Abstract
The assessment of Technosols quality in urban environments is pivotal for the maintenance of ecosystems impacted by human activities. The study was performed on Technosols constructed in experimental mesocosms in the suburban area of Naples (Southern Italy) to highlight changes in the main soil properties over eight years and to identify the most suitable indices at quality monitoring. In this study, several chemical, biological, and integrated indices were analysed to evaluate the mineral accumulation, potential ecological risk, edaphon activity, fertility, and the overall soil quality. The Technosols showed alkaline pH, nitrogen ranged from 24.5 to 39.5 g kg−1, high organic matter contents above 40 g kg−1, and there were no evident processes of soil compaction. Heavy metals (Cr, Cu, Fe, Mg, Mn, Ni, Pb, and Zn) did not exceed the thresholds defined by the Italian law for urban soils, despite their volcanic components. During eight years, the chemical indices depicted changes in the elements balance and increase in ecological risk; the biological indices indicated a reduction in the fungal fraction (fivefold) and in the resources utilisation and carbon storage. The soil quality index with all parameters highlighted the reduction in the soil quality (from 0.78 to 0.65) due to the decrease of the chemical quality, the increase of microbial stress conditions, and changes of the microbial composition, underlining the importance of integrating chemical and biological information for monitoring Technosols.
1. Introduction
Soil quality is the soil capacity to promote plant growth, to protect watersheds by regulating infiltration and precipitation, and to prevent water and air pollution by buffering inquinants [1,2]. The soil quality definition is controversial, reflecting the complexity and site specificity of the belowground part of terrestrial ecosystems as well as the many linkages between soil functions and soil-based ecosystem services [3]. Soil quality can be assessed both for agro-ecosystems where the main ecosystem service is productivity, and for natural ecosystems where major aims are maintenance of environmental quality and biodiversity conservation [4,5]. Soil quality is a dynamic concept and related to extrinsic factors such as parent material, climate, topography, and hydrology, which can change over time for effective land use or management [1,6]. In this view, the evaluation and the maintenance of soil quality in the urban environments are an important goal to reach because several urban soils are strongly affected by the addition of anthropogenic materials that modify soil native properties [7,8], and uses and ecosystem services that can potentially provide them [9]. In detail, urban soils inglobe several anthropogenic materials and all sorts of artefacts, as building debris or construction rubble made up of cement, concrete, pebbles, or other recalcitrant fragments [10]. The anthropogenic addition strongly alters the natural soil processes through contamination, depletion of living forms and changes of physical and chemical features that impact soil health by decreasing soil quality [8]. In fact, Technic is a qualifier for soils FAO classification [11] that defines Technosols as soils having ≥ 20% (by volume, weighted average) artefacts in the upper 100 cm from the soil surface or soils with ≥50% of artefacts in a layer ≥ 10 cm thick and starting ≤ 90 cm from the soil surface [11,12]. Technosols are widespread in urban contexts [8] and generally covered by ornamental woody and grassy vegetation (shrubs and lawns) with the scope to create positive influence on the microclimate of built urban environments [13]. Urban vegetation can play a crucial role for environment and society, by providing important ecosystem services such as carbon (C) sequestration, meant as a source of environmental balance to high C emissions [14], microclimate formation, pollution and dust reduction in the atmospheric air, water balance control, as well as recreational, educational, cultural, and aesthetic functions [15]. In addition, several studies have highlighted the relationships between urban soil quality and human health observing both direct (contaminant exposition, inhalation, ingestion, and consumption of plants grown in contaminated soil) and indirect (interactions among soil, microbial community, vegetation, and nutritional value of foods) effects of soil on humans [16,17,18]. Preserving or improving Technosols quality is pivotal for creating or maintaining urban green infrastructures [19] and for human health [9].
The urban soil quality was recently linked with the Technic requirement, to assess their level of ecosystem functioning as a service provider [20]. This purpose is not easy at all, due to the diversity of degradation processes and the kind of contamination, their interactions and peculiarities of each urban settlement [21]. In this context, little is known about the best applicable methods to assess the quality of Technosols or the most appropriate indicators (physical, chemical, and biological features) to engage for its assessment and utilisation. Valid tools must be identified to determine Technosol quality and the use of indices may help to monitor its variation over time, more than individual pedogenetic characteristics. In detail, a valid index is defined as “a decision tool intended to make complex information more accessible for decision makers which can point out an overall trend among many and conflicting indicator results” [22]. The integrated indices are meant as good tools and their use is very common in soil quality determinations. Indeed, they quantify the relationships between different typology indicators and soil functions [23,24] whereas focusing the attention on the selection of a minimum data set (MDS) may quicken the investigation by choosing, with a statistical approach, the most responsive indicators from the total dataset (TDS) [24]. In fact, the use of MDS reduces both the need to evaluate a large number of indicators and the data redundancy [24,25].
In this perspective, the aims of the current study performed on Technosols of the suburban area of Naples (Southern Italy) were: (1) to highlight the changes in the main soil properties over eight years, in order to envisage specific management actions and maintain the ecosystem services supply; (2) to identify the most suitable indices at quality monitoring to assess the potential use of Technosols for a sustainable green city.
2. Materials and Methods
The study was carried out on topsoils of Technosols set up in 12 experimental mesocosms located in the city of Naples (Campania region, Southern Italy), starting from a previous investigation on the effects of a single compost application on several Technosol characteristics, particularly on microbial community responses [26]. In this new study, several chemical, biological, and integrated indices were analysed as they were specific for the mineral accumulation, potential ecological risk of heavy metal contamination, edaphon activity, fertility, and the overall soil quality. The investigated indices were: geo-accumulation index (Igeo), enrichment factor (EF), pollution load index (PLI), ecological risk index (RI), and nutrients and metals availability (Availability) for chemical characteristics; microbial quotient (qCmic), fraction of fungal biomass on the microbial biomass (FB/MB ratio), metabolic quotient (qCO2), coefficient of endogenous mineralization (CEM), and biological fertility index (BFI) for biological characteristics; soil quality index on total and minimum data set (SQI and SQIMDS) and scaled soil quality index (sSQI) as integrated indices of the soil’s overall physical, chemical and biological features.
The design was chosen in order to better validate the investigated chemical, biological, and integrated indices over time and to provide simple tools to monitor soil quality in newly constructed Technosols by promoting the use of these soils for green infrastructure and green areas in urban environments [27].
2.1. Study Area
The experimental sites, reported in Figure 1, were located at University Campus of Monte Sant’Angelo, Federico II of Naples (40°50′12.63″ N, 14°10′58.0322″ E, 122 a.s.l) in the western urban area, in mesocosms set up during the campus construction in 2006, as reported in Panico et al. [26].
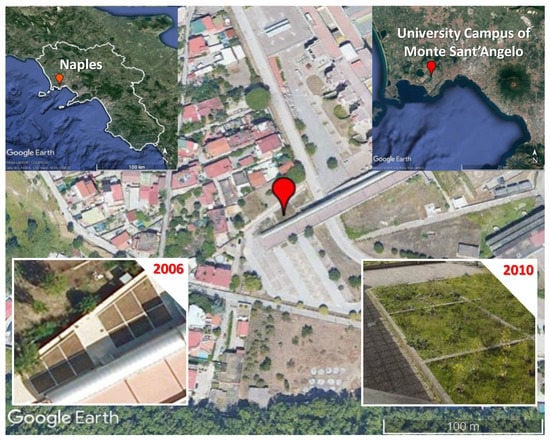
Figure 1.
The investigated study area in Naples (Campania region, Italy). The bottom panels show the experimental mesocosms in 2006 after setting up and in 2010, before the first soil sampling.
