Environmental Assessment of Recycling (EAoR) for Safe Recycling of Steelmaking Slag in the Republic of Korea: Applications, Leaching Test, and Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Experimental Procedures
2.2.1. Leaching Test
2.2.2. Toxicity Test Using Aliivibrio fischeri
3. Results and Discussion
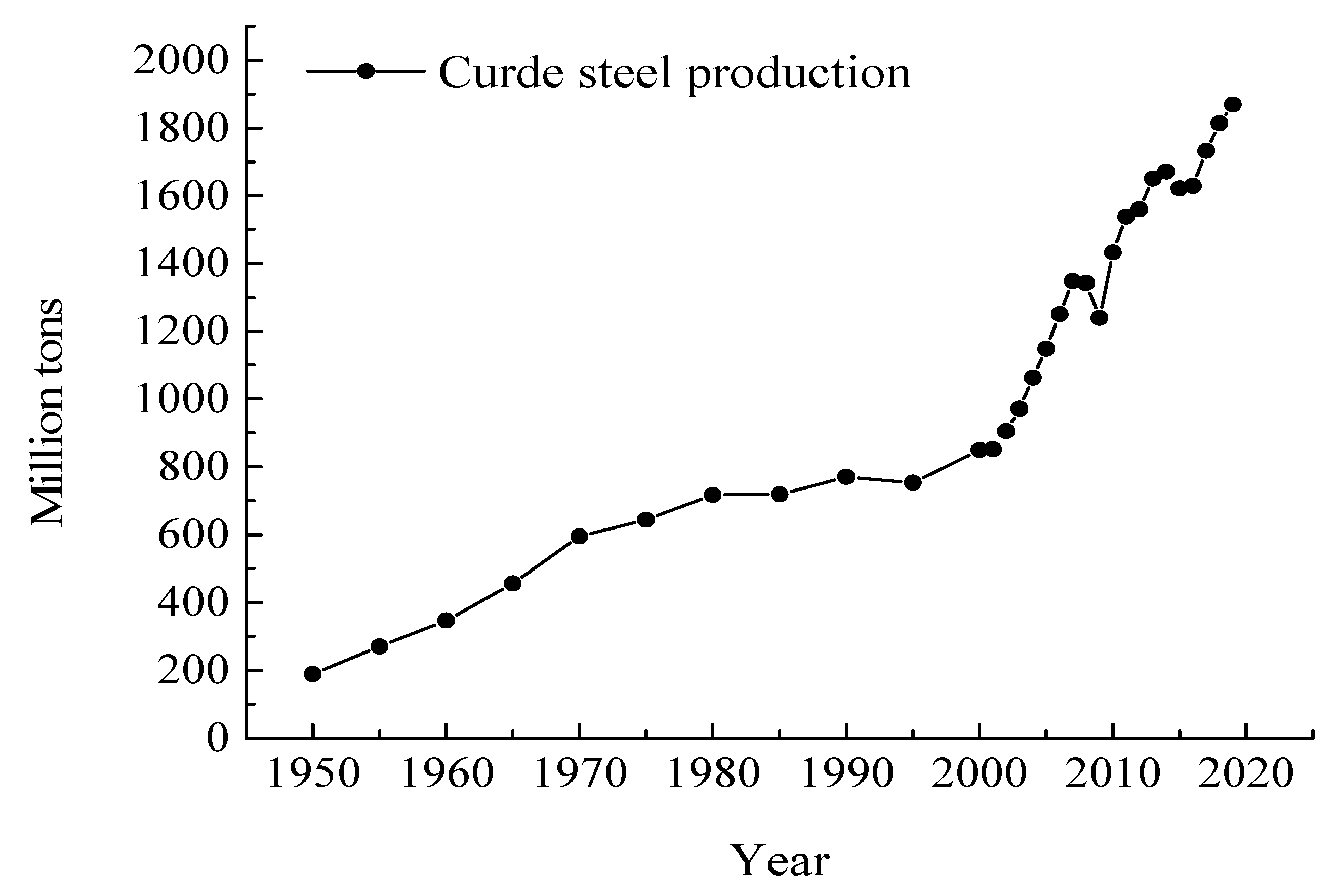
3.1. Historic Trends in the Republic of Korea
3.2. Steel Slag Management Regulations and Legal Position in the Republic of Korea
3.3. Environmental Risk Assessment of Steelmaking Slag in the Republic of Korea
3.3.1. General Physico-Chemical Estimation of Steelmaking Slag
3.3.2. Estimation of Leaching Test for Steelmaking Slag
3.3.3. Evaluation of Toxicity with Aliivibrio fischeri
3.4. Future of Steelmaking Slag Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Steel Association. Steel Statistical Yearbook 2020. Available online: https://www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook.html (accessed on 5 January 2021).
