Do Morphological Traits Predict Ecological Guilds of the Mekong Fish Fauna?
Abstract
:1. Introduction
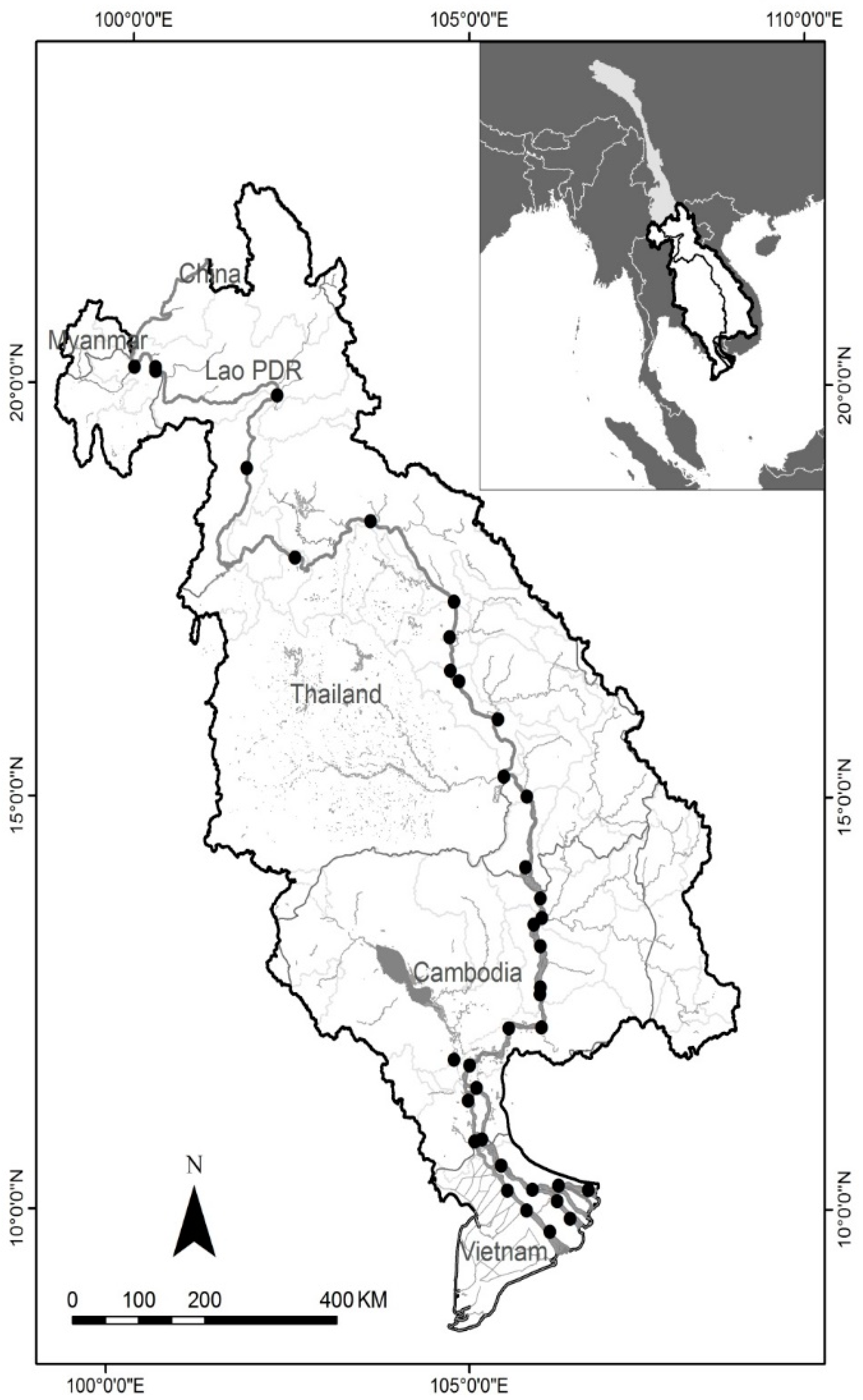
2. Materials and Methods
2.1. Fish Guilds and Morphological Trait Data
2.2. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziv, G.; Baran, E.; Nam, S.; Rodríguez-Iturbe, I.; Levin, S.A. Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin. Proc. Natl. Acad. Sci. USA 2012, 109, 5609–5614. [Google Scholar] [CrossRef] [Green Version]
- Baran, E.; So, N.; Degen, P.; Chen, X.-Y.; Starr, P. Updated information on fish and fisheries in the Mekong Basin. Catch Cult. 2013, 19, 24–25. [Google Scholar]
- Baran, E. Fish Migration Triggers in the Lower Mekong Basin and Other Tropical Freshwater Systems; MRC Technical Paper No. 14; MRC: Vientiane, Laos, 2006. [Google Scholar]
- Kummu, M.; Sarkkula, J. Impact of the Mekong River Flow Alteration on the Tonle Sap Flood Pulse. Ambio 2008, 37, 185–192. [Google Scholar] [CrossRef]
- Winemiller, K.O. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 1989, 81, 225–241. [Google Scholar] [CrossRef]
- Winemiller, K.O. Life history strategies, population regulation, and implications for fisheries management. Can. J. Fish. Aquat. Sci. 2005, 62, 872–885. [Google Scholar] [CrossRef]
- Toussaint, A.; Charpin, N.; Brosse, S.; Villéger, S. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci. Rep. 2016, 6, 22125. [Google Scholar] [CrossRef] [Green Version]
- Valbo-Jørgensen, J.; Coates, D.; Hortle, K. Fish Diversity in the Mekong River Basin. In The Mekong Biophysical Environment of An International River Basin; Campbell, I.C., Ed.; Elsevier Academic Press: New York, NY, USA, 2009; pp. 161–196. ISBN 9780123740267. [Google Scholar]
- Frimpong, E.A.; Angermeier, P.L. Trait-based approaches in the analysis of stream fish communities. Am. Fish. Soc. Symp. 2010, 73, 109–136. [Google Scholar]
- Assumpção, L.D.; Makrakis, M.C.; Makrakis, S.; Piana, P.A.; Silva, P.S.D.; Lima, A.F.D.; Fernandez, D.R. Morphological differentiation among migratory fish species from the Paraná River basin. Biota Neotrop. 2012, 12, 41–49. [Google Scholar] [CrossRef]