Each mesocosm was a cement vessel with area of 16 m2 and 2 m depth. In the vessels the native volcanic soils, identified as Lepti-Vitric Andosols [28], excavated and moved during the construction of the foundations of the university campus, were mixed with building rubble and other edification waste products. The resulting soils, made of materials deposited by humans (≥20% by weighted average in the upper 100 cm from the soil surface) and volcanic soils, were isolated below and to the sides, and contained fine earth without any contact to other soil material containing fine earth [11]. These soils were classified as Isolatic Ekranic Technosols according to the FAO-WRB system [11]. The Technosols’ profile observed in the mesocosms showed presence of artificial infill without a sharp transition along the entire section. The topsoils were different from the deeper layers and until 30–40 cm the horizons were heterogenic mixed with concrete, bricks fragments, part of iron roads and metal objects, pebbly, sandy layers with medium artefact content. The artefact content was downward decreasing and the horizons below the depth of 100 cm were almost completely free of artefacts. The particle-size classes dominating the profile were sands and sandy loams.
The plant cover is mainly represented by species from Gramineae, Leguminosae, and Malvaceae families, which quickly (about one year) and spontaneously colonized the mesocosms (Figure 1). During the research, the investigated mesocosms were left undisturbed and subjected only to the influence of natural factors.
The climate is Mediterranean, warm temperate with dry and hot summers [29] and mild, wet winters featured by annual rainfall of 929 mm and mean monthly temperatures of 11 °C in January and 26 °C in August [30]. Soils were analysed in 2010 and 2018, after four and twelve years since the mesocosms’ construction, respectively, and soil samples were collected at 0–10 cm depth with a core of 5 cm in diameter. Five subsamples from each mesocosms were picked and mixed together in order to have representative samples. Soil samples were sieved at <2 mm and analysed for physical, chemical, and biological characteristics.
2.2. Soil Analyses
All the physical, chemical, and biological analyses were performed in triplicate and converted into different units to calculate the investigated indices.
2.2.1. Physical and Chemical Analyses
All soil samples were subjected to chemical and physical laboratory analyses following the Italian Methods Manual on Soil Chemical Analyses [31]. Soil pH was determined in soil:distilled water (1:2.5 = v:v) suspension by electrometric method. Soil water content (WC) and water holding capacity (WHC) were determined in oven-dried samples at 105 °C until constant weight and by the gravimetric method respectively. Bulk density (BD) was determined as the ratio between dry soil mass and total soil volume on soil cores after drying for 48 h at 105 °C. Soil porosity was assessed according to Danielson and Sutherland [32]. Total nitrogen (Total N), total carbon (Total C), and organic carbon (organic C) were evaluated in oven-dried (105 °C until constant weight) and grounded (Fritsch Analysette Spartan 3 Pulverisette 0) samples by gas chromatography (Thermo Finnigan, CNS Analyzer). In addition, the C/N ratio was calculated from total N and C content. The organic C was evaluated by treatment with HCl 10% soil samples to remove carbonates before measurement. The soil organic matter (SOM) was obtained by multiplying organic C by 1.724 [33]. The total content and the available fractions of minerals (Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, and Zn) were evaluated in grounded and mineralized soil samples by atomic absorption spectroscopy (SpectrAA 20—Varian). The total content was evaluated after soil digestion with a mixture of HF and HNO3 (1:2 = v:v) in a microwave oven (Milestone mls 1200—Microwave Laboratory Systems). The available fractions of K, Mg, and Na were evaluated through mineralization with BaCl2 and triethanolamine at pH 8.1 [34]. The available fractions of Cu, Cr, Fe, Mn, Ni, Pb, and Zn were determined in digested soils with diethylenetriamine pentacetic acid, CaCl2, and triethanolamine at pH 7.3 [35]. Accuracy was checked by concurrent analysis of standard reference material (BCR CRM 142R—Commission of the European Communities, 1994) and recoveries ranged from 86% to 98%.
2.2.2. Biological Analyses
The soil biological activities were assessed in fresh samples within one week from the sampling. Basal microbial respiration (BR), microbial and fungal carbon (Cmic and Cfung), fungi abundance and vitality expressed as total and active fungal biomass (TFB and AFB), and active on total fungal biomass (FAR) were determined.
BR was assessed as CO2 evolved in one hour by soils and trapped in the soda (NaOH 0.1 N), determined by two-phase titration (HCl 0.05 N) after addition of distilled water and incubated at 25 °C in the dark for ten days [36]. CR is the cumulative respiration during the whole incubation period, determined according to Insermeyer [37].
Cmic was evaluated through the substrate-induced respiration method SIR as reported in Degens et al. [38], after the addition of glucose (75 mM) as an easily mineralizable substrate. Cfung was calculated from total fungal biomass on the basis of mean fungal values of the C/N ratio [39] and nitrogen content [40]. TFB was determined by the membrane filter technique through optical microscopy as intersections of fungal mycelia [41,42] stained with aniline blue, extracted from the soils, and dispersed in phosphate buffer (60 mM, pH 7.5). AFB was determined in soil dispersion as TFB, by using fluorescence microscopy after sample pretreatment with fluorescein diacetate for fungal vital mycelia colouration [43]. FAR, the fungal activity ratio, was evaluated as the percentage of AFB on TFB.
2.3. Soil Chemical Indices
The following chemical indices were calculated for the current study: geo-accumulation index (Igeo), enrichment factor (EF), pollution load index (PLI), and potential ecological risk index (RI). In addition, the percentage of mineral availability (Availability) was also calculated for the investigated urban soils.
Geo-accumulation index-Igeo expresses the degree of the specific mineral contamination in the investigated soils by comparing current concentration with the natural level used as reference [44]. It is calculated as reported in Equation (1):
where [sample] is the concentration of the investigated element in soil and [reference] is the geochemical background concentration of the same mineral [45]. The factor of 1.5 is applied to minimize the fluctuation of the values in the background due to the lithological heterogeneity. Igeo evaluation is based on seven classes [44]: Igeo 0: uncontaminated, Igeo 0–1: uncontaminated to moderately contaminated, Igeo 1–2: moderately contaminated, Igeo 2–3: moderately to strongly contaminated, Igeo 3–4: strongly contaminated, Igeo 4–5: strongly to extremely strongly contaminated, Igeo >5: extremely contaminated.
Enrichment factor (EF) evaluates the enrichment degree of the Technosols by comparing Fe concentration with uncontaminated background levels to normalize heavy metal contaminants [46].
EF was calculated according to the Equation (2) [46]:
where [element] and [normalizer] refer to the concentration of the investigated metal and Fe in the sample and background respectively. Enrichment factor is divided into five classes [46]: EF < 2: deficiency to minimal enrichment, EF 2–5: moderate enrichment, EF 5–20: significant enrichment, EF 20–40: very high enrichment, EF > 40: extremely high enrichment.
The pollution load index (PLI) assesses the degree of heavy metal contamination. It is based on the contamination factor (CF) that describes the degree of the contamination related to background values [47]. PLI was calculated as the following Equation (3) [47]:
where n is the number of the investigated minerals in the samples. PLI may assume values >1 or <1 [47] and assesses absence or existence of pollution, respectively. In this work the pollution load index included all the investigated minerals in order to assess the overall pollution due to all the measured elements.
The potential ecological risk index (RI) is useful to evaluate the impact of metals on environmental and ecological processes [48,49]. It is always used for heavy metals [50] but in the current research it includes all the investigated minerals which can have negative effects at high concentrations [49]. It was calculated as reported in Equation (4):
where is the potential ecological risk factor of the element calculated as (Equation (5)):
is the toxic response factor of the metal i which were 1 for Mn and Zn, 2 for Cr, and 5 for Cu, Ni, and Pb [50]. The for Fe, K, Mg, and Na is assumed to be 1 for their chemical characteristics. RI and are classified as follows [50]. RI < 150 means low risk, 150 ≤ RI < 300 means moderate risk, 300 ≤ RI < 600 means considerable risk, RI ≥ 600 means very high risk, while : <40 means low risk, 40 ≤ < 80 means moderate risk, 80 ≤ < 160 means considerable risk, 160 ≤ < 320 means high risk, and 320 ≤ means very high risk.
