Arbuscular Mycorrhizal Fungus Stimulates Young Field-Grown Nectarine Trees
Abstract
:1. Introduction
2. Materials and Methods
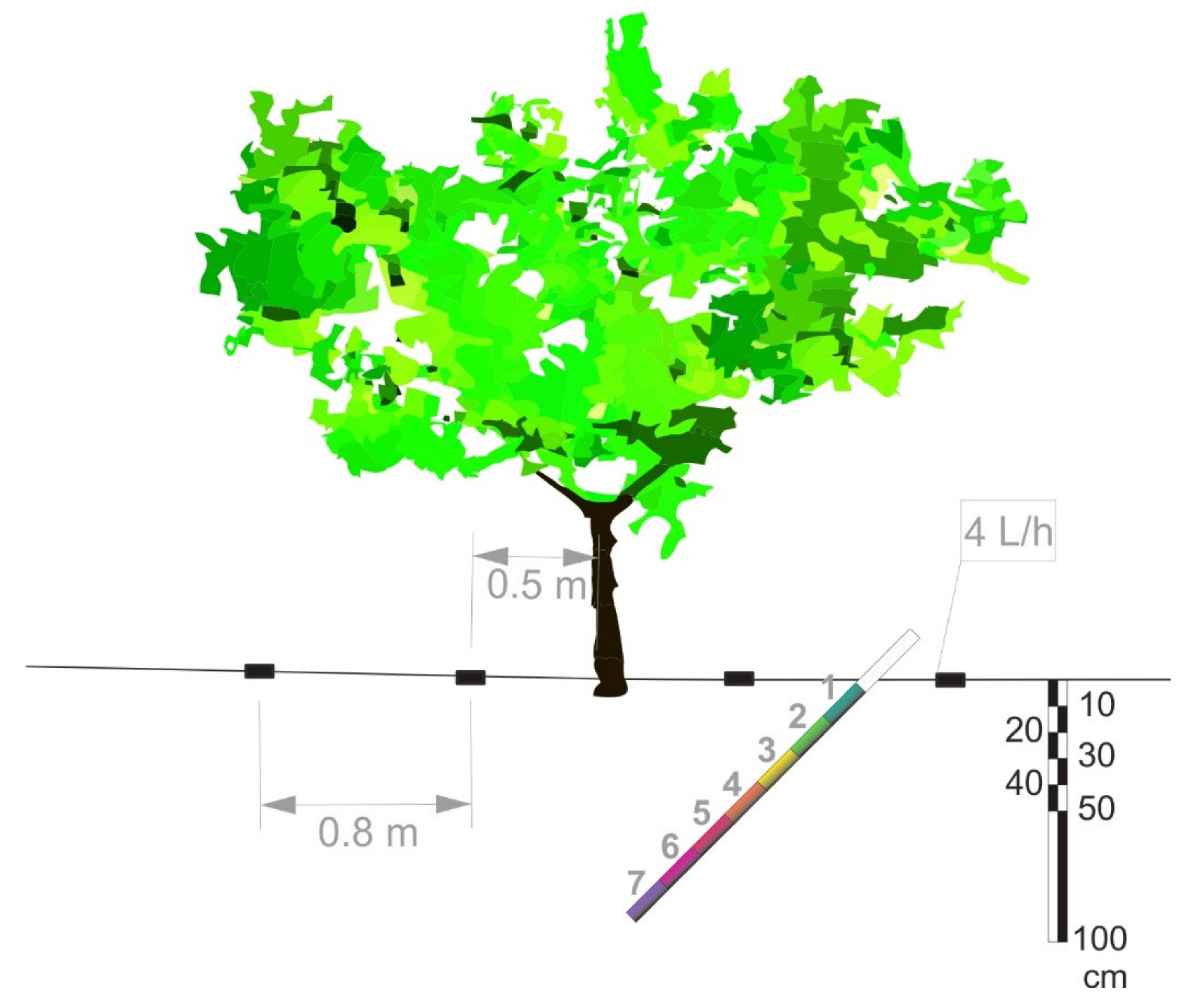
2.1. Experimental Conditions
2.2. Treatments
2.3. Measurements
2.3.1. Plant Water Status
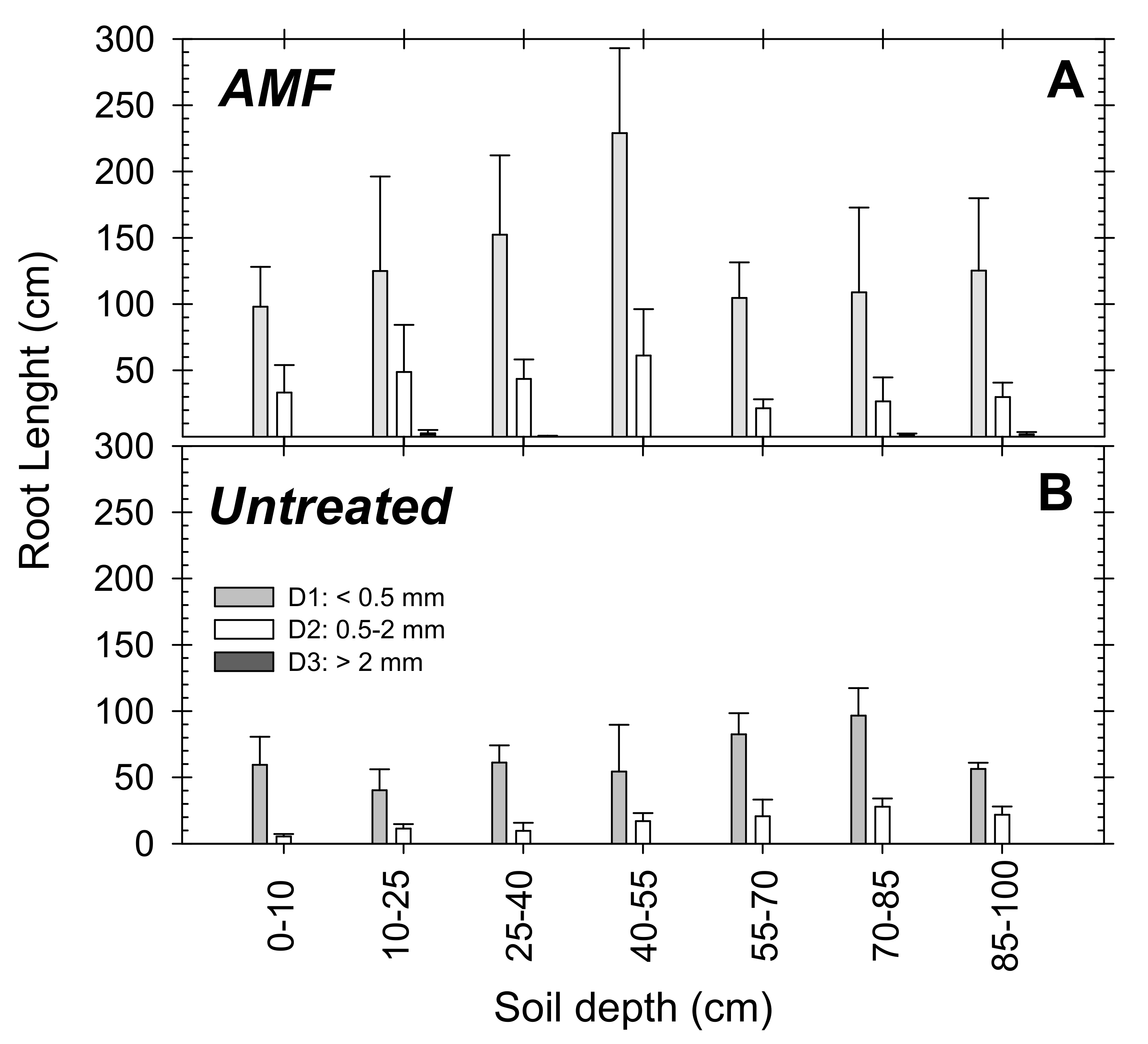