- Shuai, F.; Yu, S.; Lek, S.; Li, X. Habitat effects on intra-species variation in functional morphology: Evidence from freshwater fish. Ecol. Evol. 2018, 8, 10902–10913. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hulthén, K.; Brönmark, C.; Nilsson, P.A.; Skov, C.; Hansson, L.A.; Brodersen, J. Shape up or ship out: Migratory behaviour predicts morphology across spatial scale in a freshwater fish. J. Anim. Ecol. 2015, 84, 1187–1193. [Google Scholar] [CrossRef]
- Mekong River Commission Study on Sustainable Management and Development of the Mekong River including Impacts of Mainstream Hydropower Projects; MRC: Vientiane, Laos, 2018; Volume 4, pp. 1–129.
- So, N.; Leng, S.V.; Baran, E.; Arthur, R. An Evaluation of Fish Species and Genetic Diversity of the Tonle Sap Great Lake, Cambodia. In Proceedings of the International Workshop and Training on Fish Diversity of the Mekong River, Sendai, Japan, 17–20 November 2006. [Google Scholar]
- Phomikong, P.; Fukushima, M.; Sricharoendham, B.; Nohara, S.; Jutagate, T. Diversity and Community Structure of Fishes in the Regulated Versus Unregulated Tributaries of the Mekong River. River Res. Appl. 2015, 31, 1262–1275. [Google Scholar] [CrossRef]
- Baran, E.; Guerin, E.; Nasielski, J. Fish, Sediments, and Dams in the Mekong; WorldFish and CGIAR Research Program on Water, Land and Ecosystem (WLE): Vientiane, Laos, 2015; Volume 53, ISBN 9788578110796. [Google Scholar]
- Villéger, S.; Brosse, S.; Mouchet, M.; Mouillot, D.; Vanni, M.J. Functional ecology of fish: Current approaches and future challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Cilleros, K.; Allard, L.; Grenouillet, G.; Brosse, S. Taxonomic and functional diversity patterns reveal different processes shaping European and Amazonian stream fish assemblages. J. Biogeogr. 2016, 43, 1832–1843. [Google Scholar] [CrossRef]
- Manna, L.R.; Villéger, S.; Rezende, C.F.; Mazzoni, R. High intraspecific variability in morphology and diet in tropical stream fish communities. Ecol. Freshw. Fish 2018, 28, 41–52. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 10 June 2018).
