Interactive Effects of Nitrogen and Sulfur Nutrition on Growth, Development, and Physiology of Brassica carinata A. Braun and Brassica napus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture
2.2. Phenology Measurements
2.3. Pigments and Gas Exchange Measurements
3. Results
3.1. Plant Growth and Development
3.2. Biomass Production and Allocation
3.3. Nitrogen-Sulfur Uptake
3.4. Pigments
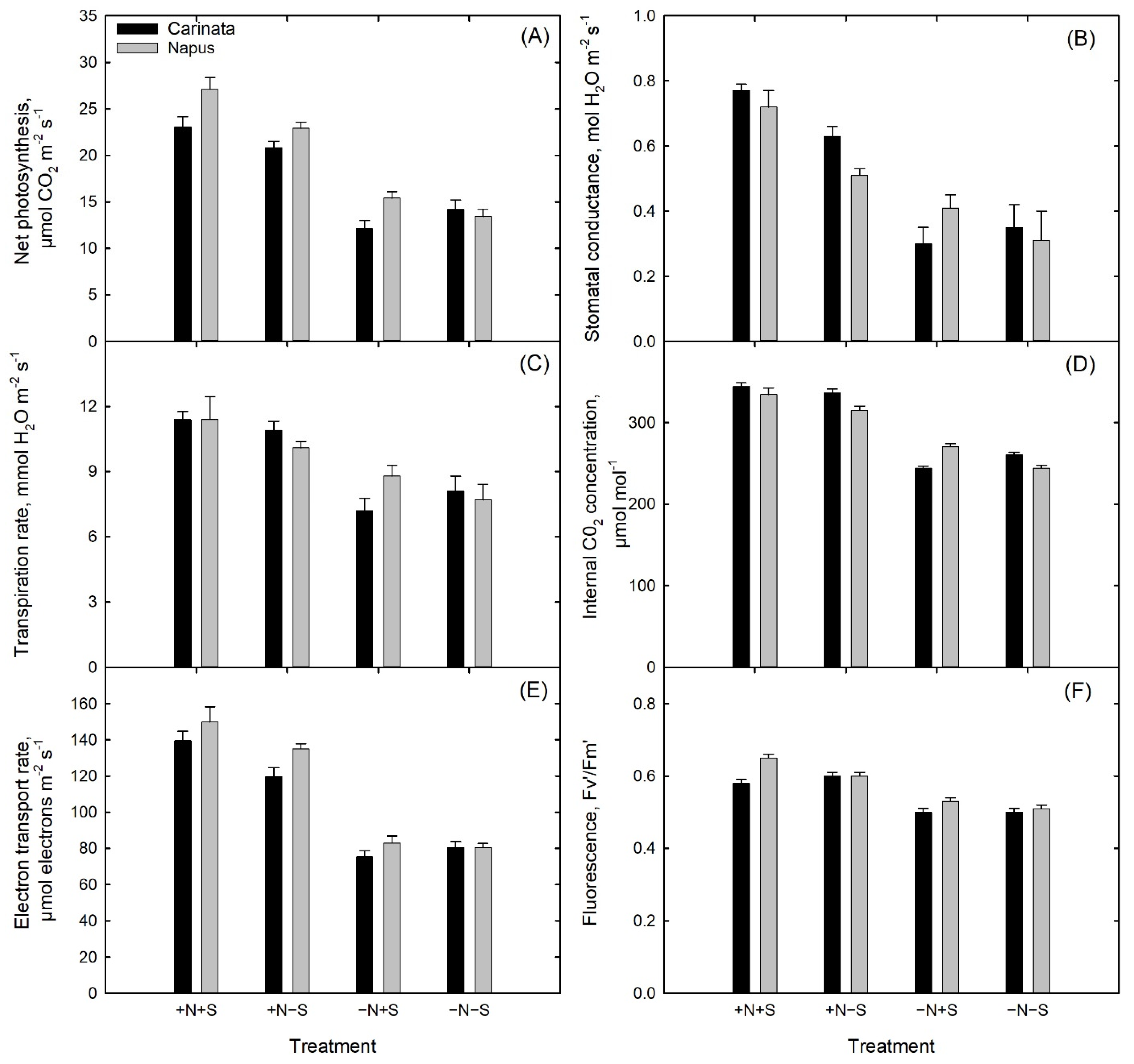
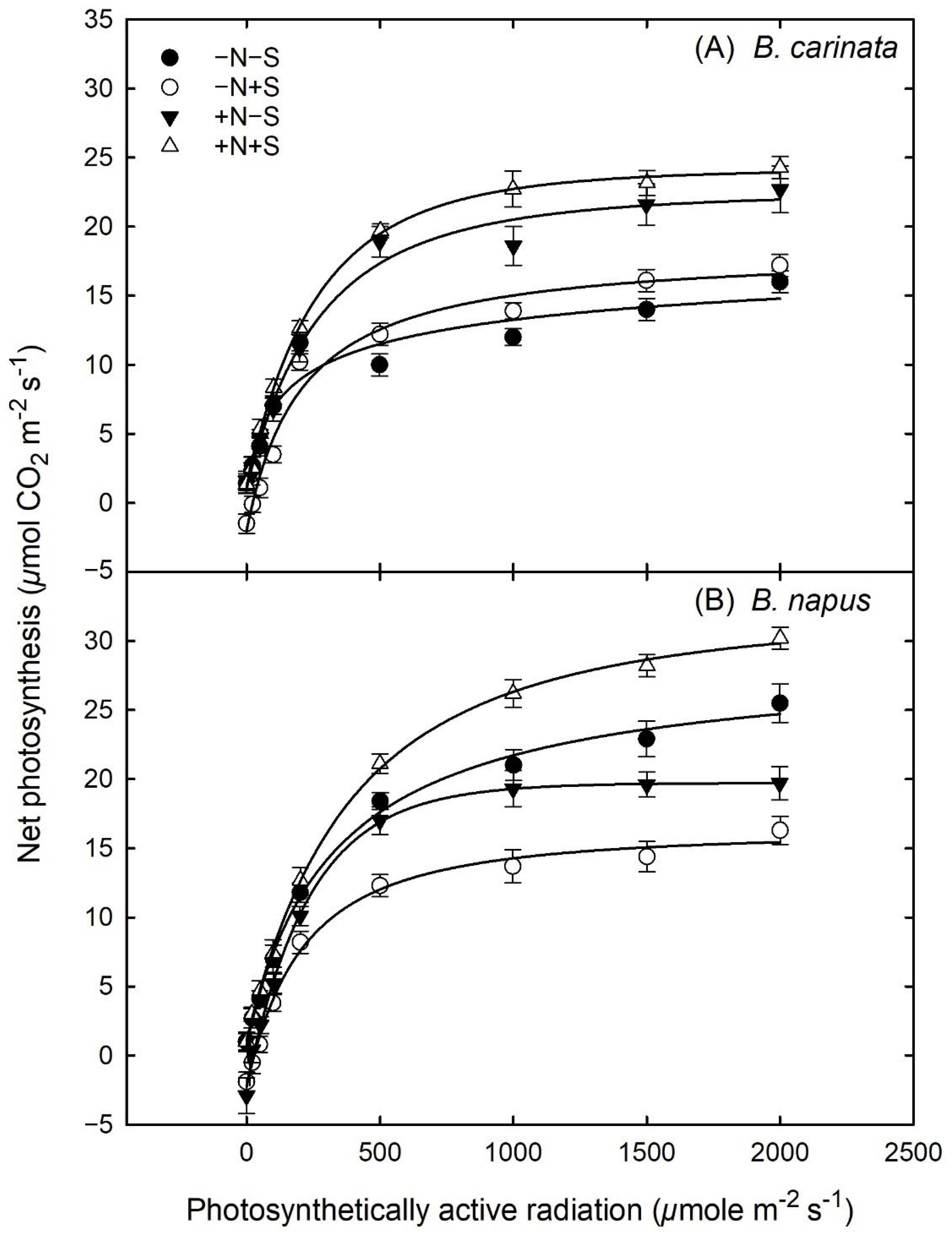
3.5. Gas Exchange and Light Response Curves
3.6. Correlation Analysis between Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marillia, E.F.; Francis, T.; Falk, K.C.; Smith, M.; Taylor, D.C. Palliser’s promise: Brassica carinata, an emerging western Canadian crop for delivery of new bio-industrial oil feedstocks. Biocatal. Agric. Biotechnol. 2014, 3, 65–74. [Google Scholar] [CrossRef]
- Mulvaney, M.J.; Leon, R.G.; Seepaul, R.; Wright, D.L.; Hoffman, T.L. Brassica carinata seeding rate and row spacing effects on morphology, yield, and oil. Agron. J. 2019, 111, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Seepaul, R.; Mulvaney, M.J.; Colvin, B.; George, S.; Marois, J.J.; Bennett, R.; Leon, R.; Wright, D.L.; Small, I.M. Brassica carinata genotypes demonstrate potential as a winter biofuel crop in South East United States. Ind. Crop. Prod. 2020, 112353. [Google Scholar] [CrossRef]
- Seepaul, R.; Kumar, S.; Iboyi, J.E.; Bashyal, M.; Stansly, T.L.; Bennett, R.; Boote, K.J.; Mulvaney, M.J.; Small, I.M.; George, S.; et al. Brassica carinata: Biology and Agronomy as a Biofuel Crop. GCB Bioenergy 2021. [Google Scholar] [CrossRef]
- Soil Health Census Report USA. Adoption of Soil Health Systems Based on Data from the 2017 U.S. Census of Agriculture. 2019. Available online: https://soilhealthinstitute.org/wp-content/uploads/2019/07/Soil-Health-Census-Report.pdf (accessed on 17 November 2020).