2.3.2. Root Measurements
2.3.3. Aboveground Measurements
Fruits
Vegetative Growth
Yield, Fruit Quality and Efficiencies at Harvest
2.4. Statistical Analysis
3. Results
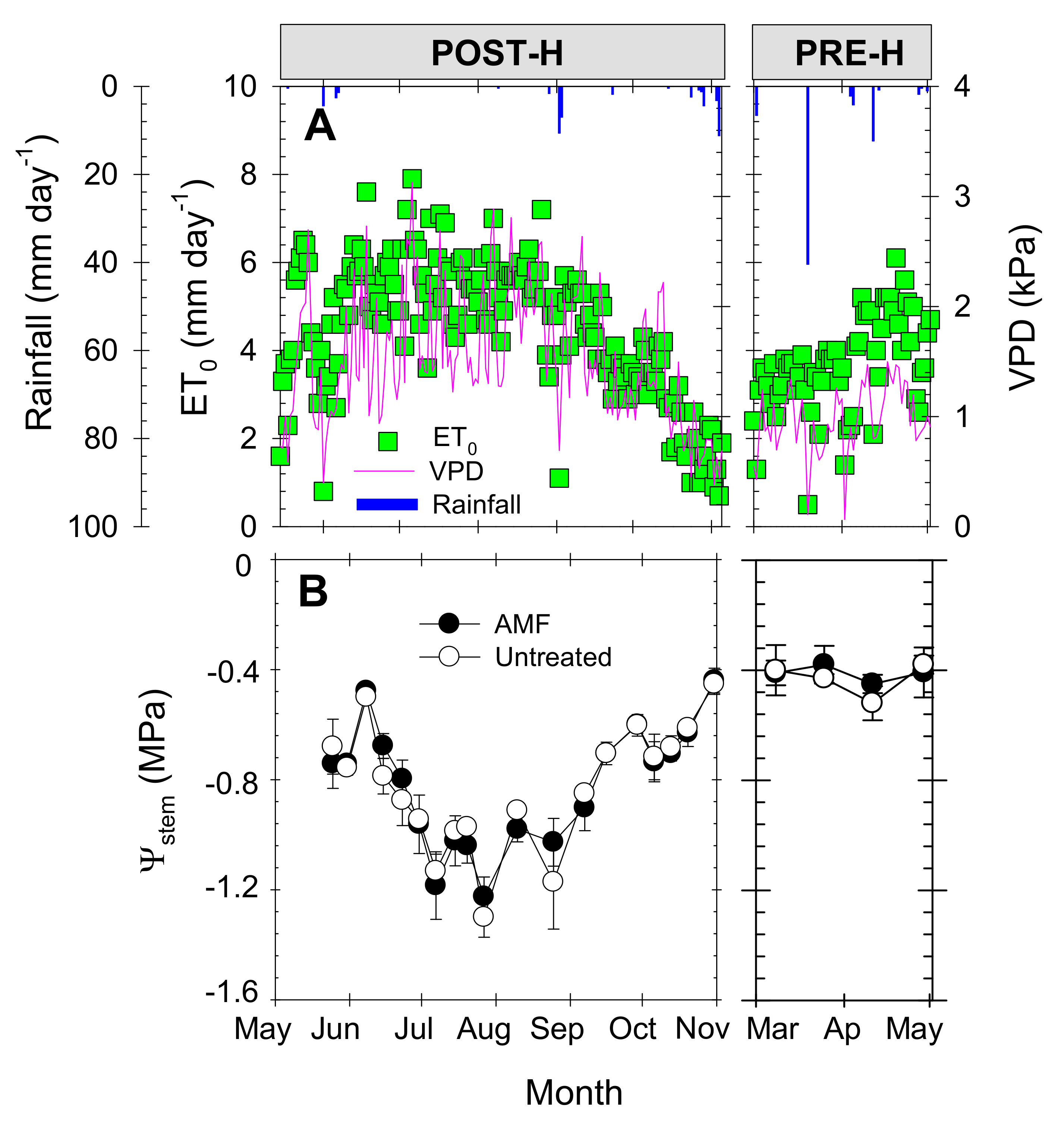
3.1. Meteorological Data and Plant Water Status
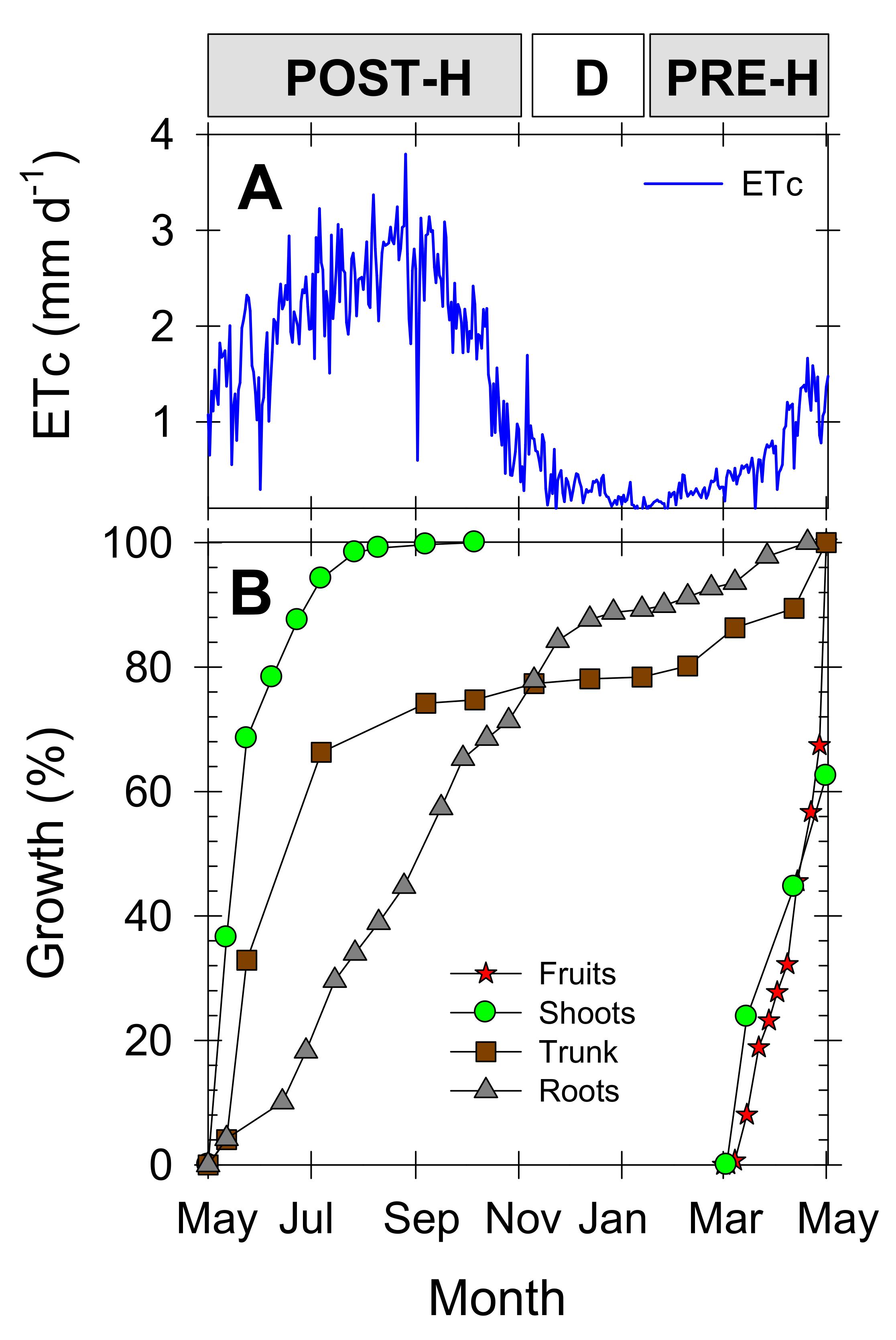
3.2. Roots vs. Aerial Growth Pattern
3.3. Yield Components and Productive Efficiencies
3.4. Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food and Agriculture Organization Statistical Data 2020. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 15 March 2021).
