Grain Density-Based Approaches to Predict the Mechanical, Thermal and Hygric Properties of Carbon-Negative Aggregate Concretes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbonation Aggregates and Environmental Impact
+zCO2 → C(x−z)SHy + zCaCo3
2.2. Multi-Scale Characterization Methodology
2.2.1. Aggregate Scale
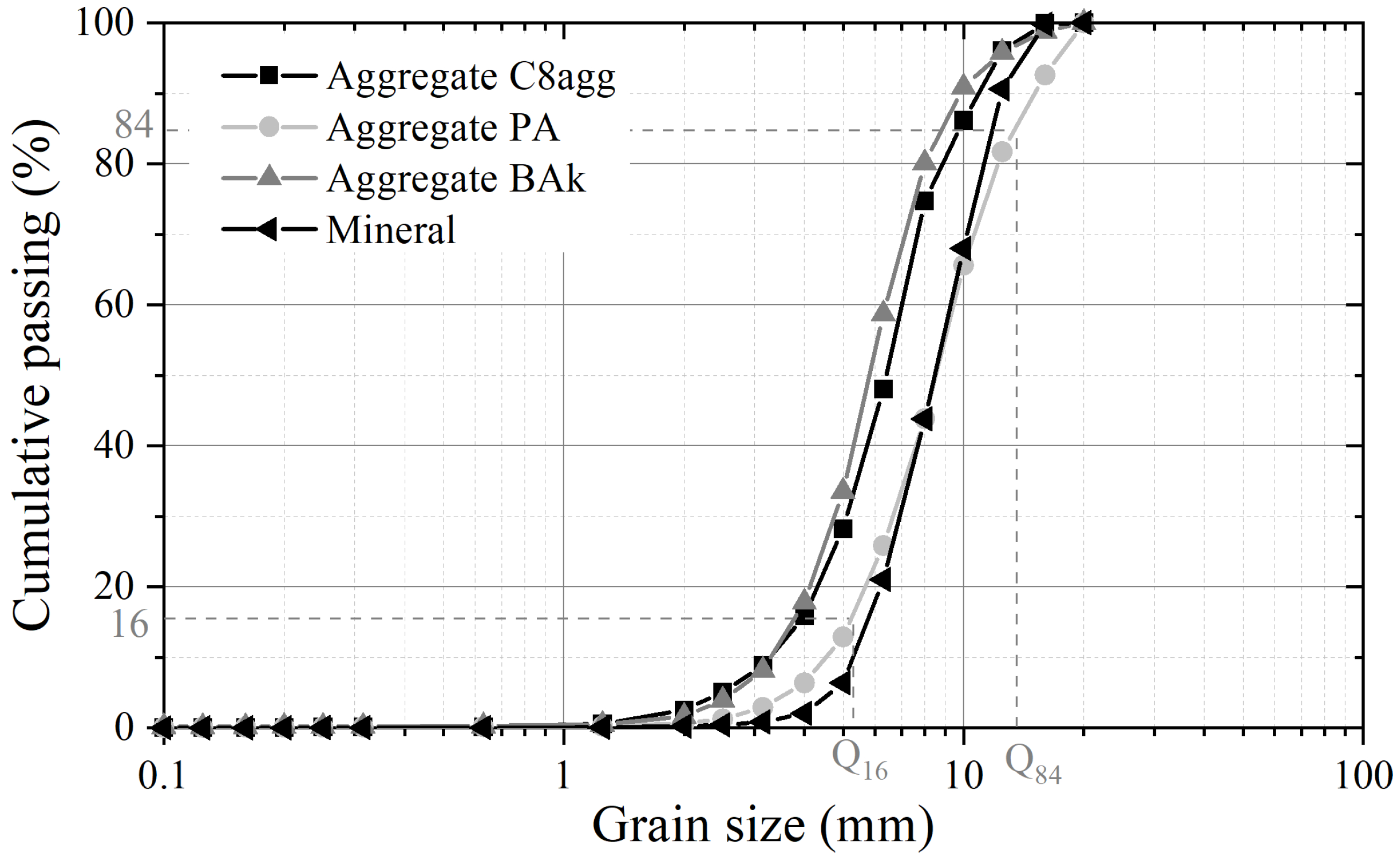
- The identification of granulometry by the sieve method, under dry conditions, according to EN 933-1. The expressions of the differential distributions and cumulative distributions are given by Equation (1), from the amount of material retained in each sieve’s i:The granular class of the aggregates is deduced from the interpretation of the granulometric curves, based on the following acceptability criteria:The granular range can be quantified using the uniformity coefficient and the curve coefficient , whose expressions are:where the percentiles, expressed in mm, are deduced from the granulometric curve. The particle size dispersion is translated by the Trask sorting index and the Folk and Ward skewness index , for which the quartiles are the of the inverse of the percentiles :The symmetry of the granulometric curves is approximated by the Krumbein asymmetry index defined as follows:
- Measurement of bulk, true and absolute densities by the pycnometric method using toluene in order to limit the risk of mineral dissolution (toluene is a non-polar solvent);
- Calculation of total, interparticle and intraparticle porosities using the following equation system:
- Identification of the crush-resistance through a Shimadzu electro-mechanical press with a nominal capacity of 250 kN, equipped with a hollow cylindrical piston (100 mm height and 200 mm2 cross-section), according to EN 13055-1.
