its Application for Cadmium Removal. Sustainability. 2021; 13(16):9307. https://doi.org/10.3390/su13169307
Chicago/Turabian StyleWang, Xiaoshu, Zheng Yan, Lingchao Song, Yangyang Wang, Jia Zhu, Nan Xu, Jinsheng Wang, Ming Chang, and Lei Wang. 2021. "Preparation and Characterization of Cattail-Derived Biochar and Its Application for Cadmium Removal" Sustainability 13, no. 16: 9307. https://doi.org/10.3390/su13169307
APA StyleWang, X., Yan, Z., Song, L., Wang, Y., Zhu, J., Xu, N., Wang, J., Chang, M., & Wang, L. (2021). Preparation and Characterization of Cattail-Derived Biochar and Its Application for Cadmium Removal. Sustainability, 13(16), 9307. https://doi.org/10.3390/su13169307