Finally, Availability refers to the degree of availability in respect to the total content of the investigated elements. It was calculated as in Equation (6) [51]:
2.4. Soil Biological Indices
The following soil biological indices were calculated for the investigated Technosols: microbial efficiency quotient (qCmic), fraction of fungal biomass on the microbial biomass (FB/MB), metabolic quotient (qCO2), and coefficient of endogenous mineralization (CEM). Additionally, the integrated biological fertility index (BFI) was evaluated and included in the biological indices for the eco-physiological indicators engaged for its determination.
qCmic is the microbial efficiency quotient and defines the equilibrium of microbial C on soil organic C [23,52]. It is the ratio between Cmic and organic C according to Sparling [53].
FB/MB measures the fraction of fungal biomass on the microbial biomass calculated as the percentage of the Cfung and Cmic ratio [54].
qCO2 is the soil metabolic quotient that is the amount of CO2 evolved from the soil (BR) as a function of the microbial biomass (Cmic) and measures the degree of the microbial community stress [55].
CEM is the coefficient of endogenous mineralization, it indicates the rate of organic C mineralization and it is calculated as the ratio between BR and organic C [56].
BFI is the biological fertility index and it is based on the equation reported by Renzi et al. [57] whose values of each variable are organized into classes according to Sequi et al. [58].
BFI was calculated as below (Equation (7)).
The biological fertility index is scored in increasing classes of soil fertility. Class I, BFI < 9, stressed soils with very low fertility; class II, 9 < BFI < 12, pre-stressed soils; class III, 13 < BFI < 18, intermediate fertility soils; class IV, 19 < BFI < 24, good fertility soils and class V, BFI > 24, very high fertility soils.
2.5. Minimum Data Set (MDS)
The overall minimum data set was determined on the total dataset, 34 indicators among physical, chemical, and biological parameters. The principal component analyses (PCA) for 2010 and 2018 were performed in order to reduce the dimensionality of the TDS and to limit the loss of information [25]. Only PCs with eigenvalues ≥ 1 were considered for MDS identification whereas principal components with eigenvalues ≤ 1 accounted for less variance than individual variables [25,59]. Within each PC, highly weighted variables were selected for factor loadings > 0.60 according to Vasu et al. [59]: if more than one indicator was chosen a Spearman rank order correlation was analysed. Only the uncorrelated variables were accepted in order to reduce the redundancy of information [25,59]. The variables in common for both the years were accounted for the overall MDS.
2.6. Soil Integrated Indices
Soil quality can be measured through the evaluation of several integrated indices calculated from the investigated physical, chemical, and biological indicators that refer to soil functioning. The investigated soil integrated indices were soil quality index (SQI) with all measured parameters, soil quality index inclusive of MDS validated parameters (SQIMDS), and scaled soil quality index (sSQI).
The soil quality index (SQI) is an integrated index that provides information about the general soil capacity of functioning [60]. It takes into account the following parameters: soil pH, WC, WHC, BD, total C, Cr, Cu, Fe, K, Mg, Mn, N, Na, Ni, Pb, and Zn, available fractions of minerals, SOM, C/N ratio, BR, Cmic, Cfung, and AFB through the “more is better” and “less is better” functions [61]. SQI is calculated in Equation (8) [22]:
where S is the score assigned to each indicator and n is the number of the investigated parameters. SQI may be classified into three different classes according to [61]: high quality (>0.70), medium quality (0.55–0.70), and low quality (<0.55).
In addition, a second SQI (SQIMDS) was calculated from parameters validated by PCA for minimum data set.
The scaled soil quality index (sSQI) integrates the investigated indicators into a single parameter to measure the soil’s vital signs [62]. For every single sample, it was assigned a value to each parameter (soil pH, BD, organic C, total Cu, Fe, K, Mg, Mn, N, Ni, Pb, Zn, BR, and Cmic) [57,62,63]. The minimum and the maximum assigned values (SQI min and SQI Max respectively), as well as the sum of the overall assigned values (ΣSQI) for each year were used for the calculation of the scaled soil quality index (sSQI) as follows in Equation (9) [62,63]:
The index was then normalized between 0 and 1. The investigated soils were classified as reported for SQI or SQIMDS.
2.7. Statistical Analyses
The normality of the data distribution was assessed by the Shapiro–Wilk test. The paired t-test and the Mann–Whitney rank test for normal or non-normal data distribution respectively were performed to evaluate the differences between 2010 and 2018. The statistical tests were considered to be significant for p < 0.05.
The PCA was performed on all measured physical, chemical, and biological data for 2010 and 2018, in order to select the soil quality indicators for MDS. The Spearman rank order correlation were performed to test correlations among the indicators and among the integrated soil quality indices. More details are reported in Section 2.5.
Systat_SigmaPlot_12.2 software (Jandel Scientific, San Rafael, CA, USA) was used for statistical analyses and graphs whereas Past 4.03 (Øyvind Hammer, Oslo, Norway) was used for the PCA.
3. Results
3.1. Technosols Characteristics
Table 1 and Supplementary Material Table S1 report the Technosols main physical and chemical characteristics. During the study years, the urban soils showed a significant increase in pH from 7.47 in samples of 2010 to 8.15 in 2018 and a significant reduction in organic matter content (SOM) ranging from 79.95 to 47 g kg−1, and in C/N ratio ranged from 22.12 to 9.08 in 2010 and 2018 respectively. Additionally, water content (WC) and water holding capacity (WHC) reduced from 2010 to 2018, decreasing from 33.40 to 28.80% d.w. and from 42.30 to 35.57% d.w., respectively. In contrast, bulk density (BD) and porosity remained essentially unchanged (Table 1).

Table 1.
The main physical and chemical characteristics of the Technosols (0–10 cm depth) sampled in the study years (2010 and 2018). Values are means (n = 36, twelve mesocosms in triplicate) ± standard errors. Different letters show statistically significant differences between years (t-test, p < 0.05). Data for year 2010 were also reported in Panico et al. [26]. Reproduced with permission from Panico S.C., Memoli V., Napoletano P., Esposito F., Colombo C., Maisto G., De Marco A., Applied Soil Ecology; published by Elsevier, 2019.
In addition, the N content of Technosols almost doubled from 24.54 in 2010 to 39.53 g kg−1 in 2018 (see Supplementary Material, Table S1). The Technosols sampled in 2018 were also richer in the total content and available fraction of many investigated elements. The exceptions were the total Mg and Mn contents that had the highest values (4.35 mg g−1 and 780 µg g−1, respectively) in 2010 (Supplementary Material, Table S1).
In 2018, the Technosols showed a significant decrease of fungal component (Cfung, total and active biomass, fungal activity ratio) and a significant increase of microbial respiration (BR) (Supplementary Material, Table S2). In detail, Cfung showed values of 13.31 in 2010 and 2.94 µg g−1 in 2018, fungal activity ratio (FAR) was 28% in 2010 and 13% in 2018, and BR increased from 5.60 to 11.54 µg CO2 g−1 dw h−1 in 2010 and 2018 respectively (Supplementary Material, Table S2).
3.2. Chemical Indices
The following chemical indices were calculated for the current study: geo-accumulation index (Igeo), enrichment factor (EF), pollution load index (PLI), potential ecological risk index (RI), and availability for each element. The results are reported in Figure 2 and for each index the appropriate reference classes to describe soil quality are given.
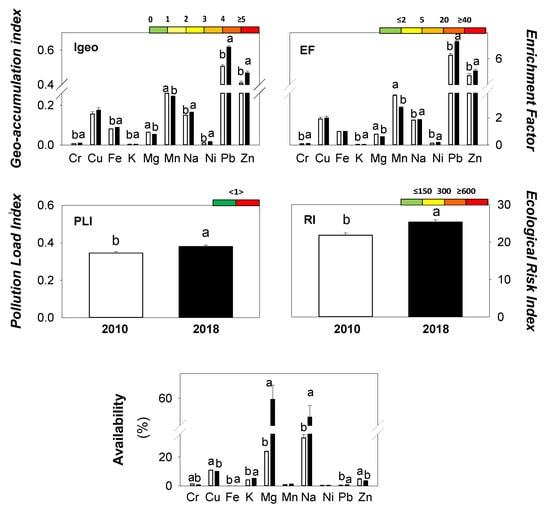
Figure 2.