2.2.2. Concrete Scale
3. Experimental Identification of Multi-Physic Properties
3.1. Physico-Mechanical Properties
3.2. Thermal Performances
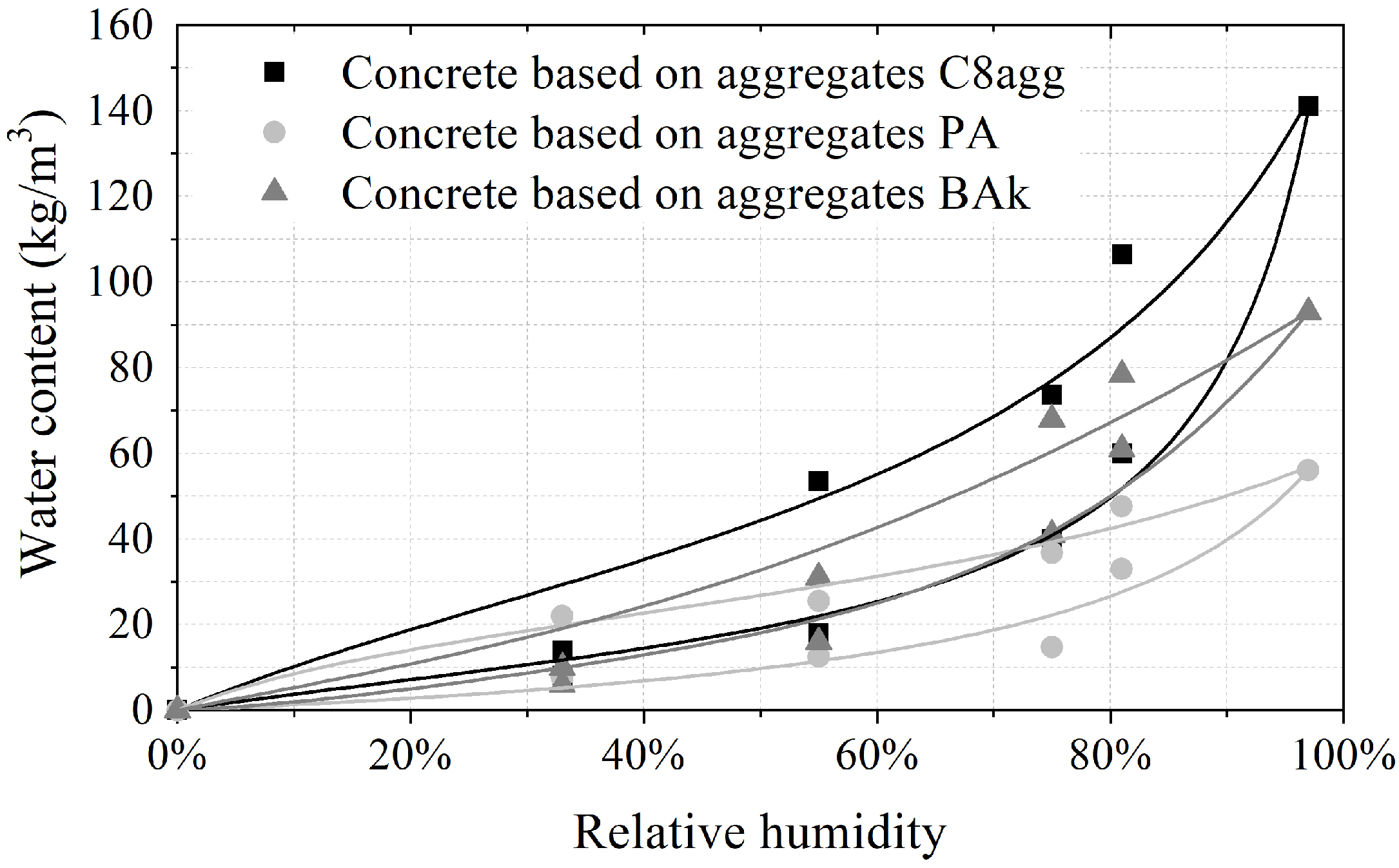
3.3. Hygric Characterization
4. Mechanical, Thermal and Hygric Predictive Approaches
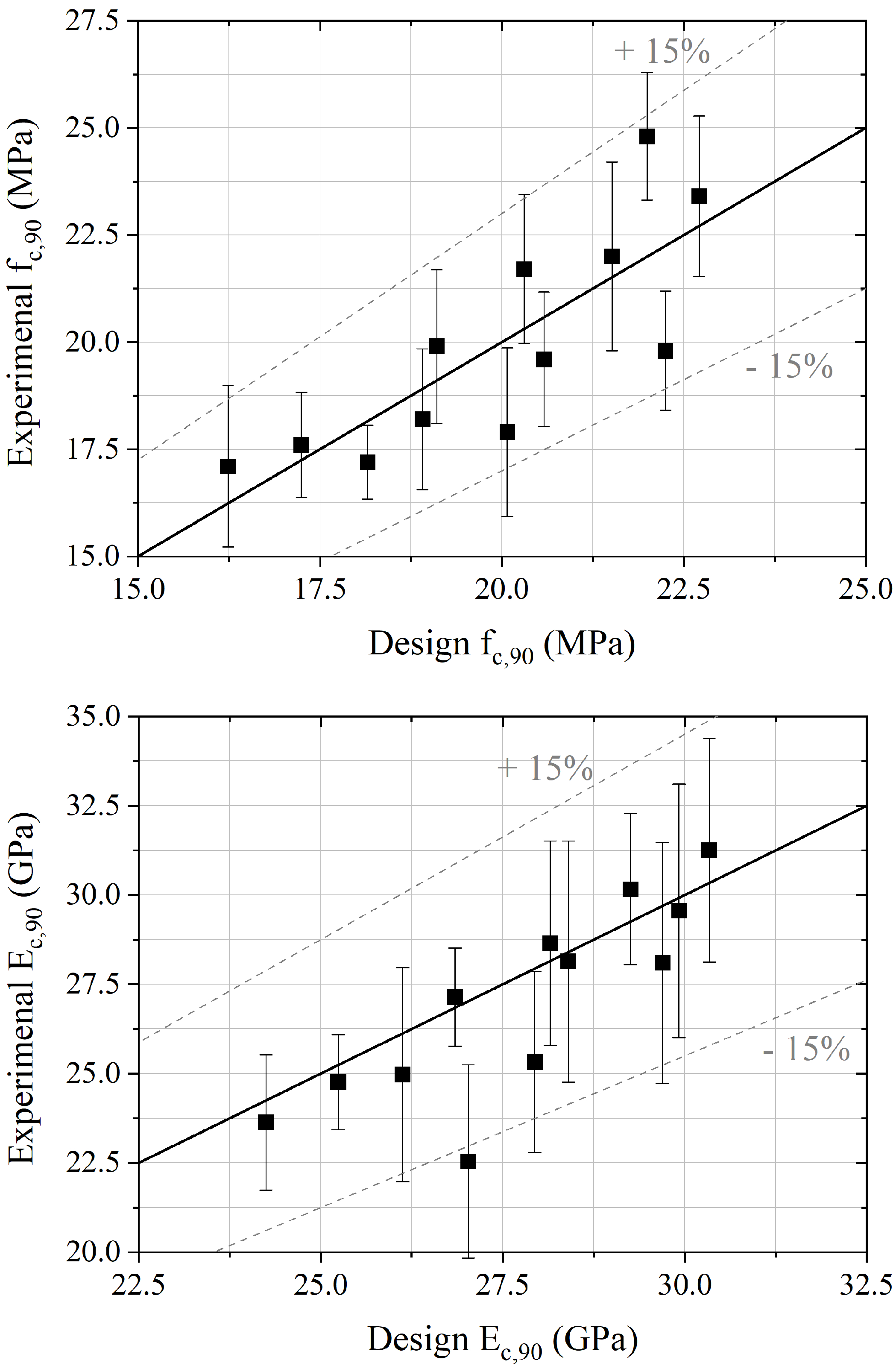
4.1. Empirical Mechanical Model
4.2. Prediction of the Thermal Characteristics
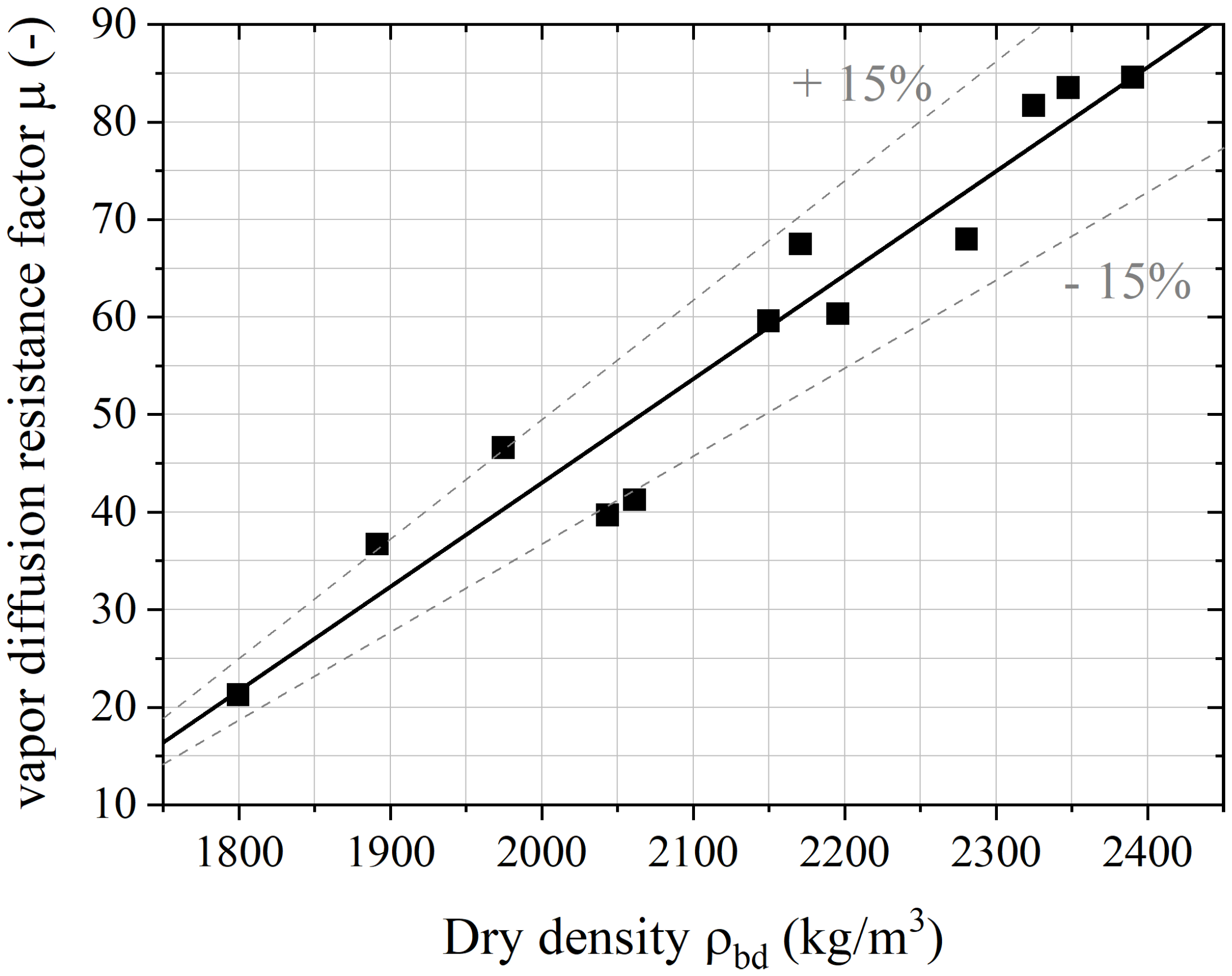
4.3. Simplified Theoretical Approach of the Hygric Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| porosity | |
| total | |
| interparticle | |
| intraparticle | |
| absolute | |
| true | |
| density, kg/m | |
| a | thermal diffusivity, m/s |
| E | thermal effusivity, J/(mK·s) |
| specific heat capacity, J/(kg·K) | |
| compressive strength, MPa | |
| modulus of elasticity, MPa | |
| carbonated | |
| mineral | |
| substitution rate, % | |
| experimental | |
| aggregate | |
| s | solid |
| hygric diffusivity, m/s | |
| moisture buffer value, kg/(m%RH) | |
| hygric effusivity, kg/(mPa·s) | |
| vapor saturation pressure, Pa | |
| vapor permeability, kg/(m·s·Pa) |
References
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Characterization of lightweight aggregates manufactured from washing aggregate sludge and fly ash. Resour. Conserv. Recycl. 2009, 53, 571–581. [Google Scholar] [CrossRef]
- Behera, M.; Bhattacharyya, S.; Minocha, A.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar]
- Evangelista, L.; de Brito, J. Mechanical behaviour of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2007, 29, 397–401. [Google Scholar] [CrossRef]
- Poon, C.; Kou, S.; Lam, L. Use of recycled aggregates in molded concrete bricks and blocks. Constr. Build. Mater. 2002, 16, 281–289. [Google Scholar] [CrossRef]
- Rao, A.; Jha, K.N.; Misra, S. Use of aggregates from recycled construction and demolition waste in concrete. Resour. Conserv. Recycl. 2007, 50, 71–81. [Google Scholar] [CrossRef]