- Lakkineni, K.C.; Abrol, Y.P. Sulphur requirement of crop plants: Physiological analysis. Fertil. News 1994, 39, 11–18. [Google Scholar]
- Fazili, I.S.; Jamal, A.; Ahmad, S.; Masoodi, M.; Khan, J.S.; Abdin, M.Z. Interactive effect of sulfur and nitrogen on nitrogen accumulation and harvest in oilseed crops differing in nitrogen assimilation potential. J. Plant Nutr. 2008, 31, 1203–1220. [Google Scholar] [CrossRef]
- Franzen, D.W. Fertilizing Mustard and Canola. Available online: https://www.ag.ndsu.edu/publications/crops/fertilizing-canola-and-mustard (accessed on 15 April 2021).
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Weisler, F.; Behrens, T.; Horst, W.J. The role of nitrogen-efficient cultivars in sustainable agriculture. Sci. World J. 2001, 1. [Google Scholar] [CrossRef]
- Malagoli, P.; Laine, P.; Rossato, L.; Ourry, A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest: I. Global N flows between vegetative and reproductive tissues in relation to leaf fall and their residual N. Ann. Bot. 2005, 95, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Rathke, G.W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Jamal, A.; Moon, Y.S.; Zainul Abdin, M. Sulphur-a general overview and interaction with nitrogen. Aust. J. Crop Sci. 2010, 4, 523. [Google Scholar]
- Gammelvind, L.H.; Schjoerring, J.K.; Mogensen, V.O.; Jensen, C.R.; Bock, J.G.H. Photosynthesis in leaves and siliques of winter oilseed rape (Brassica napus L.). Plant Soil 1996, 186, 227–236. [Google Scholar] [CrossRef]
- Momoh, E.J.; Song, W.J.; Li, H.Z.; Zhou, W.J. Seed yield and quality responses of winter oilseed of rape (Brassica napus) to plant density and nitrogen fertilization. Indian J. Agric. Sci. 2004, 74, 420–424. [Google Scholar]