The investigated chemical indices: geo-accumulation index (Igeo), enrichment factor (EF), pollution load index (PLI), ecological risk index (RI) and availability (% total content). White bars refer to measurements performed in 2010; black bars refer to measurements performed in 2018. Different letters show statistically significant differences between years (t-test, p < 0.05). Different colours indicate specific classes of soil quality increasing from green to red.
The geo-accumulation index (Igeo), pollution load index (PLI), and ecological risk index (RI) for both 2010 and 2018 showed low values and did not indicate any degree of risk due to pollution (Figure 2). Igeo showed values below 1 for all the investigated elements; the lowest values were calculated for K (0.0037 and 0.0042 in 2010 and 2018 respectively), the highest values for Pb (0.51 and 0.62 in 2010 and 2018 respectively). Igeo, for several elements, showed significantly higher values in 2018 as compared to 2010. Igeo for Mg and Mn showed opposite trends. However, PLI and RI were significantly higher in 2018 (0.38 and 25.3 respectively) than in 2010 (0.35 and 21.7 respectively) (Figure 2).
In the two studied years, EF showed a moderate enrichment for Cu, Mn, and Na with values ranging between 2 and 5. For Pb and Zn, EF exceeded 5, indicating significant enrichment in both 2010 and 2018 (Figure 2). In addition, EF showed significantly higher values in 2018 than 2010 except for Mg and Mn (Figure 2).
In the investigated soils, the percentages of available Fe, Ni, and Pb for both years were below 1%, whereas the highest availability were measured for Mg (23.91 and 59.56% in 2010 and 2018 respectively) and Na (33.39 and 52.33% in 2010 and 2018 respectively). The percentage of available elements showed higher values in 2018 than 2010 except for Cr, Cu, and Zn, which had opposite trends (Figure 2).
3.3. Biological Indices
The biological indices (qCmic, FB/MB, qCO2, CEM, and BFI) calculated for the Technosols are shown in Figure 3.
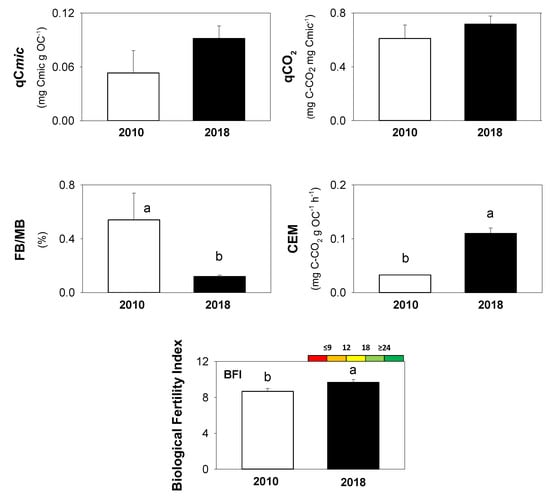
Figure 3.
The investigated biological indices: microbial quotient (qCmic), fraction of fungal biomass on the microbial biomass (FB/MB), metabolic quotient (qCO2), coefficient of endogenous mineralization (CEM), and biological fertility index (BFI). Different letters mean statistically significant differences between years (t-test, p < 0.05). Different colours indicate specific classes of soil quality increasing from red to green. qCO2 and CEM for year 2010 were also reported in Panico et al. [26]. Reproduced with permission from Panico S.C., Memoli V., Napoletano P., Esposito F., Colombo C., Maisto G., De Marco A., Applied Soil Ecology; published by Elsevier, 2019.
qCmic and qCO2 did not show any statistical difference between 2010 and 2018. The fungal fraction in the microbial biomass showed higher values in soils of 2010 (0.54%) than 2018 (0.12%). By contrast, the mineralization rate (CEM) and biological fertility index (BFI) were higher in 2018 as compared to 2010. CEM ranged from 0.033 to 0.11 mg C-CO2 g OC−1 h−1, and BFI ranged from 8.67 to 9.67 (Figure 3).
3.4. Minimum Data Set and Integrated Indices
Principal component analysis (PCA) for 2010 and 2018 indicated the soil properties to select as minimum dataset (MDS). In Table 2, the highly weighted variables for 2010 and 2018 are reported in bold. The uncorrelated indicators in common for both the years and selected for MDS were pH, WHC, BD, C/N, and Cfung.

Table 2.
Results of principal component analysis (PCA) for 2010 and 2018, showing the indicators selected as the minimum dataset (MDS). The data in bold indicate the highly weighted variables for 2010 and 2018 respectively. The underlined data indicate uncorrelated variables for the same PC. Grey lines highlight the uncorrelated indicators in common for both the years.
The integrated indices (SQI, SQIMDS, and sSQI) are reported in Figure 4. SQI decreased over time and ranged between 0.78 and 0.65, i.e., “high quality” and “medium quality” classes for 2010 and 2018 respectively. SQIMDS and sSQI did not show any significant change between the years. No significant correlation was found between SQI and the other integrated soil quality indices (Supplementary Material, Table S3).
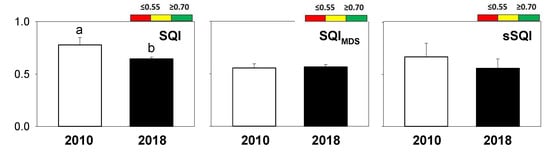
Figure 4.
The investigated integrated indices: soil quality index (SQI), soil quality index calculated by minimum data set (SQIMDS), and scaled soil quality index (sSQI). Different letters mean significant statistical differences between years (t-test, p < 0.05). Different colours indicate specific classes of soil quality increasing from red to green.
4. Discussion
4.1. Technosols Characteristics
Monitoring soil quality is essential to ensure that soil functions are maintained for current and potential future uses [23]. Generally, soil quality is not simple to assess and urban soils, especially Technosols, are very complex as they are made up of a combination of natural and man-made materials that determine soil processes and functionality. Soil substrata are usually affected by strong alterations during the early stages of evolution, thus by influencing the main soil physico-chemical and biological processes [64]. Therefore, although the investigated Technosols are very young, it is known that the evolution of urban soils is rapid and could be further accelerated in mesocosms by the volcanic origin of the soils used for their construction [65]. In any case, the results about several characteristics of the investigated Technosols confirmed values commonly reported in the research comparing urban and natural soils. However, these physical, chemical, and biological characteristics show different trends over time.
Firstly, starting from 2010 up to 2018, a significant reduction of soil organic matter content and a decrease of C/N ratio were observed in the Technosols (Table 1). However, the investigated soils showed an interesting accumulation of organic matter comparable to those measured in many forests and agriculture soils [66,67,68]. SOM is a fertility indicator which responds well to changes in soil processes [67] and drives the main soil properties such as hydrological and nutritional status, and heavy metal availability [67,69]. In fact, the decrease of soil organic substrates reflects a reduction of water holding capacity and water content, probably by losing the polar and aliphatic groups that usually bind water molecules [66]. In addition, there are no evident processes of soil compaction according to the bulk density close to 0.9 kg/dm3, typical of urban soils, that could create impermeable layers and reduce water infiltration [8,10]. The non-limiting values of soil bulk density and porosity and the lack of change for these parameters over time confirmed the low process of aggregation in these soils [70].
The urban soil pH is slightly to very strongly alkaline, similarly to many urban soils generally due to the increase of calcium carbonate [8], and usually varies from 7.4 to 8.6 with most of values in the narrow range of 8.0–8.2 [71]. In this context, the increase of alkalinity in the investigated soils, from 7.5 to 8.2 (2010 and 2018 respectively), can be commonly attributed to the filling materials such as construction wastes, concrete, and cement that release carbonates in either the short or long term. This finding is in accordance with the values measured for C content in Technosols due to natural and anthropogenic inputs and also depends on its stability or transformation rate in soils [19]. In detail, the soil organic C can be loss through excavation and relocation of the topsoil layer [7] and by the fast microbial mineralization of plant debris, mainly represented by herbaceous species established on young Technosols [64]. By contrast, the high content of soil inorganic C in urban topsoil is due to the input from lime dust, cement, and concrete particles from building sites and cement factories [72]. Moreover, a variable contribution to the C accumulation in urban soils is given by asphalt and bitumen resins used for road construction [73], plastics and polymers used for construction (e.g., tubes and pipes) and food industries [74], and polycyclic aromatic hydrocarbons and synthetic rubber, coming from chemical industries and transport [75]. The slow or absent natural degradation of these materials determines their long-term persistence in the urban soils and a stress condition for edaphic communities [19].