- Dray, S.; Dufour, A.B. The ade4 package:implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Dodédec, S.; Chessel, D. Recent developments in linear ordination methods in environmental sciences. Adv. Ecol. 1991, 1, 133–155. [Google Scholar]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Caret: Classification and Regreession Training 2020. R Package Version 6.0-86. Available online: https://CRAN.R-project.org/package=caret (accessed on 10 June 2018).
- R Core Team R:A Language and Environment for Stastical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018.
- Blake, R.W. Fish functional design and swimming performance. J. Fish Biol. 2004, 65, 970–983. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of fish swimming modes for aquatic locomotion. IEEE J. Ocean Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Dumay, O.; Tari, P.S.; Tomasini, J.A.; Mouillot, D. Functional groups of lagoon fish species in Languedoc Roussillon, Southern France. J. Fish Biol. 2004, 64, 970–983. [Google Scholar] [CrossRef]
- Gatz, A.J. Community Organization in Fishes as Indicated by Morphological Features. Ecology 1979, 60, 711–718. [Google Scholar] [CrossRef]
- Rainboth, W. The Family Cyprinidae (Carps). Mekong Fish. Netw. Newsl. 1998, 3, 1–2. [Google Scholar]
- Lucas, M.C.; Baras, E.; Thom, T.J.; Duncan, A.; Slavik, O. Migration of Freshwater Fishes; Blackwell Science Ltd.: Oxford, UK, 2001; ISBN 0632057548. [Google Scholar] [CrossRef]
- Tamario, C.; Sunde, J.; Petersson, E.; Tibblin, P.; Forsman, A. Ecological and Evolutionary Consequences of Environmental Change and Management Actions for Migrating Fish. Front. Ecol. Evol. 2019, 7, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ngor, P.B.; Grenouillet, G.; Phem, S.; So, N.; Lek, S. Spatial and temporal variation in fish community structure and diversity in the largest tropical flood-pulse system of South-East Asia. Ecol. Freshw. Fish 2018, 27, 1087–1100. [Google Scholar] [CrossRef]