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Korea Iron & Steel Association. Production and Uses of Steel Slag in the Republic of Korea. Available online: https://www.kosa.or.kr/statistics/.jsp (accessed on 9 February 2020).
- Ministry of Land Infrastructure and Transport Republic of Korea. Production and Demand of Aggregate in the Republic of Korea. 2019. Available online: https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1223 (accessed on 19 July 2021).
- Geiseler, J. Use of steelworks slag in Europe. Waste Manag. 1996, 16, 59–63. [Google Scholar] [CrossRef]
- Topkaya, Y.; Sevinç, N.; Günaydın, A. Slag treatment at Kardemir integrated iron and steel works. Int. J. Miner. Process. 2004, 74, 31–39. [Google Scholar] [CrossRef]
- Diao, J.; Zhou, W.; Ke, Z.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Ortiz, N.; Pires, M.A.F.; Bressiani, J.C. Use of steel converter slag as nickel adsorber to wastewater treatment. Waste Manag. 2001, 21, 631–635. [Google Scholar] [CrossRef]
- Ponsot, I.; Bernardo, E. Self glazed glass ceramic foams from metallurgical slag and recycled glass. J. Clean. Prod. 2013, 59, 245–250. [Google Scholar] [CrossRef]
- Sarfo, P.; Wyss, G.; Ma, G.; Das, A.; Young, C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Sáez-De-Guinoa Vilaplana, A.; García-Armingol, T.; Aranda-Usón, A.; Lausín-González, C.; López-Sabirón, A.M.; Ferreira, G. Evaluation of the steel slag incorporation as coarse aggregate for road construction: Technical requirements and environmental impact assessment. J. Clean. Prod. 2016, 130, 175–186. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. Sustainable solutions for road pavements: A multi-scale characterization of warm mix asphalts containing steel slags. J. Clean. Prod. 2017, 166, 835–843. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Adhikari, R.; Chen, Y.-H.; Li, P.; Chiang, P.-C. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Tong, Z.; Xue, Z. Microwave-assisted selective leaching behavior of calcium from Basic Oxygen Furnace (BOF) slag with ammonium chloride solution. J. Min. Metall. Sect. B Metall. 2017, 53, 139–146. [Google Scholar] [CrossRef]
- Fujisawa, N.; Fukushima, M.; Yamamoto, M.; Iwai, H.; Komai, T.; Kawabe, Y.; Liu, D. Structural alterations of humic acid fractions in a steel slag-compost fertilizer during fertilization. Analysis by pyrolysis/methylation-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2012, 95, 126–133. [Google Scholar] [CrossRef]
- Gómez-Nubla, L.; Aramendia, J.; Fdez-Ortiz de Vallejuelo, S.; Carrero, J.A.; Madariaga, J.M. Focused ultrasound energy over steel slags as a fast tool to assess their environmental risk before and after their reuse in agriculture and civil constructions. Microchem. J. 2017, 132, 268–273. [Google Scholar] [CrossRef]
- Drissen, P.; Ehrenberg, A.; Kühn, M.; Mudersbach, D. Recent Development in Slag Treatment and Dust Recycling. Process. Metall. 2009, 80, 737–745. [Google Scholar]
- Nakase, K.; Matsui, A.; Kikuchi, N.; Miki, Y.; Kishimoto, Y.; Goto, I.; Nagasaka, T. Fundamental Research on a Rational Steelmaking Slag Recycling System by Phosphorus Separation and Collection. J. Manuf. Sci. Prod. 2013, 13, 39–45. [Google Scholar] [CrossRef]
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 463. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Enviornment Republic of Korea. Guidelines for Recycling Steel Slag and Coal Ash Discharging Business. 2009. Available online: https://www.law.go.kr/LSW/admRulLsInfoP.do?admRulSeq=2100000202184#:~:text=1.%20%EC%B2%A0%EA%B0%95%EC%8A%AC%EB%9E%98%EA%B7%B8%20%3A%20%EC%B2%A0%EA%B0%95%EC%8A%AC%EB%9E%98%EA%B7%B8,%EC%95%84%EB%8B%88%ED%95%98%EA%B3%A0%20%EC%9E%AC%ED%99%9C%EC%9A%A9%ED%95%A0%20%EC%88%98%20%EC%9E%88%EB%8B%A4 (accessed on 2 February 2021).