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.M.; Tsimplis, M.; et al. The Mediterranean climate: An overview of the main characteristics and issues. In Mediterranean Climate Variability; Lionello, P., Malanotte-Rizzoli, P., Boscolo, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 1–26. [Google Scholar]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef] [Green Version]
- English, M.J.; Solomon, K.H.; Hoffman, G.J. A Paradigm Shift in Irrigation Management. J. Irrig. Drain. Eng. 2002, 128, 267–277. [Google Scholar] [CrossRef]
- Passioura, J.B. Water transport in and to roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 245–256. [Google Scholar] [CrossRef]
- Vamerali, T.; Saccomani, M.; Bona, S.; Mosca, G.; Guarise, M.; Ganis, A. A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids. Plant Soil 2003, 157–167. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. (Eds.) Colonization of roots and anatomy of arbuscular mycorrhiza. In Mycorrhizal Symbiosis; Academic Press: London, UK, 2008; pp. 42–90. [Google Scholar]
- Sánchez-Blanco, M.J.; Álvarez, S.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Root System Response to Drought and Salinity: Root Distribution and Water Transport. In Root Engineering, Soil Biology; Springer: Berlin/Heidelberg, Germay, 2014; pp. 325–352. [Google Scholar] [CrossRef]
- Bucher, M.; Wegmüller, S.; Drissner, D. Chasing the structures of small molecules in arbuscular mycorrhizal signaling. Curr. Opin. Plant Biol. 2009, 12, 500–507. [Google Scholar] [CrossRef]
- Lǚ, L.-H.; Zou, Y.-N.; Wu, Q.-S. Mycorrhizas Mitigate Soil Replant Disease of Peach Through Regulating Root Exudates, Soil Microbial Population, and Soil Aggregate Stability. Commun. Soil Sci. Plant Anal. 2019, 50, 909–921. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis On Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Calvet, C.; Pinochet, J.; Hernández-Dorrego, A.; Estaún, V.; Camprubi, A. Field microplot performance of the peach-almond hybrid GF-677 after inoculation with arbuscular mycorrhizal fungi in a replant soil infested with root-knot nematodes. Mycorrhiza 2001, 10, 295–300. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, J.M.R.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Fernández, C.; Aroca, R.; Barea, J.M. Influence of arbuscular mycorrhizal fungi and water regime on the development of endemic Thymus species in dolomitic soils. Appl. Soil Ecol. 2011, 48, 31–37. [Google Scholar] [CrossRef]
- Posta, K.; Duc, N.H. Benefits of Arbuscular Mycorrhizal Fungi Application to Crop Production under Water Scarcity. 2020. Available online: https://www.intechopen.com/books/droughtdetection-and-solutions/benefits-of-arbuscular-mycorrhizal-fungi-application-to-crop-production-underwater-scarcity (accessed on 15 March 2020).
- Franco, J.A.; Abrisqueta, J.M. A comparison between minirhizotron and soil coring methods of estimating root distribution in young almond trees under trickle irrigation. J. Hortic. Sci. 1997, 72, 797–805. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Vicente-Sánchez, J.; Martín, F.F.; Bañón, S.; Sánchez-Blanco, M.J. Protective effects of Glomus iranicum var. tenuihypharum on soil and Viburnum tinus plants irrigated with treated wastewater under field conditions. Mycorrhiza 2015, 25, 399–409. [Google Scholar] [CrossRef]