- Chea, R.; Lek, S.; Ngor, P.; Grenouillet, G. Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol. Freshw. Fish 2017, 26, 575–585. [Google Scholar] [CrossRef]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef]
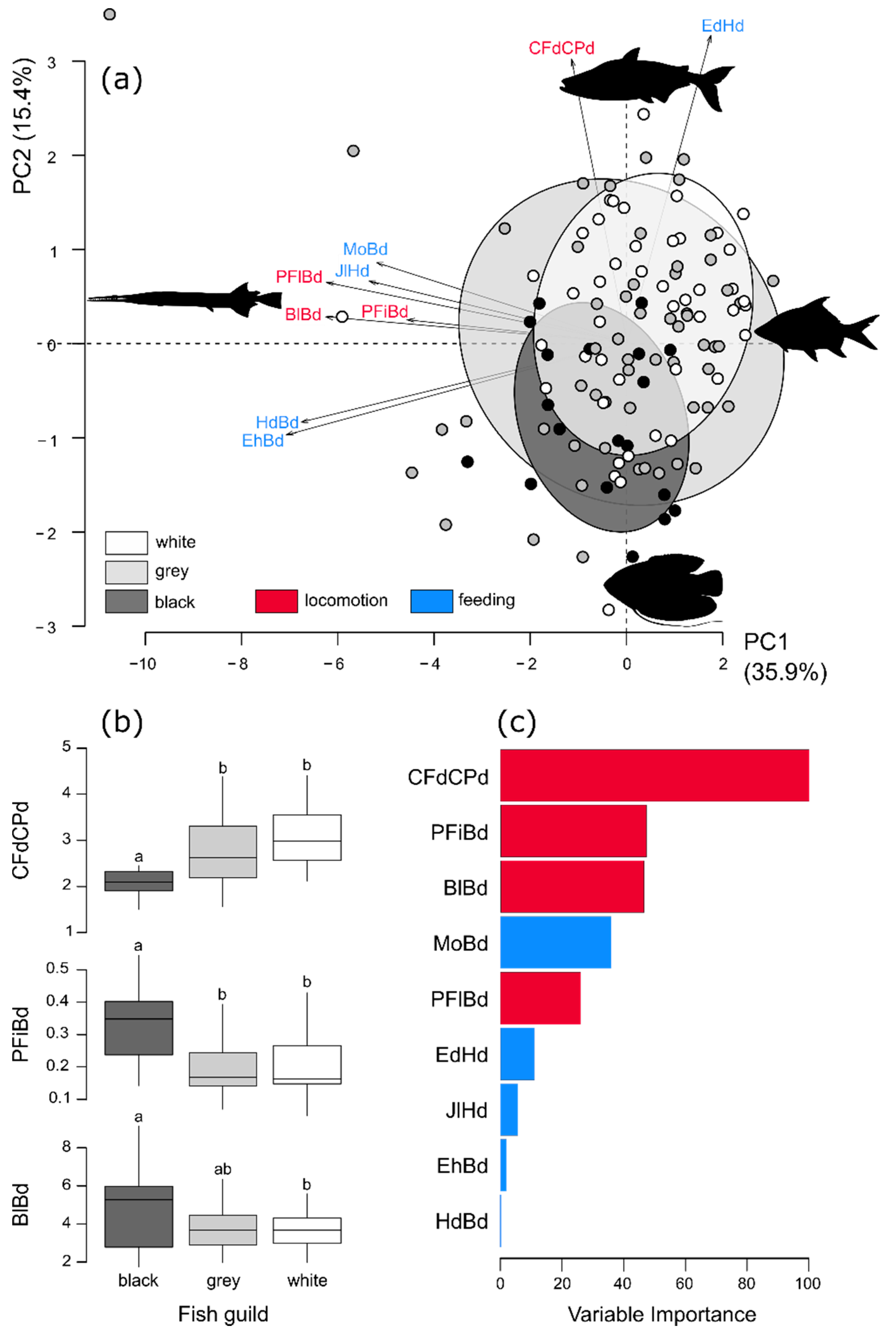
| Traits | Code | Description | Potential Links to Fish Functions |
|---|---|---|---|
| Bl/Bd | BlBd | Body elongation | Hydrodynamism |
| Eh/Bd | EhBd | Eye vertical position | Position of fish and/or of its prey in the water column |
| Ed/Hd | EdHd | Relative eye size | Visual acuity |
| Mo/Bd | MoBd | Oral gape position | Feeding position in the water column |
| Jl/Hd | JlHd | Relative maxillary length | Size of mouth and strength of jaw |
| Hd/Bd | HdBd | Body lateral shape | Hydrodynamism and head size |
| PFi/Bd | PFiBd | Pectoral fin vertical position | Pectoral fin use for swimming |
| PFl/Bd | PFlBd | Pectoral fin size | Pectoral fin use for swimming |
| CFd/CPd | CFdCPd | Caudal peduncle throttling | Caudal propulsion efficiency through reduction of drag |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chea, R.; Brosse, S.; Lek, S.; Grenouillet, G. Do Morphological Traits Predict Ecological Guilds of the Mekong Fish Fauna? Sustainability 2021, 13, 8401. https://doi.org/10.3390/su13158401
Chea R, Brosse S, Lek S, Grenouillet G. Do Morphological Traits Predict Ecological Guilds of the Mekong Fish Fauna? Sustainability. 2021; 13(15):8401. https://doi.org/10.3390/su13158401
Chicago/Turabian StyleChea, Ratha, Sebastien Brosse, Sovan Lek, and Gaël Grenouillet. 2021. "Do Morphological Traits Predict Ecological Guilds of the Mekong Fish Fauna?" Sustainability 13, no. 15: 8401. https://doi.org/10.3390/su13158401
APA StyleChea, R., Brosse, S., Lek, S., & Grenouillet, G. (2021). Do Morphological Traits Predict Ecological Guilds of the Mekong Fish Fauna? Sustainability, 13(15), 8401. https://doi.org/10.3390/su13158401