- Nippon Slag Association. Production and Uses of Blast Furnace Slag in Japan. Available online: https://www.slg.jp/pdf/Blast%20Furnace%20Slag%202017FY%20rev.pdf (accessed on 22 February 2021).
- Euro Slag Association. Euroslag Statistics 2018. Available online: https://www.euroslag.com/products/statistics/statistics-2018/ (accessed on 5 June 2018).
- United State Geological Survey. Iron and steel slag. Available online: https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/mcs-2019-fesla.pdf (accessed on 19 February 2019).
- Mayes, W.M.; Younger, P.L.; Aumônier, J. Hydrogeochemistry of Alkaline Steel Slag Leachates in the UK. Water Air Soil Pollut. 2008, 195, 35–50. [Google Scholar] [CrossRef]
- Hou, D.; Li, D.; Xu, G.; Zhang, Y. Superposition model for analyzing the dynamic ground subsidence in mining area of thick loose layer. Int. J. Min. Sci. Technol. 2018, 28, 663–668. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Proctor, D.M.; Shay, E.C.; Fehling, K.A.; Finley, B.L. Assessment of Human Health and Ecological Risks Posed by the Uses of Steel-Industry Slags in the Environment. Hum. Ecol. Risk Assess. Int. J. 2010, 8, 681–711. [Google Scholar] [CrossRef]
- Spanka, M. Minimizing the release of Chromium, Molybdenum, Vanadium, and Fluoride from Steelwork Slags. 2018. Available online: https://kups.ub.uni-koeln.de/8583/1/Spanka%20%282018%29.pdf (accessed on 30 July 2021).
- Fang, H.; Huang, L.; Wang, J.; He, G.; Reible, D. Environmental assessment of heavy metal transport and transformation in the Hangzhou Bay, China. J. Hazard Mater. 2016, 302, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Chaurand, P.; Rose, J.; Briois, V.; Olivi, L.; Hazemann, J.L.; Proux, O.; Domas, J.; Bottero, J.-Y. Environmental impacts of steel slag reused in road construction: A crystallographic and molecular (XANES) approach. J. Hazard Mater. 2007, 139, 537–542. [Google Scholar] [CrossRef]
- Mayes, W.M.; Younger, P.L.; Aumonier, J. Buffering of Alkaline Steel Slag Leachate across a Natural Wetland. Environ. Sci. Technol. 2006, 40, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Mayes, W.M.; Baxter, H.A.; Jarvis, A.P.; Burke, I.T.; Stewart, D.I.; Rogerson, M. Options for managing alkaline steel slag leachate: A life cycle assessment. J. Clean. Prod. 2018, 202, 401–412. [Google Scholar] [CrossRef]
- Hull, S.L.; Oty, U.V.; Mayes, W.M. Rapid recovery of benthic invertebrates downstream of hyperalkaline steel slag discharges. Hydrobiologia 2014, 736, 83–97. [Google Scholar] [CrossRef]
- Burke, I.T.; Mayes, W.M.; Peacock, C.L.; Brown, A.P.; Jarvis, A.P.; Gruiz, K. Speciation of arsenic, chromium, and vanadium in red mud samples from the Ajka spill site, Hungary. Environ. Sci. Technol. 2012, 46, 3085–3092. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams Intercomparison of Thermodynamic Databases Geological Survey of Japan Open File Report No.419. 2005. Available online: https://www.gsj.jp/researches/openfile/openfile2005/openfile0419.html (accessed on 22 July 2021).