- Kayali, O. Fly ash lightweight aggregates in high performance concrete. Constr. Build. Mater. 2008, 22, 2393–2399. [Google Scholar] [CrossRef]
- Radonjanin, V.; Malešev, M.; Marinković, S.; Al Malty, A. Green recycled aggregate concrete. Constr. Build. Mater. 2013, 47, 1503–1511. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef] [Green Version]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- INSEE. Indicateurs de Développement Durable en Champagne-Ardenne: La Production des Granulats; Insee dossier n°34; INSEE: Malakoff, France, 2011; pp. 21–22. [Google Scholar]
- Chen, T.; Bai, M.; Gao, X. Carbonation curing of cement mortars incorporating carbonated fly ash for performance improvement and CO2 sequestration. J. CO2 Util. 2021, 51, 101633. [Google Scholar] [CrossRef]
- Cerezo, V. Propriétés Mécanique, Thermique et Acoustique d’un Matériau à Base de Particules Végétales: Approche Expérimentale et Modélisation Théorique. Ph.D. Thesis, Université de Lyon, Lyon, France, 2005. [Google Scholar]
- Latif, E.; Lawrence, M.; Shea, A.; Walker, P. Moisture buffer potential of experimental wall assemblies incorporating formulated hemp-lime. Build. Environ. 2015, 93, 199–209. [Google Scholar] [CrossRef] [Green Version]
- BPEL-91. Régles Techniques de Conception et de Calcul des Ouvrages et Constructions en Béton; Eyrolles: Paris, France, 1993. [Google Scholar]
- Arnould, M.; Virlogeux, M. Granulats et Bétons Légers; Presse de l’ENPC: Toulon, France, 1986. [Google Scholar]
- ACI Committee 318. Building Code Requirements for Structural Concrete and Commentary (ACI 318M-11); ACI Committee: Farmington Hills, MI, USA, 2011; p. 520. [Google Scholar] [CrossRef]
- EN 1992-1-1. EuroCode 2: Design of Concrete Structures: General Rules and Rules for Buildings. 2007. Available online: https://www.phd.eng.br/wp-content/uploads/2015/12/en.1992.1.1.2004.pdf (accessed on 21 July 2021).