- Nicolás, E.; Maestre-Valero, J.F.; Alarcón, J.J.; Pedrero, F.; Vicente-Sánchez, J.; Bernabé, A.; Fernández, F. Effectiveness and persistence of arbuscular mycorrhizal fungi on the physiology, nutrient uptake and yield of Crimson Seedless grapevine. J. Agric. Sci. 2015, 153, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.-L.; Liang, S.-M.; Chu, X.-N.; Yang, Y.-L.; Wu, Q.-S. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant Soil Environ. 2020, 66, 624–631. [Google Scholar] [CrossRef]
- Bland, W.L.; Mesarch, M.A. Counting error in the line-intercept method of measuring root length. Plant Soil 1990, 125, 155–157. [Google Scholar] [CrossRef]
- Abrisqueta, I.; Conejero, W.; López-Martínez, L.; Vera, J.; Sánchez, M.C.R. Root and aerial growth in early-maturing peach trees under two crop load treatments. Span. J. Agric. Res. 2017, 15, e0803. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.M. Minirhizotron Observation Tubes: Methods and Applications for Measuring Rhizosphere Dynamics; American Society of Agronomy: Madison, WI, USA, 1987; 143p. [Google Scholar]
- Upchurch, D.R. Conversion of minirhizotron-root intersections to root length density. In Minirhizotron Observation Tubes: Methods and Applications for Measuring Rhizosphere Dynamics; Taylor, H.M., Ed.; Wiley: Hoboken, NJ, USA, 1987; pp. 51–65. [Google Scholar]
- Machado, R.M.A.; Oliveira, M.D.R.G. Comparison of tomato root distributions by minirhizotron and destructive sampling. Plant Soil 2003, 255, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hammer, R.D.; Blanchar, R.W. Minirhizotron quantification of soybean root growth as affected by reduced A horizon in soil. J. Plant Nutr. Soil Sci. 2003, 166, 708–711. [Google Scholar] [CrossRef]
- Abrisqueta, J.; Mounzer, O.; Álvarez, S.; Conejero, W.; García-Orellana, Y.; Tapia, L.; Vera, J.; Abrisqueta, I.; Sánchez, M.C.R. Root dynamics of peach trees submitted to partial rootzone drying and continuous deficit irrigation. Agric. Water Manag. 2008, 95, 959–967. [Google Scholar] [CrossRef]
- Wells, C.E.; Glenn, D.M.; Eissenstat, D.M. Changes in the risk of fine-root mortality with age: A case study in peach, Prunus persica (Rosaceae). Am. J. Bot. 2002, 89, 79–87. [Google Scholar] [CrossRef]
- Bernier, P.Y.; Robitaille, G. A plane intersect method for estimating fine root productivity of trees from minirhizotron images. Plant Soil 2004, 265, 165–173. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Martín-Aranda, J.; Fereres, E. Olive-tree root dynamics under different soil water regimes. Agric. Mediterr. 1992, 122, 225–235. [Google Scholar]
- Conesa, M.R.; Martínez-López, L.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M. Summer pruning of early-maturing Prunus persica: Water implications. Sci. Hortic. 2019, 256, 108539. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; Available online: http://www.fao.org/docrep/x0490e/x0490e00.htm (accessed on 20 April 2011).
- Abrisqueta, I.; Abrisqueta, J.; Tapia, L.; Munguía, J.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M. Basal crop coefficients for early-season peach trees. Agric. Water Manag. 2013, 121, 158–163. [Google Scholar] [CrossRef]