- Hernández, P.; Dorador, A.; Martínez, M.; Toro, N.; Castillo, J.; Ghorbani, Y. Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching. Minerals 2020, 10, 477. [Google Scholar] [CrossRef]
- Susset, B.; Grathwohl, P. Leaching standards for mineral recycling materials-A harmonized regulatory concept for the upcoming German Recycling Decree. Waste Manag. 2011, 31, 201–214. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; Meeussen, J.C.L.; Comans, R.N.J. Leaching of Heavy Metals from Contaminated Soils: An Experimental and Modeling Study. Environ. Sci. Technol. 2004, 38, 4390–4395. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; van der Sloot, H.A.; Comans, R.N.J. The leaching of major and trace elements from MSWI bottom ash as a function of pH and time. Appl. Geochem. 2006, 21, 335–351. [Google Scholar] [CrossRef]
- Grathwohl, P.; Susset, B. Comparison of percolation to batch and sequential leaching tests: Theory and data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Delapp, R.C.; Kosson, D.S.; van der Sloot, H.A.; Liu, J. Release of heavy metals during long-term land application of sewage sludge compost: Percolation leaching tests with repeated additions of compost. Chemosphere 2017, 169, 271–280. [Google Scholar] [CrossRef] [PubMed]








| Type | Regulation and Standard |
|---|---|
| R-1 | Original form or simply repair and reuse |
| R-2 | Simply repair or wash and reuse |
| R-3 | Can be made into a recoverable state or used for recycling |
| R-4 | Directly recoverable |
| R-5 | Recycling for agriculture or soil improvement |
| R-6 | Recycling of organic materials to improve soil quality |
| R-7 | Recycling as embankment materials, cover material, road layer material, and filling material in soil or public water |
| R-8 | Directly recovered as energy, or processed to be recoverable |
| R-9 | Can be recovered as energy |
| R-10 | A type of intermediate processed waste for product manufacturing |
| Area | Usage | Republic of Korea | Japan | USA | EU | Australia | China | Remark |
|---|---|---|---|---|---|---|---|---|
| Raw material | Cement raw material | ○ | ○ | ○ | ○ | ○ | ○ | |
| Concrete admixture | ○ | ○ | ○ | ○ | ○ | ○ | ||
| Silicate fertilizer | ○ | ○ | ○ | ○ | ○ | - | ||
| Aggregate | Brick clay | ○ | - | ○ | - | ○ | ○ | |
| Concrete aggregate | ○ | ○ | ○ | ○ | ○ | ○ | ||
| Banking | ○ | ○ | ○ | - | ○ | ○ | ||
| Soil covering | ○ | - | ○ | - | ○ | ○ | ||
| Dike construction | ○ | - | - | ○ | - | - | ||
| Public water surface backfilling | ○ | ○ | ○ | - | ○ | ○ | ||
| Road aggregate | ○ | ○ | ○ | ○ | ○ | ○ | ||
| Chemical construction | ○ | - | - | - | - | - | ||
| Dewatering aggregate | ○ | ○ | - | ○ | ○ | - | ||
| Non-skid aggregate | ○ | - | ○ | - | ○ | - | ||
| Retaining wall and backfilling | ○ | - | ○ | ○ | ○ | ○ | ||
| Rubble stone | ○ | - | ○ | - | ○ | ○ | Road construction | |
| Other | Rockwool | ○ | - | ○ | ○ | ○ | - | |
| Pigment | ○ | ○ | - | - | - | - | ||
| Soil amendment | ○ | ○ | ○ | ○ | - | - | ||
| Chemical additive | ○ | - | ○ | - | - | - | ||
| Processing material | ○ | ○ | - | - | - | - | Magnetic separation | |
| Railway material | - | ○ | ○ | ○ | - | ○ | ||