- Zhang, M.; Gjorv, O. Mechanical properties of high-strength lightweight concrete. ACI Mater. J. 1991, 88, 240–247. [Google Scholar] [CrossRef]
- Slate, F.; Nilson, A.; Martinez, S. Mechanical properties of high-strength lightweight concrete. J. Am. Concr. Inst. 1986, 83, 606–613. [Google Scholar] [CrossRef]
- Ke, Y. Caractérisation du Comportement Mécanique des Bétons de Granulats Légers: Expérience et Modélisation. Ph.D. Thesis, Université de Cergy Pontoise, Cergy, France, 2008. [Google Scholar]
- Ren, M.; Wen, X.; Gao, X.; Liu, Y. Thermal and mechanical properties of ultra-high performance concrete incorporated with microencapsulated phase change material. Constr. Build. Mater. 2021, 273, 121714. [Google Scholar] [CrossRef]
- Collet, F.; Pretot, S. Thermal conductivity of hemp concretes: Variation with formulation, density and water content. Constr. Build. Mater. 2014, 65, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Rahim, M. Analyse et Caractérisation du Comportement Hygrothermique de Parois Agro-Sourcées à L’échelle 1: Expérimentation et Modélisation. Ph.D. Thesis, Universite de Picardie Jules Verne, Amiens, France, 2015. [Google Scholar]
- Hashin, Z.; Shtrikman, S. A variational approach to the theory of the elastic behaviour of multiphase materials. J. Mech. Phys. Solids 1963, 11, 127–140. [Google Scholar] [CrossRef]
- Bederina, M.; Marmoret, L.; Mezreb, K.; Khenfer, M.; Bali, A.; Quéneudec, M. Effect of the addition of wood shavings on thermal conductivity of sand concretes: Experimental study and modelling. Constr. Build. Mater. 2007, 21, 662–668. [Google Scholar] [CrossRef]
- Rode, P. Moisture Buffering of Building Materials; Report BYG DTU R-126; Deparment of Civil Engineering Technical University of Denmark: Lyngby, Denmark, 2005. [Google Scholar]