- Fereres, E.; Martinich, D.A.; Aldrich, T.M.; Castel, J.R.; Holzapfel, E.; Schulbach, H. Drip irrigation saves money in young almond orchards. Calif. Agric. 1982, 36, 12–13. [Google Scholar]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Moens, M. First Report of the Cereal Cyst Nematode Heterodera latipons on Wheat in Morocco. Plant Dis. 2012, 96, 774. [Google Scholar] [CrossRef]
- Hsiao, T.C. Measurement of tree water status. In Irrigation of Agricultural Crops. Agronomy Monograph; Steward, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 243–279. [Google Scholar]
- Merrill, S.D.; Upchurch, D.R. Converting Root Numbers Observed at Minirhizotrons to Equivalent Root Length Density. Soil Sci. Soc. Am. J. 1994, 58, 1061–1067. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Good Practice Guidance for Land Use, Land-Use Change and Forestry; Penman, J., Gytarsky, M., Hiraishi, T., Krug, T., Kruger, D., Pipatti, R., Buendía, L., Miwa, K., Ngara, T., Tanabe, K., et al., Eds.; IPCC: Geneva, Switzerland, 2003. [Google Scholar]
- Carvajal, M.; Mota, C.; Alcaráz-López, C.; Iglesias, M.; Martínez-Ballesta, M.C. Investigación sobre la absorción de CO2 por los cultivos más representativos de la Región de Murcia. In Etiquetado de Carbono en Las Explotaciones y Productos Agrícolas: La Iniciativa Agricultura Murciana Como Sumidero de CO2; Consejería de Agricultura y Agua (CARM): Murcia, Spain, 2010; pp. 65–92. ISBN 978-84-693-6838-1. [Google Scholar]
- Hooker, J.E.; Munro, M.; Atkinson, D. Vesicular-arbuscular mycorrhizal fungi induced alteration in poplar root system morphology. Plant Soil 1992, 145, 207–214. [Google Scholar] [CrossRef]
- Alarcón, A.L.; Gómez-Bellot, M.J.; Bernabe, A.J.; Calvo, G.; Martín, F.F. Changes in root architecture and productivity of melon (Cucumis melo L. cv. Hispano Nunhems) promoted by Glomus iranicum var. tenuihypharum. J. Hortic. Sci. Biotechnol. 2019, 95, 364–373. [Google Scholar] [CrossRef]
- Alvarado-Raya, H.E. Peach seedling growth with mycorrhiza and vermicompost. Tecnociencia 2017, 9, 48–57. [Google Scholar]
- Giovanetti, M.; Mosse, B. An evolution of techniques to measure vesicular-arbuscular infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; Li, G.H.; Zou, Y.N. Roles of arbuscular mycorrhizal fungi on growth and nutrient acquisition of peach (Prunus persica L. Batsch) seedlings. J. Anim. Plant. Sci. 2011, 21, 746–750. [Google Scholar]
- Abbott, L.; Robson, A. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Baldi, E.; Amadei, P.; Pelliconi, F.; Tosell, M. Use of Tricho derma spp. and arbuscular mycorrhizal fungi to increase soil beneficial population of bacteria in a nectarine commercial orchard: Effect on root growth, nutrient acquisition and replanting disease. J. Plant Nutr. 2015, 39, 1147–1155. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gianinazzi-Pearson, V.; Tisserant, B.; Lemoine, M.C. Protein Activities as Potential Markers of Functional Endomycorrhizae in Planta. In Mycorrhizas in Ecosystems; Read, D.J., Lewis, D.H., Fitter, A.H., Alexander, I.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 333–339. [Google Scholar]