| Wastewater treatment | - | - | ○ | ○ | - | - | Remove nitrous and phosphorus | |
| Filter | - | - | ○ | - | ○ | - | Trout farm | |
| Land fill material | ○ | - | ○ | - | - | - | ||
| Mining wastewater treatment | - | - | ○ | ○ | - | - | ||
| Building roof materials | - | - | ○ | ○ | - | - |
| Country | Slag Type | Regulation and Guideline About Slag Aging |
|---|---|---|
| Republic of Korea | Steel slag | - Size ≦ 100 mm, stockpiled for at least 1 month - Size ≧100 mm, stockpiled for at least 3 months |
| USA (DOT) | Air-cooled slag or blast furnace slag | - Stockpiled for a minimum of 1 month - Must pass the test of leachate determination (bucket test) |
| Steelmaking slag | - Stockpiled for 6 months to allow hydration and expansion - ASTM D4792 [00(2006) Standard Test Method for Potential Expansion of Aggregates from Hydration Reactions] | |
| Belgium | Steelmaking slag | - Stockpiled for a minimum of 12 months - Free CaO: ≦ 4.5% |
| Netherlands | Steelmaking slag | - Stockpiled for a minimum of 12 months - Free CaO: ≦ 4.5% |
| Australia | Steelmaking slag | - Stockpiled for 1~3 months |
| New Zealand | Steelmaking slag | - Stockpiled for 1~3 months |
| Brazil | Steelmaking slag | - Stockpiled for a minimum of 6 months |
| Country | Main Content | Remark |
|---|---|---|
| Republic of Korea | Slag is classified as a “by-product” in the Resource Conservation and Recycling Promotion Act | Article 3 under Waste Management Act in Republic of Korea |
| Japan | - Law promoting effective use of resources: slag material is a co-product of the steel industry and is defined as a special resource - Act on the Promotion of Effective Utilization of Resources: slag is not waste if used as a beneficial material | |
| US | Slag is a co-product rather than a by-product or waste, according to federal government and in certain states | United States |
| Department of Environmental Protection (DEP): slag is a co-product rather than a by-product or waste | Pennsylvania state | |
| Compo Laws § 324.11506 (I)(f). “Slag” is defined as “the nonmetallic product resulting from melting or smelting operations for iron or steel” | Michigan state | |
| The policy also notes that blast furnace slag generally does not have negative environmental effects when used as a construction material | Ohio state | |
| Steelmaking slag is not covered by solid waste regulations in Indiana or other states | Indiana, Illinois state | |
| UK | Blast furnace slag defined as a by-product. BFS produced in the UK could be classified as a by-product | www.environment-agency.gov.uk (accessed on 6 July 2021) |
| EU | - Granulated slag from the production of iron and steel is considered to be a product in Austria and in many Organizations for Economic Co-operation and Development member countries - In Europe, steel slag and blast furnace slag products have been eliminated from the European Waste Catalogue - Slag treated with granulation, palletization, foaming, separation, crushing, sieving, and milling are excluded | European works council 10 02 01 waste the processing of slag 10 02 02 unprocessed slag |
| Australia | Slag is a by-product of the iron and steel manufacturing process |