Moreover, in the studied Technosols, N content increased significantly over 8 years, confirming that larger N reserves are generally reported in urban soils than in natural soils [72,75]. Vasenev and Kuzyakov [19] reported that the N inputs are abundant in residential and city public zones due to the atmospheric N deposition as nitrogen oxides (NOx), and ammonia (NH3) emissions from fuel combustion, and industrial and urban traffic. In addition, among the spontaneous vegetation established on the Technosols, leguminous were abundantly represented, and certainly contributed to enriching the soil with the fixation of atmospheric N [76].
In addition, the alkaline soil in urban ecosystems can cause nutrient imbalance and change the metal speciation and activities [8]. Heavy metals in urban soil come from parent material and exogenous input including household, garbage disposal, transportation, mining and processing, manufacture, and fossil fuel burning [8]. In detail, the investigated Technosols are developed on volcanic deposits with a high baseline content of some trace elements and are rich in inorganic and organic compounds that bind elements [77]. Nevertheless, for both years of study, heavy metals and minerals do not exceed the thresholds defined by the Italian law (D.L. 152/2006) for urban soils. In addition, the Technosols showed higher amounts of both total content and available fraction for many investigated elements in 2018 than 2010. Metal deposition and accumulation in Technosols may vary according to natural and human sources. Indeed, whether Fe and Ni probably increase because of parent materials, Cr, Pb, and Zn surely increase due to human activities occurring in cities such as traffic, combustion of gasoline, or abrasion of Zn-added tires [8,78]. The soil K content could derive either from the plant or microorganism activities that release it (as exchangeable and non-exchangeable form) locked in primary and secondary minerals [79], but also inherited from the construction wastes and material of the roadside soils [10]. Sodium content is usually a little higher than K in earth’s crust and it significantly raised as total content and available fraction in the investigated Technosols over time, probably for effect of precipitations or/and run-off waters from roads, being in a site far few kilometres from the sea [79]. However, the Na content measured in the Technosols appears not to cause salinity problems [10]. By contrast, in the investigated soils, the Mg and Mn behaviour, whose total content of both decrease over time, appear to be influenced by the pH values and soil biological activities through the mediation of plants and microorganisms that engaged these elements for their metabolic processes [80].
However, the general trend of increasing elements (minerals and heavy metals) impacted the biological activities occurring in the investigated Technosols. The response of the microbial community is widely studied in soil quality evaluations because it rapidly responds to environmental stressors and hazardous environments [53]. Despite the increasing release of elements and the lowering of SOM content, the investigated Technosols show better biological features if compared to agricultural soils [81] and quite similar to forest soils [82] of the Mediterranean area. After 8 years of monitoring, the microbial community changed in composition but not in biomass. In detail, variation in SOM amount and quality reduced activity and abundance of specialized microorganisms as fungi (Cfung, TFB, and AFB) in favour of generalist species such as bacteria that may release compounds by inhibiting the fungal growth [26]. In addition, soil bacteria have a relative resistance to external influences and extreme environmental conditions whereas fungi, in contrast, are more sensitive to various anthropogenic influences [82]. Additionally, the increase in microbial respiration over time may be a response of the edaphic community to increased stress conditions in the investigated Technosols [81,82]. Indeed, the increase of soil Cr, Pb, and Zn contents can affect the soil microbial community with negative effects on microbial biomass and basal respiration, and changes of the microbial community structure [77].
4.2. Variation of Chemical Indices
The geo-accumulation index showed a moderate enrichment of heavy metal in the Technosols, particularly Pb and Zn, probably as dissolution or the weathering of the soil substrata, but also due to input of particulate and atmospheric deposition from anthropogenic urban activities such as intense traffic of the suburban area of Naples and waste debris [69,83]. The enrichment factor classifies the Cr and Ni content in the investigated soils as “deficiency to minimal enrichment” while Pb and Zn as “significant enrichment”. Similarities are reported by Charzyński et al. [69] for Igeo (Pb and Zn) and EF (Pb) in some sealed Polish Ekranic Technosols. In summary Pb and Zn show the highest values for Igeo and EF showed an increase over time in the investigated Technosols, confirming the relationship between the intensity of urbanization and the environmental damage [84,85]. This indicates that the studied Technosols showed a moderate anthropogenic input, with potential adverse effects on the health of urban green plants [86].
A similar trend is also shown from the pollution load index and the ecological risk index that assess the contamination level and the potential ecological risk of the measured elements (heavy metals and nutrients) in the study area [48]. In fact, the two indices confirmed that the investigated soils are not polluted and their increase from 2010 to 2018 indicated a worsening of soil biological conditions and an increase in risk for the occurring processes. The indices trend would be particularly affected by several heavy metals whose content depends on the weathering of volcanic parent material as well as the technogenic depositions in the surface layers [69].
Moreover, the Igeo and EF indices show high values which highlighted moderate enrichment for Mn and Na. In addition, Mn shows a decrease over time for the two mentioned indices. The soil Mn content often follows the organic matter trend and can be rapidly used by plants and soil microorganisms, especially at alkaline pH by reducing its availability [80].
Soil pH has a dominant effect on solubility, availability, and potential phytotoxicity of ions, nutrients, as well as toxic elements [87]. In fact, low pH shifts the equilibrium toward free metal cations and protonated anions, higher pH favours carbonate or hydroxyl complexes [87]. In addition, the oxidative activity of soil microorganisms can affect the availability of many micronutrients [87]. In this way the increase of Mn, K, and Fe availability during soil monitoring may be due to the biological activities of soil communities that improve the bio-accessibility of those elements incorporating them in their metabolic processes [87].
4.3. Variation of Biological Indices
Soil chemical properties (i.e., pH, SOM content, micro and macronutrients) create specific niche conditions for microorganisms and thus indirectly affect both biological activity and microbial diversity in the soil environment [23]. It should be emphasized that extreme soil conditions (as in the case of polluted soils) usually have an inhibitory impact on soil biological properties. Moreover, soil microbiota rapidly responds to environmental change and biological indices give evidence on soil functionality and on the interactions with all the components of the ecosystem [23].
Microbial (qCmic) and metabolic (qCO2) quotients have been employed for the eco-physiological characterization of the microbial community “performance” in a particular soil category [88,89]. qCmic is an index that reflects substrate availability to soil microbes: high values are indicative of soil labile C accumulation and favourable environment for microbial growth whereas a low ratio is due to the poor quality of the organic matter [23]. In the investigated Technosols, values of qCmic for 2010 and 2018 are lower than agricultural or other urban soils [70,90,91]. Generally, in managed soils, the microbial quotient decreases [92] and it is assumed that values below 0.2 mg g−1 indicate limited organic substrate availability for the microbial community [89]. A measure of the efficiency of microbial biomass in C substrates utilisation is supplied by qCO2 that tends to increase in response to stress conditions, in which the prevalence of catabolic versus anabolic processes reduces C accumulation by soil microorganisms [55]. The stress condition in the investigated Technosols is confirmed by the values of qCO2 higher than reported by Marzaioli et al. [61] for arable and forest soils and Renella et al. [70] for urban de-sealed soils. In addition, an increase of the metabolic quotient in response to metal contamination has been reported by other authors [77,82]. The stressful conditions occurring in the study sites were, however, confirmed by both low qCmic and high qCO2 values. In fact, the qCO2 depicts the energetic efficiency of the microbial community and a high qCO2 indicates a high energy demand for microbial biomass maintenance [55]. The lowest qCmic would further suggest a scarce efficiency of microbes to convert the soil organic carbon into microbial biomass [93,94]. The results confirmed the influence of SOM on microbial amount and activity [94]. In fact, qCmic and qCO2 did not significantly change over time in the investigated Technosols, probably due to the decrease of soil organic matter content and the increase of its quality observed from 2010 to 2018.