- Amaya-Carpio, L.; Davies, F.; Fox, T.; He, C. Arbuscular mycorrhizal fungi and organic fertilizer influence photosynthesis, root phosphatase activity, nutrition, and growth of Ipomoea carnea ssp. Fistulosa. Photosynthetica 2009, 47, 1–10. [Google Scholar] [CrossRef]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Hendrick, R.L.; Fogel, R. The demography of fine roots in response to patches of water and nitrogen. New Phytol. 1993, 125, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.; Campbell, C.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [Green Version]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Ross, N.W.; Catlin, P.B. Rootstocks and root physiology. In Almond Orchard Management; Micke, W., Kester, D., Eds.; Division of Agricultural Sciences, University of California: Berkeley, CA, USA, 1978; pp. 25–29. [Google Scholar]
- Pastor, A.P.; Sánchez, M.C.R.; Domingo, R.; Torrecillas, A. Growth and phenological stages of Búlida apricot trees in south-east Spain. Agronomie 2004, 24, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, D.J.; Van den Ende, B. Productivity of peach trees. Factors affecting dry weight distribution during tree growth. Ann. Bot. 1975, 39, 423–432. [Google Scholar] [CrossRef]
- Sorrenti, G.; Muzzi, E.; Toselli, M. Root growth dynamic and plant performance of nectarine trees amended with biochar and compost. Sci. Hortic. 2019, 257, 108710. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No 1221/2008 of 5 December 2008. Amending Regulation (EC) No 1580/2007 laying down implementing rules of Council Regulations (EC) No 2200/96, (EC) No 2201/96 and (EC) No 1182/2007 in the fruit and vegetable sector as regards marketing standards. Off. J. Eur. Union 2008, 51, 80. [Google Scholar]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Slawomir, S.; Aleksander, S. The influence of mycorrhizal fungi on the growth and yield of plum and sour cherry trees. J. Fruit Ornam. Plant Res. 2010, 18, 71–77. [Google Scholar]
- Sharma, S.D.; Bhutani, V.P.; Dohroo, N.P. Occurrence of VAM fungi under old apple orchards. J. Indian Soc. Soil Sci. 1998, 46, 143–144. [Google Scholar]
- Sharma, S.; Sharma, N.; Sharma, C.; Sood, R.; Singh, R. Studies on Correlations Between Endomycorrhizal and Azotobacter Population With Growth, Yield And Soil Nutrient Status Of Apple (Malus domestica Borkh) Orchards in Himachal Pradesh. Acta Hortic. 2005, 283–287. [Google Scholar] [CrossRef]
- Awasthi, R.P.; Godara, R.K.; Kaith, N.S. Correlation between VA-mycorrhizae spore number, root colonization, Azotobacter population and fruit yield of July Elberta peach. J. Hill Res. 1999, 12, 1–4. [Google Scholar] [CrossRef]
- Shrestha, Y.H.; Ishii, T.; Matsumoto, I.; Kadoya, K. Effects of Vesicular-Arbuscular Mycorrhizal Fungi on Satsuma Mandarin Tree Growth and Water Stress Tolerance and on Fruit Development and Quality. J. Jpn. Soc. Hortic. Sci. 1996, 64, 801–807. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995, 95, 472–478. [Google Scholar] [CrossRef]