- Gunning, P.; Hills, C.; Carey, P. Production of lightweight aggregate from industrial waste and carbon dioxide. Waste Manag. 2009, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Fernández Bertos, M.; Simons, S.; Hills, C.; Carey, P. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Rahmouni, I.; Promis, G.; R’mili, A.; Beji, H.; Limam, O. Effect of carbonated aggregates on the mechanical properties and thermal conductivity of eco-concrete. Constr. Build. Mater. 2019, 197, 241–250. [Google Scholar] [CrossRef]
- Rode, C.; Clorius, C. Modeling of moisture transport in wood with hysteresis and temperature dependent sorption characteristics. In Performance of Exterior Envelopes of Whole Buildings; International Conference Clearwater: Clearwater, FL, USA, 2004; Volume 80. [Google Scholar]
- Rode, C.; Peuhkuri, R.; Time, B.; Svennberg, K.; Ojanen, T. Moisture buffer value of building materials. J. ASTM Int. 2007, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rode, C.; Holm, A.; Padfield, T. A Review of Humidity Buffering in the Interior Spaces. J. Therm. Envel. Build. Sci. 2004, 27, 221–226. [Google Scholar] [CrossRef]
- Bourdot, A.; Promis, G.; Tran Le, A.; Douzane, O.; Benazzouk, A.; Rosquoët, F.; Langlet, T. Hygrothermal properties of blocks based on eco-aggregates: Experimental and numerical study. Constr. Build. Mater. 2016, 125, 279–289. [Google Scholar] [CrossRef]
- Rahmouni, I. Optimisation de la Formulation de Bétons a Base D’éco-Granulats Obtenus par Carbonatation Accélérée de Déchets D’incinérateur. Ph.D. Thesis, Université de PIcardie Jules Verne, Amiens, France, 2019. [Google Scholar]
- Tran Le, A.D. Etude des Transferts Hygrothermiques Dans le Béton de Chanvre et leur Application au Bâtiment: Simulation Numérique et Approche Expérimentale. Ph.D. Thesis, Université de Reims Champagne-Ardenne, Reims, France, 2010. [Google Scholar]
- Peuhkuri, R.; Rode, C.; Hansen, K.K. Non-isothermal moisture transport through insulation materials. Build. Environ. 2008, 43, 811–822. [Google Scholar] [CrossRef]