The more labile SOM induces a faster mineralization over time, measured through the significant increase of coefficient of endogenous mineralization. In 2018, the Technosols’ microbial community probably tended to immobilise less C into the microbial biomass and to lose it through CO2 emissions, showing a reduction over time in the efficient use of C resources. This result is corroborated by the observed shift in the microbial community’s composition, enhancing the bacterial rather than the fungal abundance in soil. In this view, the percentage of FB/MB and the fungal activity ratio gives specific information about shifts in the soil community structure [54] and show both significant decrease from 2010 to 2018. Fungi and bacteria represent 95% of soil microbial biomass and differ in occupation of ecological niches in soil, consumption of organic matter, and rates of nutrient turnover [53,66]. Soil fungi contribute to the stabilization of soil organic C, with approximately 55% of C-use efficiency and incorporate more soil C in their biomass than bacteria, so that fungal cell walls are more recalcitrant than bacterial cell walls [54]. Therefore, C sequestration may be more intense in soils dominated by fungal communities than in those whose communities are essentially dominated by bacteria [95]. Consequently, low values of C/N ratio favour bacteria and they dominate in N-rich soils [95]. Therefore, our results confirmed that soil organic matter content is the primary factor influencing soil fungal abundance and activity [75] and in urban soil the decrease of SOM quantity and quality leads to less fungal fraction on total microbes, by inhibiting fungal activity [96]. The lower efficiency of the investigated soils to C sequestration and the microbial activity linked with the faster bacterial turnover compared to the fungal one is also highlighted by the biological fertility index (BFI). In fact, the soils are classified as “stressed with very low fertility” for 2010 and “pre-stressed” for 2018, classes I and II respectively [58]. According to Khanghahi et al. [97] and Renzi et al. [57], BFI increases linearly with the composing variables related to soil microbial activity, but also seems to be poorly affected by microbial biomass and qCO2. However, BFI depends mainly on the rise of CEM or BR over time. Probably this index is more sensitive to spatially varying soil fertility than microbial activity or microbial biomass alone and allows large-scale assessment of arable soil quality at varying levels of human disturbance [58], but it is less informative for a time scale evaluation and for other disturbances than agricultural practise.
4.4. Soil Quality Variation through Integrated Indices
The investigated integrated indices highlight medium to good quality for the studied Technosols but different results are evident over time due to the different approaches.
The results of the SQI reflect a reduction in the soil chemical quality, such as a decrease in the quantity and stability of organic matter and an increase in Cu, Pb, and Zn content, with an improved stress condition for the microbial community, mainly represented by bacteria less efficient in the C utilisation and storage than fungi.
The selection of a minimum data set does not seem to be sufficiently informative for the assessment of the Technosols’ quality over time, and the lack of statistically significant correlations between SQI and SQIMDS confirms this result. It is likely that a limited number of parameters such as those selected in the MDS cannot be fully representative of changes in the quality of recently established and poorly developed Technosols under investigation [98].
The quality reduction of the investigated soils over time is also shown by the sSQI index. In fact, the fluctuations between the maximum and minimum values of the indicators used for sSQI calculation increase the variability of the index and cover both the differences between the years and the statistical relationships between the SQI and sSQI indices.
5. Conclusions
This study highlights the need to monitor the quality of urban soils over time, even when neither pollution nor stress conditions are detected. The continuous anthropogenic impact and the great variability of the urban environment can induce a gradual deterioration of soil quality through the increase of pollutant concentrations, nutrient loss, and the fast organic matter turnover. These variations can affect the microbial community, which shows a low efficiency in the resource utilisation and C storage. The decreasing of the carbon sequestration function in relatively low polluted urban systems should be considered in the development of urban assessment and future soil monitoring.
In addition, our study provided evidence about the usefulness of an integrate approach of chemical and biological indices to evaluate the stressful conditions of Technosols.
The high spatial and temporal variability of urban soils and the continuous and persistent influence of human activity make some indicators unsuitable for providing information on soil quality. On the other hand, the soil variability must be considered together with broader effects as variations of soil chemical composition due to the contribution of parent material background which is often ignored in urban ecosystems.
In conclusion, starting from the investigated Technosols, our study suggests that in urban areas the integration of physical, chemical, and biological characteristics is essential to obtain more information about both the general soil functionality and ecosystem services it is providing. In fact, an integrated approach is recommended in order to maintain soil quality over time, ensure the soil use, and prevent damages to the environmental and human health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13169101/s1. Table S1: Total content and available fractions of the elements measured in Technosols during the study years (2010 and 2018), Table S2: Microbial and fungal carbon, basal respiration, percentage of active fungal biomass on total fungal biomass measured in Technosols, Table S3: Relationships among the integrated soil quality indices (Spearman correlations).
Author Contributions
Conceptualization, A.D.M. and P.N.; methodology, software, C.C., A.D.M. and P.N.; validation, V.M., S.C.P., L.S., E.D.I., A.G.R. and G.M.; formal analysis, P.N., V.M. and S.C.P.; data curation, P.N. and A.D.M.; writing—original draft preparation, A.D.M. and P.N.; writing—review and editing, A.D.M., P.N., E.D.I. and C.C.; supervision, A.D.M., C.C. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Sims, J.T.; Cunningham, S.D.; Summer, M.E. Assessing Soil Quality for Environmental Purposes: Roles and Challenges for Soil Scientists. J. Environ. Qual. 1997, 26, 20–25. [Google Scholar] [CrossRef]
- Nortcliff, S. Standardisation of soil quality attributes. Agric. Ecosyst. Environ. 2002, 88, 161–168. [Google Scholar] [CrossRef]
- Dominati, E.; Patterson, M.; Mackay, A. A framework for classifying and quantifying the natural capital and ecosystem services of soils. Ecol. Econ. 2010, 69, 1858–1868. [Google Scholar] [CrossRef]
- Schwilch, G.; Bernet, L.; Fleskens, L.; Giannakis, E.; Leventon, J.; Marañón, T.; Mills, J.; Short, C.; Stolte, J.; van Delden, H.; et al. Operationalizing ecosystem services for the mitigation of soil threats: A proposed framework. Ecol. Indic. 2016, 67, 586–597. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Raciti, S.M.; Hutyra, L.R.; Finzi, A.C. Depleted soil carbon and nitrogen pools beneath impervious surfaces. Environ. Pollut. 2012, 164, 248–251. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhang, G.L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61, 30–46. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Sun, G.-X.; Ren, Y.; Luo, X.-S.; Zhu, Y.-G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Jim, C.Y. Urban soil characteristics and limitations for landscape planting in Hong Kong. Landsc. Urban Plan. 1998, 40, 235–249. [Google Scholar] [CrossRef]