- Conesa, M.; Espinosa, P.; Pallarés, D.; Pérez-Pastor, A. Influence of Plant Biostimulant as Technique to Harden Citrus Nursery Plants before Transplanting to the Field. Sustainability 2020, 12, 6190. [Google Scholar] [CrossRef]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. Hortic. Rev. 2010, 111–165. [Google Scholar] [CrossRef]
- Conesa, M.R.; Conejero, W.; Vera, J.; Agulló, V.; García-Viguera, C.; Ruiz-Sánchez, M.C. Irrigation management practices in nectarine fruit quality at harvest and after cold storage. Agric. Water Manag. 2021, 243, 106519. [Google Scholar] [CrossRef]
- López, G.; Echeverria, G.; Bellvert, J.; Mata, M.; Behboudian, M.H.; Girona, J.; Marsal, J. Water stress for a short period before harvest in nectarine: Yield, fruit composition, sensory quality, and consumer acceptance of fruit. Sci. Hortic. 2016, 211, 1–7. [Google Scholar] [CrossRef]


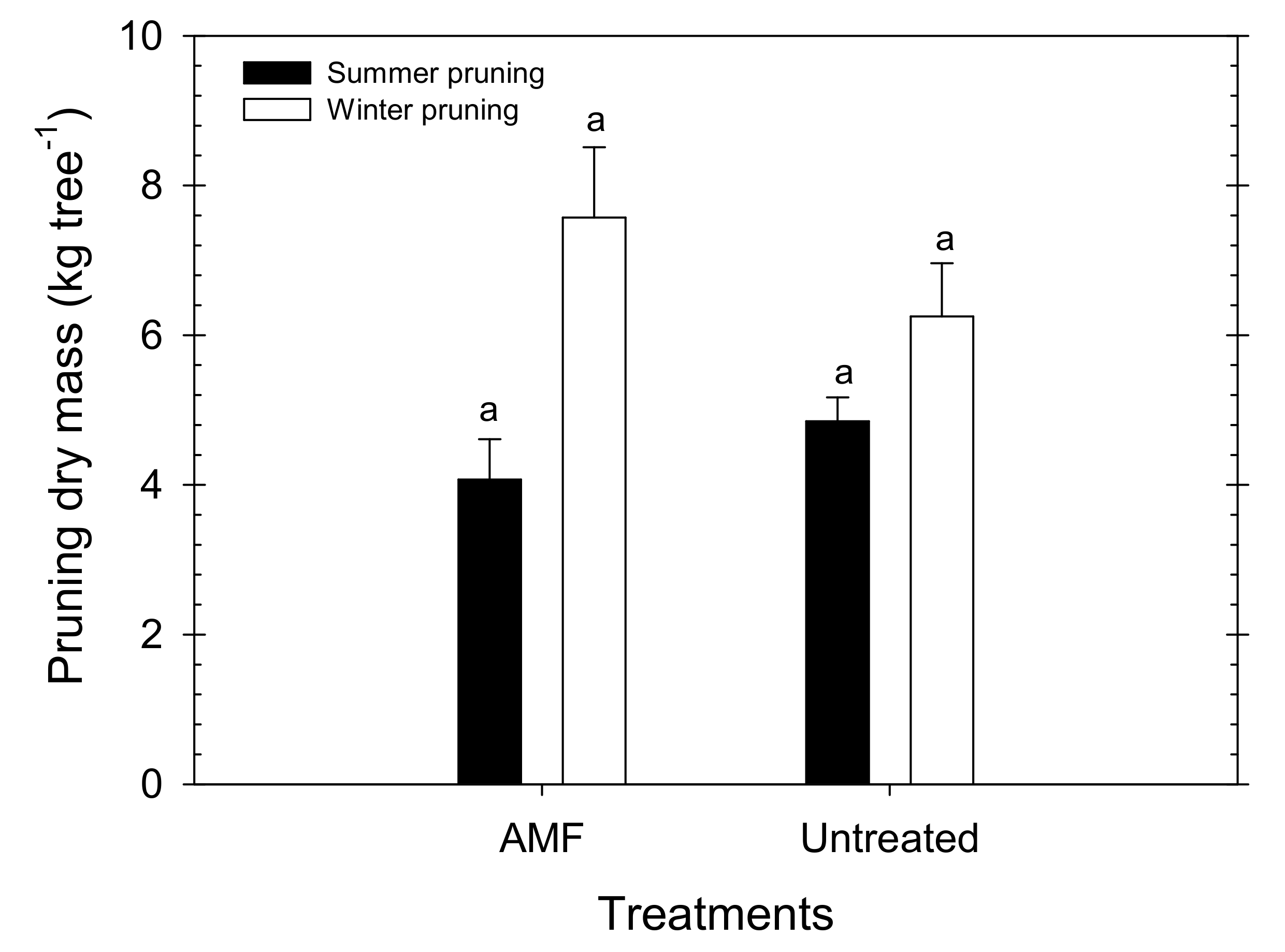
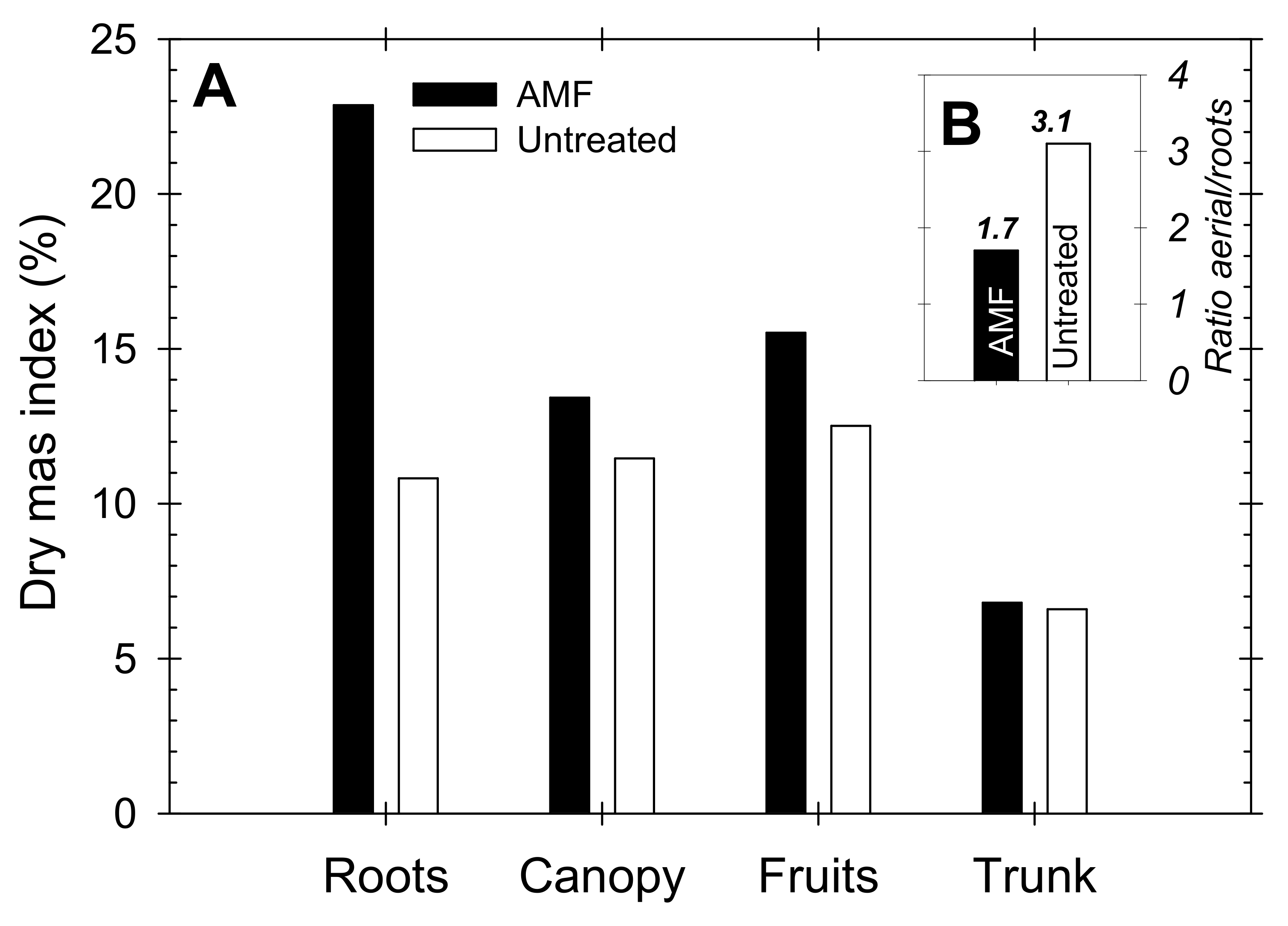
| Yield Components | AMF | Untreated | ANOVA |
|---|---|---|---|
| Yield (kg tree−1) | 20.57 a | 16.59 b | * |
| First picking date (%) | 54 | 52 | ns |
| Second picking date (%) | 46 | 48 | ns |
| Crop load (fruit tree−1) | 146 a | 116 b | * |
| Canopy area (m2) | 7.57 | 6.83 | ns |
| Canopy volume (m3) | 16.20 | 13.83 | ns |
| Trunk cross sectional area (TCSA, cm−2) | 62.09 | 58.38 | ns |
| Crop load efficiency (CLE, fruit m−2 canopy) | 21.37 a | 17.01 b | * |
| Productive efficiency (PE, kg cm−2) | 0.33 | 0.28 | ns |
| Irrigation water use efficiency (IWUE. kg m−3) | 4.63 a | 3.73 b | * |
| First Picking | Second Picking | Average | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit Components | AMF | Untreated | ANOVA | AMF | Untreated | ANOVA | AMF | Untreated | ANOVA |
| Fruit height (mm) | 61.24 | 61.02 | ns | 60.97 | 62.05 | ns | 61.13 | 61.54 | ns |
| Fruit diameter (mm) | 65.66 a | 64.84 b | * | 63.99 | 63.66 | ns | 65.25 a | 64.25 b | * |
| Fruit mass (g) | 149.27 | 147.74 | ns | 139.46 | 138.69 | ns | 148.51 | 143.22 | ns |
| TSS (°Brix) | 11.83 | 12.12 | ns | 13.66 | 13.65 | ns | 11.98 | 12.89 | ns |
| Lightness (L*) | 37.93 b | 41.42 a | * | 37.52 | 38.16 | ns | 39.68 | 39.79 | ns |
| Chrome (C*) | 24.45 | 27.04 | ns | 27.01 | 27.77 | ns | 25.75 | 27.41 | ns |
| Hue angle (h°) | 17.76 b | 26.93 a | * | 23.32 | 22.17 | ns | 22.35 | 24.55 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, M.R.; López-Martínez, L.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Arbuscular Mycorrhizal Fungus Stimulates Young Field-Grown Nectarine Trees. Sustainability 2021, 13, 8804. https://doi.org/10.3390/su13168804
Conesa MR, López-Martínez L, Conejero W, Vera J, Ruiz-Sánchez MC. Arbuscular Mycorrhizal Fungus Stimulates Young Field-Grown Nectarine Trees. Sustainability. 2021; 13(16):8804. https://doi.org/10.3390/su13168804
Chicago/Turabian StyleConesa, María R., Lidia López-Martínez, Wenceslao Conejero, Juan Vera, and María Carmen Ruiz-Sánchez. 2021. "Arbuscular Mycorrhizal Fungus Stimulates Young Field-Grown Nectarine Trees" Sustainability 13, no. 16: 8804. https://doi.org/10.3390/su13168804
APA StyleConesa, M. R., López-Martínez, L., Conejero, W., Vera, J., & Ruiz-Sánchez, M. C. (2021). Arbuscular Mycorrhizal Fungus Stimulates Young Field-Grown Nectarine Trees. Sustainability, 13(16), 8804. https://doi.org/10.3390/su13168804