- Abadie, M.; Mendonça, K. Moisture performance of building materials: From material characterization to building simulation using the Moisture Buffer Value concept. Build. Environ. 2009, 44, 388–401. [Google Scholar] [CrossRef]
- Arfvidsson, J. Moisture penetration for periodically varing relative humidity at boundary. Acta Phys. Aedificiorum 1999, 2. [Google Scholar]
- Baroghel-Bouny, V. Water vapour sorption experiments on hardened cementitious materials. Part II: Essential tool for assessment of transport properties and for durability prediction. Cem. Concr. Res. 2007, 37, 438–454. [Google Scholar] [CrossRef]

| Environmental Indicators | Unit | Aggregates | ||
|---|---|---|---|---|
| Carbonated | Mineral | |||
| Global warming | (kg CO2-eq) | 259 | 343 | −24% |
| Ozone depletion | (kg CFC11-eq) | 8.82 | 8.67 | 2% |
| Acidification of soil and water | (kg SO2-eq) | 0.346 | 0.643 | −46% |
| Eutrophication | (kg PO43−-eq) | 0.046 | 3.800 | −99% |
| Photochemical ozone creation | (kg C2H4-eq) | 0.024 | 0.046 | −47% |
| Depletion of abiotic resources—elements | (kg Sb-eq) | 1.79 | 4.19 | −59% |
| Depletion of abiotic resources—fossil fuels | (MJ) | 603 | 1400 | −57% |
| Water pollution | (m3) | 5480 | 9400 | −42% |
| Air pollution | (m3) | 4760 | 5760 | −17% |
| Aggregate | C8agg | PA | BAk | Mineral | |
|---|---|---|---|---|---|
| Granular | d | 2.5 | 2.5 | 2.5 | 2.5 |
| class | D | 16 | 16 | 16 | 16 |
| Shape | 0.53 | 0.54 | 0.50 | 0.65 | |
| of curve | 0.95 | 0.97 | 0.98 | 1.09 | |
| Sorting | 1.31 | 1.31 | 1.36 | 1.27 | |
| indices | 0.47 | 0.62 | 0.66 | 0.50 | |
| 0.03 | 0.00 | 0.02 | 0.02 | ||
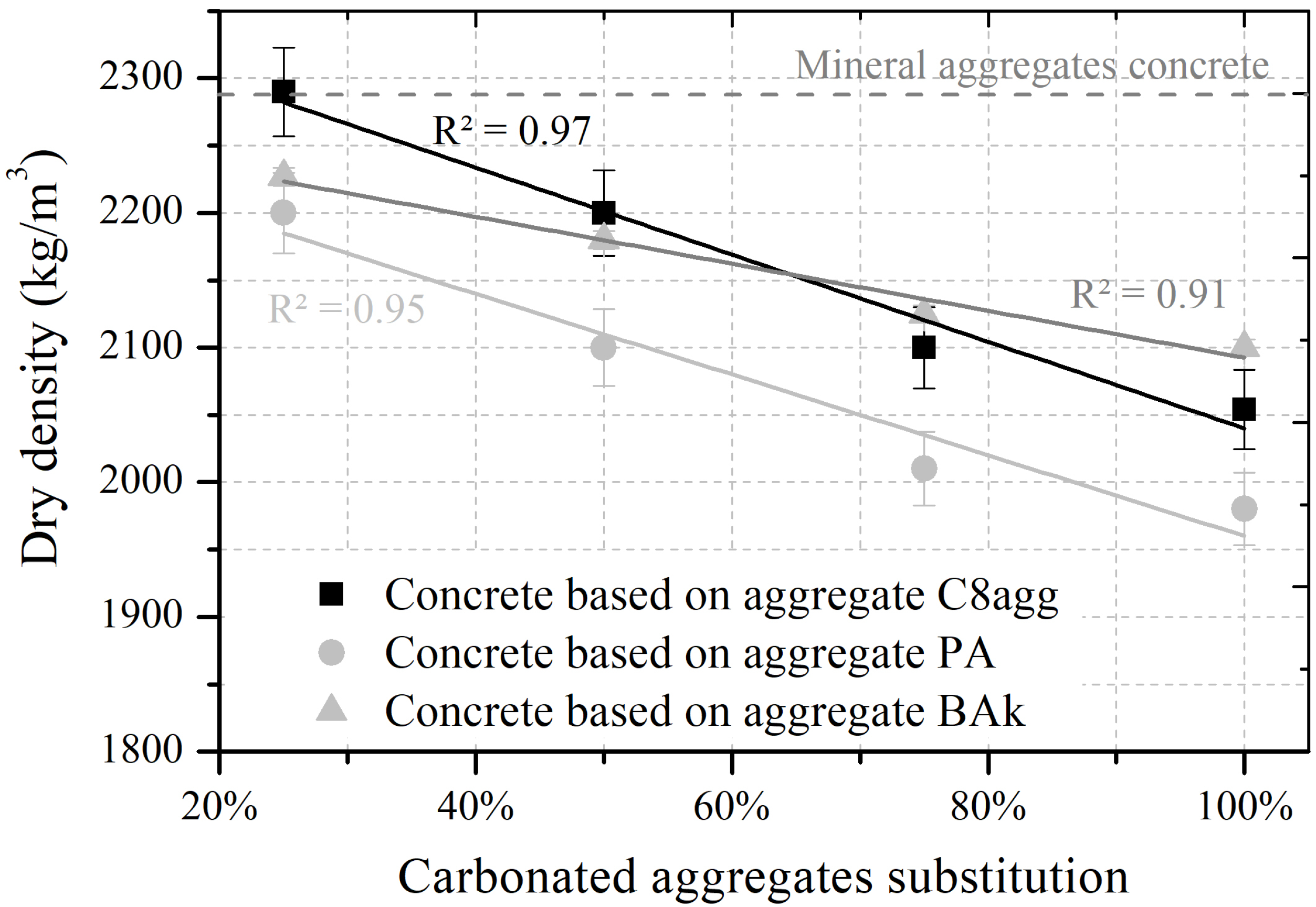
| Density | bulk | 1056 | 779 | 1186 | 1362 |
| (kg/m) | rd | 1816 | 1541 | 2040 | 2500 |
| absolute | 2561 | 2436 | 2556 | 2590 | |
| Porosity | total | 0.59 | 0.68 | 0.55 | 0.49 |
| (%) | interparticle | 0.42 | 0.50 | 0.44 | 0.46 |
| intraparticle | 0.17 | 0.18 | 0.11 | 0.03 | |
| Concrete Based on | Substitution Rate | (MPa) | (MPa) | (MPa) | (MPa) | (MPa) |