- Albert, B.; Le Cahérec, F.; Niogret, M.F.; Faes, P.; Avice, J.C.; Leport, L.; Bouchereau, A. Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta 2012, 236, 659–676. [Google Scholar] [CrossRef] [Green Version]
- Seepaul, R.; George, S.; Wright, D.L. Comparative response of Brassica carinata and B. napus vegetative growth, development and photosynthesis to nitrogen nutrition. Ind. Crop. Prod. 2016, 94, 872–883. [Google Scholar] [CrossRef]
- Jackson, G.D. Effects of nitrogen and sulfur on canola yield and nutrient uptake. Agron. J. 2000, 92, 644–649. [Google Scholar] [CrossRef]
- Malagoli, P.; Lainé, P.; Le Deunff, E.; Rossato, L.; Ney, B.; Ourry, A. Modeling nitrogen uptake in oilseed rape cv Capitol during a growth cycle using influx kinetics of root nitrate transport systems and field experimental data. Plant Physiol. 2004, 134, 388–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, C.R.; Mogensen, V.O.; Mortensen, G.; Andersen, M.N.; Schjoerring, J.K.; Thage, J.H.; Koribidis, J. Leaf photosynthesis and drought adaptation in field-grown oilseed rape (Brassica napus L.). Funct. Plant Biol. 1996, 23, 631–644. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Sulphur uptake, yield responses and the interactions between nitrogen and sulphur in winter oilseed rape (Brassica napus). J. Agric. Sci. 1996, 126, 53–62. [Google Scholar] [CrossRef]
- Zhao, F.J.; Salmon, S.E.; Withers, P.J.A.; Monaghan, J.M.; Evans, E.J.; Shewry, P.R.; McGrath, S.P. Variation in the breadmaking quality and rheological properties of wheat in relation to sulphur nutrition under field conditions. J. Cereal Sci. 1999, 30, 19–31. [Google Scholar] [CrossRef]
- Jamal, A.; Fazli, I.S.; Ahmad, S.; Abdin, M.Z.; Yun, S.J. Effect of sulphur and nitrogen application on growth characteristics, seed and oil yields of soybean cultivars. Korean J. Crop Sci. 2005, 50, 340. [Google Scholar]