- IUSS. IUSS Working Group WRB: World Reference Base for Soil Resources 2014, Update 2015; World Soil Resources Report No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Schad, P. Technosols in the World Reference Base for Soil Resources–history and definitions. Soil Sci. Plant Nutr. 2018, 64, 138–144. [Google Scholar] [CrossRef]
- Kumar, K.; Hundal, L.S. Soil in the City: Sustainably Improving Urban Soils. J. Environ. Qual. 2016, 45, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, M.; Young, J.; Coldren, C.; Slaughter, L.; Longing, S. Soil physiochemical properties and carbon sequestration of Urban landscapes in Lubbock, TX, USA. Urban For. Urban Green. 2020, 56, 126847. [Google Scholar] [CrossRef]
- Hegetschweiler, K.T.; de Vries, S.; Arnberger, A.; Bell, S.; Brennan, M.; Siter, N.; Olafsson, A.S.; Voigt, A.; Hunziker, M. Linking demand and supply factors in identifying cultural ecosystem services of urban green infrastructures: A review of European studies. Urban For. Urban Green. 2017, 21, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Reid, B.J.; Meharg, A.A.; Banwart, S.A.; Fu, B.J. Optimizing Peri–URban Ecosystems (PURE) to re–couple urban–rural symbiosis. Sci. Total Environ. 2017, 586, 1085–1090. [Google Scholar] [CrossRef]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Vasenev, V.; Kuzyakov, Y. Urban soils as hot spots of anthropogenic carbon accumulation: Review of stocks, mechanisms and driving factors. Land Degrad. Dev. 2018, 29, 1607–1622. [Google Scholar] [CrossRef]
- Fourvel, G.J.; Vidal-Beaudet, L.; Le Bocq, A.; Thery, F.; Brochier, V.; Cannavo, P. Fertility of Technosols constructed with dam sediments for urban greening and land reclamation. J. Soils Sediments 2019, 19, 3178–3192. [Google Scholar] [CrossRef]
- Fergusson, L. Anthrosols and Technosols: The Anthropogenic Signature of Contaminated Soils and Sediments in Australia. Water Air Soil Pollut. 2017, 228, 1–14. [Google Scholar] [CrossRef]
- Andrews, S.S.; Flora, C.B.; Mitchell, J.P.; Karlen, D.L. Growers’ perceptions and acceptance of soil quality indices. Geoderma 2003, 114, 187–213. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Coleman, D.C.; Crossley, D., Jr.; Clark, J.S. Biological indices of soil quality: An ecosystem case study of their use. For. Ecol. Manag. 2000, 138, 357–368. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Moradian, S. Assessment of soil quality indices for salt-affected agricultural land in Kurdistan Province, Iran. Ecol. Indic. 2017, 83, 482–494. [Google Scholar] [CrossRef]
- Rezaei, S.A.; Gilkes, R.J.; Andrews, S.S. A minimum data set for assessing soil quality in rangelands. Geoderma 2006, 136, 229–234. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Napoletano, P.; Esposito, F.; Colombo, C.; Maisto, G.; De Marco, A. Variation of the chemical and biological properties of a Technosol during seven years after a single application of compost. Appl. Soil Ecol. 2019, 138, 156–159. [Google Scholar] [CrossRef]
- Ruiz, F.; Cherubin, M.R.; Ferreira, T.O. Soil quality assessment of constructed Technosols: Towards the validation of a promising strategy for land reclamation, waste management and the recovery of soil functions. J. Environ. Manag. 2020, 276, 111344. [Google Scholar] [CrossRef]
- Di Gennaro, A. I Sistemi di Terre della Campania; Assessorato Regionale alla Ricerca Scientifica: Firenze, Italy, 2002. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Sebastiani, A.; Buonocore, E.; Franzese, P.P.; Riccio, A.; Chianese, E.; Nardella, L.; Manes, F. Modelling air quality regulation by green infrastructure in a Mediterranean coastal urban area: The removal of PM10 in the Metropolitan City of Naples (Italy). Ecol. Model. 2021, 440, 109383. [Google Scholar] [CrossRef]
- Colombo, C.; Miano, T. Metodi di Analisi Chimica del Suolo; Pubblicità & Stampa: Bari, Italy, 2015. [Google Scholar]
- Danielson, R.E.; Sutherland, P.L. Porosity. In Methods of Soil Analysis. Part 1-Physical and Mineralogical Methods, 2nd ed.; Agronomy Monographs, 9(1); Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 443–462. [Google Scholar]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Schwertfeger, D.M.; Hendershot, W.H. Determination of Effective Cation Exchange Capacity and Exchange Acidity by a One-Step BaCl 2 Method. Soil Sci. Soc. Am. J. 2009, 73, 737–743. [Google Scholar] [CrossRef]
- Lindsay, W.N.; Norwell, W.A. Development of a DTPA micronutrient soil test. Agron. Abstr. 1969, 69–87. [Google Scholar] [CrossRef]
- Froment, A. Soil respiration in a mixed oak forest. Oikos 1972, 23, 273–277. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Für Pflanz. Düngung Bodenkd. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Duncan, L.C. Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol. Biochem. 2001, 33, 1143–1153. [Google Scholar] [CrossRef]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994; p. 242. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystem; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Sundman, V.; Sivelä, S. A comment on the membrane filter technique for estimation of length of fungal hyphae in soil. Soil Biol. Biochem. 1978, 10, 399–401. [Google Scholar] [CrossRef]
- Olson, F.C.W. Quantitative estimates of filamentous algae. Trans. Am. Microsc. Soc. 1950, 69, 272–279. [Google Scholar] [CrossRef]
- Söderström, B.E. Seasonal fluctuations of active fungal biomass in horizons of a podzolized pine-forest soil in central Sweden. Soil Biol. Biochem. 1979, 11, 149–154. [Google Scholar] [CrossRef]
- Mueller, L.; Schindler, U.; Mirschel, W.; Graham Shepherd, T.; Ball, B.C.; Helming, K.; Rogasik, J.; Eulenstein, F.; Wiggering, H. Assessing the productivity function of soils. A review. Agron. Sustain. Dev. 2010, 30, 601–614. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Okedeyi, O.O.; Dube, S.; Awofolu, O.R.; Nindi, M.M. Assessing the enrichment of heavy metals in surface soil and plant (Digitaria eriantha) around coal-fired power plants in South Africa. Environ. Sci. Pollut. Res. 2014, 21, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Pejman, A.; Nabi Bidhendi, G.; Ardestani, M.; Saeedi, M.; Baghvand, A. A new index for assessing heavy metals contamination in sediments: A case study. Ecol. Indic. 2015, 58, 365–373. [Google Scholar] [CrossRef]
- da Silva Lobato, A.K.; Alvino Lima, E.J.; Guedes Lobato, E.M.S.; Maciel, G.M.; Marques, D.J. Tolerance of Plants to Toxicity Induced by Micronutrients. Abiotic Biot. Stress Plants Recent Adv. Futur. Perspect. 2016, 9, 229–246. [Google Scholar] [CrossRef] [Green Version]
- Ahamad, M.I.; Song, J.; Sun, H.; Wang, X.; Mehmood, M.S.; Sajid, M.; Su, P.; Khan, A.J. Contamination level, ecological risk, and source identification of heavy metals in the hyporheic zone of the weihe river, China. Int. J. Environ. Res. Public Health 2020, 17, 1070. [Google Scholar] [CrossRef] [Green Version]
- Massas, I.; Ehaliotis, C.; Gerontidis, S.; Sarris, E. Elevated heavy metal concentrations in top soils of an Aegean island town (Greece): Total and available forms, origin and distribution. Environ. Monit. Assess. 2009, 151, 105–116. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; De Marco, A.; D’Ascoli, R.; Castaldi, S.; Gentile, A.; Virzo De Santo, A. Impact of fire on fungal abundance and microbial efficiency in C assimilation and mineralisation in a Mediterranean maquis soil. Biol. Fertil. Soils 2007, 44, 377–381. [Google Scholar] [CrossRef]
- Insam, H.; Haselwandter, K. Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 1989, 79, 174–178. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Renzi, G.; Canfora, L.; Salvati, L.; Benedetti, A. Validation of the soil Biological Fertility Index (BFI) using a multidimensional statistical approach: A country-scale exercise. Catena 2017, 149, 294–299. [Google Scholar] [CrossRef]
- Sequi, P.; Benedetti, A.; Dell’Abate, M.T. ATLAS. Atlante di Indicatori della Qualità del Suolo; Franco Angeli: Milano, Italy, 2006. [Google Scholar]