|---|---|---|---|---|---|---|
| C8agg | 100% | 7.7 | 17.8 | 19.9 | 23.8 | 22.54 |
| 75% | 8.4 | 20.1 | 21.7 | 25.4 | 28.65 | |
| 50% | 10.9 | 20.8 | 22.0 | 26.1 | 30.16 | |
| 25% | 19.1 | 23.6 | 23.4 | 26.2 | 31.25 | |
| PA | 100% | 15.2 | 16.6 | 17.1 | 19.8 | 23.63 |
| 75% | 15.3 | 16.8 | 17.2 | 18.0 | 24.97 | |
| 50% | 15.4 | 16.8 | 17.9 | 20.3 | 25.32 | |
| 25% | 18.5 | 21.8 | 23.8 | 24.3 | 28.10 | |
| BAk | 100% | 10.3 | 14.0 | 17.6 | 20.6 | 24.76 |
| 75% | 11.9 | 15.6 | 18.2 | 22.6 | 27.14 | |
| 50% | 12.3 | 16.0 | 19.6 | 21.0 | 28.14 | |
| 25% | 12.7 | 16.3 | 19.8 | 21.1 | 29.56 | |
| Mineral aggregates | - | 26.9 | 33.2 | 38.5 | 42.2 | 37.44 |
| Aggregate | |||
|---|---|---|---|
| (W/(m·K)) | (W/(m·K)) | (W/(m·K)) | |
| C8agg | 0.19 | 0.54 | 0.90 |
| PA | 0.15 | 0.50 | 0.92 |
| BAk | 0.20 | 0.59 | 0.85 |
| Mineral aggregates | 0.25 | 0.84 | 0.91 |
| Concrete Based on | Substitution Rate | (W/(m·K)) | (J/(kg·K)) | a (m/s) | E (J/(mK·s)) |
|---|---|---|---|---|---|
| C8agg | 100% | 0.78 | 1 640 | 0.42 | 1134 |
| 75% | 0.87 | 1 263 | 0.48 | 1178 | |
| 50% | 1.12 | 1 200 | 0.53 | 1239 | |
| 25% | 1.21 | 1 025 | 0.54 | 1310 | |
| PA | 100% | 0.83 | 1 560 | 0.51 | 1133 |
| 75% | 0.96 | 1 461 | 0.55 | 1179 | |
| 50% | 1.04 | 1 445 | 0.55 | 1207 | |
| 25% | 1.07 | 1 372 | 0.59 | 1287 | |
| BAk | 100% | 0.91 | 1 090 | 0.54 | 1018 |
| 75% | 0.87 | 996 | 0.58 | 1145 | |
| 50% | 1.07 | 952 | 0.65 | 1287 | |
| 25% | 1.09 | 882 | 0.63 | 1356 | |
| Mineral aggregates | - | 1.23 | 1 815 | 0.72 | 1449 |
| Concrete Based on | Substitution Rate | MBV | MBV | MBV | Classification |
|---|---|---|---|---|---|
| (g/(m%RH)) | |||||
| C8agg | 100% | 2.14 | 2.62 | 2.38 | Excellent |
| 75% | 1.43 | 2.38 | 1.91 | Good | |
| 50% | 1.80 | 2.10 | 1.95 | Good | |
| 25% | 1.43 | 2.14 | 1.79 | Good | |
| PA | 100% | 1.95 | 2.43 | 2.19 | Excellent |
| 75% | 1.19 | 1.90 | 1.55 | Good | |
| 50% | 1.19 | 1.67 | 1.43 | Good | |
| 25% | 1.11 | 1.43 | 1.27 | Good | |