| Component | Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Republic of Korea | USA | Australia | Turkey | Japan | Brazil | Sweden | France | China | |
| CaO | 46.1 | 40–52 | 35 | 38.62 | 45.8 | 45.2 | 45.0 | 47.71 | 42.92 |
| SiO2 | 41.8 | 2.1 | 14 | 19.28 | 11.10 | 12.2 | 11.1 | 13.25 | 11.51 |
| Al2O3 | - | 1–3 | 5.5 | 2.71 | 1.9 | 0.8 | 1.9 | 3.04 | 1.4 |
| MgO | 6.3 | 5–10 | 7.7 | 8.05 | 6.5 | 5.5 | 9.6 | 6.37 | 4.36 |
| MnO | 5.4 | 5–8 | 5.7 | 7.52 | 5.3 | 7.1 | 3.1 | 2.64 | 4.04 |
| P2O5 | - | 0.5–1 | - | - | 1.7 | - | 0.23 | 1.47 | 0.83 |
| Total iron (Fet) | - | - | - | 22.61 | 17.4 | 18.8 | 23.9 | 24.36 | 23.74 |
| S | 0.08 | - | 0.1 | 0.28 | 0.06 | 0.07 | - | - | 0.07 |
| K2O | - | - | 0.1 | - | - | - | - | - | - |
| Cr2O3 | - | - | 1 | - | - | - | - | - | - |
| V2O5 | - | - | 0.3 | - | - | - | - | - | - |
| Fe2O3 | - | 10–40 | 29 | - | - | - | - | - | - |
| Usage | Remark | |
|---|---|---|
| Raw materials (cement, admixture, fertilizer, rockwool, ceramic) | Use is specified in current recycling guidelines | |
| Aggregate (brick, concrete, road, ascon, rubble) | ||
| Filling caissons | ||
| Anti-slip aggregate | ||
| Iron recovery | Not yet possible (lack of technology) | |
| Railroad ballast | Excluded from review (sold according to road aggregate standards) | |
| Building roof materials | Excluded from review (ceramic material) | |
| Water treatment | Wastewater treatment, filter | Excluded from review (patent application from a specific company, involving atomizing a fine powder) Sold as a product |
| Mine wastewater | Excluded from review | |
| Ocean | Artificial reef | Excluded from review Designated by other law (Ministry of Food, Agriculture, Forestry and Fisheries) |
| Tetrapod block | ||
| In situ capping | Reviewing | |
| Materials for red tide prevention | Reviewing | |
| Soil conditioner | Reviewing | |
| Fine aggregate substituted for sand in sewage pipe installation | Reviewing | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-Y.; Kang, J.-H.; Hwang, D.-G.; Yoon, Y.-S.; Yoo, M.-S.; Jeon, T.-W. Environmental Assessment of Recycling (EAoR) for Safe Recycling of Steelmaking Slag in the Republic of Korea: Applications, Leaching Test, and Toxicity. Sustainability 2021, 13, 8805. https://doi.org/10.3390/su13168805
Lee M-Y, Kang J-H, Hwang D-G, Yoon Y-S, Yoo M-S, Jeon T-W. Environmental Assessment of Recycling (EAoR) for Safe Recycling of Steelmaking Slag in the Republic of Korea: Applications, Leaching Test, and Toxicity. Sustainability. 2021; 13(16):8805. https://doi.org/10.3390/su13168805
Chicago/Turabian StyleLee, Min-Yong, Jang-Hyun Kang, Dong-Gun Hwang, Young-Sam Yoon, Myung-Soo Yoo, and Tae-Wan Jeon. 2021. "Environmental Assessment of Recycling (EAoR) for Safe Recycling of Steelmaking Slag in the Republic of Korea: Applications, Leaching Test, and Toxicity" Sustainability 13, no. 16: 8805. https://doi.org/10.3390/su13168805
APA StyleLee, M.-Y., Kang, J.-H., Hwang, D.-G., Yoon, Y.-S., Yoo, M.-S., & Jeon, T.-W. (2021). Environmental Assessment of Recycling (EAoR) for Safe Recycling of Steelmaking Slag in the Republic of Korea: Applications, Leaching Test, and Toxicity. Sustainability, 13(16), 8805. https://doi.org/10.3390/su13168805