- Vasu, D.; Tiwari, G.; Sahoo, S.; Dash, B.; Jangir, A.; Sharma, R.P.; Naitam, R.; Tiwary, P.; Karthikeyan, K.; Chandran, P. A minimum data set of soil morphological properties for quantifying soil quality in coastal agroecosystems. Catena 2021, 198, 105042. [Google Scholar] [CrossRef]
- Liebig, M.A.; Varvel, G.; Doran, J. A simple performance-based index for assessing multiple agroecosystem functions. Agron. J. 2001, 93, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Amacher, M.C.; O’Neill, K.P.; Perry, C.H. Soil vital signs: A new soil quality index (SQI) for assessing forest soil health. USDA For. Serv. Res. Pap. RMRS-RP 2007, 1–12. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of soil quality index using three methods. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef] [Green Version]
- Séré, G.; Schwartz, C.; Ouvrard, S.; Renat, J.C.; Watteau, F.; Villemin, G.; Morel, J.L. Early pedogenic evolution of constructed Technosols. J. Soils Sediments 2010, 10, 1246–1254. [Google Scholar] [CrossRef]
- Solleiro-Rebolledo, E.; Sedov, S.; Cabadas-Báez, H. Use of soils and palaeosols on volcanic materials to establish the duration of soil formation at different chronological scales. Quat. Int. 2015, 376, 5–18. [Google Scholar] [CrossRef]
- Ventorino, V.; De Marco, A.; Pepe, O.; Virzo De Santo, A.; Moschetti, G. Impact of innovative agriculturalpractices of carbon sequestration on soilmicrobial community. In Carbon Sequestration in Agricultural Soils. Amultidisciplinary Approach to Innovative Methods; Piccolo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–177. [Google Scholar]
- Osman, K.T. Forest Soils: Properties and Management; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–217. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Esposito, F.; Maisto, G.; De Marco, A. Plant cover and management practices as drivers of soil quality. Appl. Soil Ecol. 2018, 129, 34–42. [Google Scholar] [CrossRef]
- Charzyński, P.; Plak, A.; Hanaka, A. Influence of the soil sealing on the geoaccumulation index of heavy metals and various pollution factors. Environ. Sci. Pollut. Res. 2017, 24, 4801–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renella, G. Evolution of physico-chemical properties, microbial biomass and microbial activity of an urban soil after de-sealing. Agriculture 2020, 10, 596. [Google Scholar] [CrossRef]
- Nannoni, F.; Rossi, S.; Protano, G. Soil properties and metal accumulation by earthworms in the Siena urban area (Italy). Appl. Soil Ecol. 2014, 77, 9–17. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Managing soil carbon stocks to enhance the resilience of urban ecosystems. Carbon Manag. 2015, 6, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Kida, K.; Kawahigashi, M. Influence of asphalt pavement construction processes on urban soil formation in Tokyo. Soil Sci. Plant Nutr. 2015, 61, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Skariyachan, S.; Megha, M.; Kini, M.N.; Mukund, K.M.; Rizvi, A.; Vasist, K. Selection and screening of microbial consortia for efficient and ecofriendly degradation of plastic garbage collected from urban and rural areas of Bangalore, India. Environ. Monit. Assess. 2015, 187, 4174. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, F.; Yu, Y.; Zhang, J.; Wang, R.; Srinivasulu, M.; Vasenev, V.I. Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. J. Soils Sediments 2017, 17, 1116–1125. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Pei, K.; Zhou, J.; Peixoto, L.; Gunina, A.; Zeng, Z.; Zang, H.; Rasmussen, J.; Kuzyakov, Y. Nitrogen rhizodeposition by legumes and its fate in agroecosystems: A field study and literature review. L. Degrad. Dev. 2021, 32, 410–419. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Santorufo, L.; De Marco, A.; Barile, R.; Maisto, G. Total and fraction content of elements in volcanic soil: Natural or anthropogenic derivation. Sci. Total Environ. 2018, 625, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcke, W.; Müller, S.; Kanchanakool, N.; Zech, W. Urban soil contamination in Bangkok: Heavy metal and aluminium partitioning in topsoils. Geoderma 1998, 86, 211–228. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium-sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef] [Green Version]
- Panico, S.C.; Esposito, F.; Memoli, V.; Vitale, L.; Polimeno, F.; Magliulo, V.; Maisto, G.; De Marco, A. Variations of agricultural soil quality during the growth stages of sorghum and sunflower. Appl. Soil Ecol. 2020, 152, 103569. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Esposito, F.; Panico, S.C.; Barile, R.; Maisto, G. Seasonality, altitude and human activities control soil quality in a national park surrounded by an urban area. Geoderma 2019, 337, 1–10. [Google Scholar] [CrossRef]
- Maisto, G.; Alfani, A.; Baldantoni, D.; De Marco, A.; Virzo De Santo, A. Trace metals in the soil and in Quercus ilex L. leaves at anthropic and remote sites of the Campania Region of Italy. Geoderma 2004, 122, 269–279. [Google Scholar] [CrossRef]
- Cicchella, D.; De Vivo, B.; Lima, A. Background and baseline concentration values of elements harmful to human health in the volcanic soils of the metropolitan and provincial areas of Napoli (Italy). Geochem. Explor. Environ. Anal. 2005, 5, 29–40. [Google Scholar] [CrossRef]
- Shifaw, E. Review of heavy metals pollution in China in agricultural and urban soils. J. Health Pollut. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Gong, Z.; Zhang, G.; Burghardt, W. Concentrations and chemical speciations of Cu, Zn, Pb and Cr of urban soils in Nanjing, China. Geoderma 2003, 115, 101–111. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Czerwonka, G.; Konieczna, I.; Zarnowiec, P.; Zieliński, A.; Malinowska-Gniewosz, A.; GaŁuszka, A.; Migaszewski, Z.; Kaca, W. Characterization of microbial communities in acidified, sulfur containing soils. Pol. J. Microbiol. 2017, 66, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, T.H. Microbial eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Yuangen, Y.; Paterson, E.; Campbell, C.D. Urban soil microbial features and their environmental significance as exemplified by Aberdeen City, UK. Chin. J. Geochem. 2001, 20, 34–44. [Google Scholar] [CrossRef]
- Lago, M.d.C.F.; Barreal, M.E.; Gallego, P.P.; Briones, M.J.I. Legacy Effects of Agricultural Practices Override Earthworm Control on C Dynamics in Kiwifruit Orchards. Front. Environ. Sci. 2020, 8, 545609. [Google Scholar] [CrossRef]
- Nelson, K.L.; Lynch, D.H.; Boiteau, G. Assessment of changes in soil health throughout organic potato rotation sequences. Agric. Ecosyst. Environ. 2009, 131, 220–228. [Google Scholar] [CrossRef]
- Shukurov, N.; Pen-Mouratov, S.; Steinberger, Y. The influence of soil pollution on soil microbial biomass and nematode community structure in Navoiy Industrial Park, Uzbekistan. Environ. Int. 2006, 32, 1–11. [Google Scholar] [CrossRef]
- Antisari, L.V.; Ferronato, C.; De Feudis, M.; Natali, C.; Bianchini, G.; Falsone, G. Soil biochemical indicators and biological fertility in agricultural soils: A case study from northern Italy. Minerals 2021, 11, 219. [Google Scholar] [CrossRef]
- Malik, A.A.; Chowdhury, S.; Schlager, V.; Oliver, A.; Puissant, J.; Vazquez, P.G.M.; Jehmlich, N.; von Bergen, M.; Griffiths, R.I.; Gleixner, G. Soil fungal: Bacterial ratios are linked to altered carbon cycling. Front. Microbiol. 2016, 7, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slukovskaya, M.V.; Vasenev, V.I.; Ivashchenko, K.V.; Dolgikh, A.V.; Novikov, A.I.; Kremenetskaya, I.P.; Ivanova, L.A.; Gubin, S.V. Organic matter accumulation by alkaline-constructed soils in heavily metal-polluted area of Subarctic zone. J. Soils Sediments 2021, 21, 2071–2088. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Murgese, P.; Strafella, S.; Crecchio, C. Soil biological fertility and bacterial community response to land use intensity: A case study in the Mediterranean Area. Diversity 2019, 11, 211. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Chen, J.; Sun, Z.; Tan, M. Establishing a minimum dataset for soil quality assessment based on soil properties and land-use changes. Acta Ecol. Sin. 2007, 27, 2715–2724. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).