| BAk | 100% | 1.90 | 2.62 | 2.26 | Excellent |
| 75% | 1.90 | 2.62 | 2.26 | Excellent | |
| 50% | 1.43 | 2.39 | 1.90 | Good | |
| 25% | 1.43 | 1.90 | 1.67 | Good | |
| Mineral aggregates | - | 0.71 | 1.19 | 0.95 | Moderate |
| Concrete Based on | Substitution Rate | (kg/(Pa·ms)) | (kg/(Pa·m·s)) | (m/s) | |
|---|---|---|---|---|---|
| C8agg | 100% | 5.33 | 4.85 | 8.27 | 41 |
| 75% | 4.28 | 2.96 | 4.80 | 67 | |
| 50% | 4.37 | 2.94 | 4.53 | 68 | |
| 25% | 4.01 | 2.36 | 3.48 | 85 | |
| PA | 100% | 4.90 | 9.40 | 3.67 | 21 |
| 75% | 3.47 | 4.29 | 1.53 | 47 | |
| 50% | 3.20 | 3.36 | 1.10 | 60 | |
| 25% | 2.84 | 2.45 | 7.40 | 82 | |
| BAk | 100% | 5.06 | 5.44 | 1.16 | 37 |
| 75% | 5.06 | 5.04 | 9.91 | 40 | |
| 50% | 4.26 | 3.31 | 6.07 | 60 | |
| 25% | 3.74 | 2.39 | 4.10 | 84 | |
| Mineral aggregates | - | 2.13 | 4.92 | 5.34 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmouni, I.; Promis, G.; Douzane, O.; Rosquoet, F. Grain Density-Based Approaches to Predict the Mechanical, Thermal and Hygric Properties of Carbon-Negative Aggregate Concretes. Sustainability 2021, 13, 8194. https://doi.org/10.3390/su13158194
Rahmouni I, Promis G, Douzane O, Rosquoet F. Grain Density-Based Approaches to Predict the Mechanical, Thermal and Hygric Properties of Carbon-Negative Aggregate Concretes. Sustainability. 2021; 13(15):8194. https://doi.org/10.3390/su13158194
Chicago/Turabian StyleRahmouni, Imen, Geoffrey Promis, Omar Douzane, and Frédéric Rosquoet. 2021. "Grain Density-Based Approaches to Predict the Mechanical, Thermal and Hygric Properties of Carbon-Negative Aggregate Concretes" Sustainability 13, no. 15: 8194. https://doi.org/10.3390/su13158194
APA StyleRahmouni, I., Promis, G., Douzane, O., & Rosquoet, F. (2021). Grain Density-Based Approaches to Predict the Mechanical, Thermal and Hygric Properties of Carbon-Negative Aggregate Concretes. Sustainability, 13(15), 8194. https://doi.org/10.3390/su13158194