- Jamal, A.; Fazli, I.S.; Ahmad, S.; Kim, K.T.; Oh, D.G.; Abdin, M.Z. Effect of sulfur on nitrate reductase and ATP sulfurylase activities in groundnut (Arachis hypogea L.). J. Plant Biol. 2006, 49, 513–517. [Google Scholar] [CrossRef]
- Tandon, H.L.S. Sulphur Research and Agricultural Production in India. Available online: http://eprints.icrisat.ac.in/13314/1/RP%203272.pdf (accessed on 20 April 2021).
- Ahmad, A.; Khan, I.; Anjum, N.A.M.; Diva, I.; Abdin, M.Z.; Iqbal, M. Effect of timing of sulfur fertilizer application on growth and yield of rapeseed. J. Plant Nutr. 2005, 28, 1049–1059. [Google Scholar] [CrossRef]
- Asare, E.; Scarisbrick, D.H. Rate of nitrogen and sulphur fertilizers on yield, yield components and seed quality of oilseed rape (Brassica napus L.). Field Crop. Res. 1995, 44, 41–46. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Orsini, F.; Bottino, A.; Barbieri, G. Sulphur fertilisation affects yield and quality in friarielli (Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort.) grown in a floating system. J. Hortic. Sci. Biotechnol. 2008, 83, 743–748. [Google Scholar] [CrossRef]
- Ahmad, G.; Jan, A.; Arif, M.; Jan, M.T.; Khattak, R.A. Influence of nitrogen and sulfur fertilization on quality of canola (Brassica napus L.) under rainfed conditions. J. Zhejiang Univ. Sci. 2007, B8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Malhi, S.S.; Gan, Y.; Raney, J.P. Yield, seed quality, and sulfur uptake of Brassica oilseed crops in response to sulfur fertilization. Agron. J. 2007, 99, 570–577. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Fismes, J.; Vong, P.C.; Guckert, A.; Frossard, E. Influence of sulfur on apparent N-use efficiency, yield and quality of oilseed rape (Brassica napus L.) grown on a calcareous soil. Eur. J. Agron. 2000, 12, 127–141. [Google Scholar] [CrossRef]
- Sachdev, M.S.; Deb, D.L. Nitrogen and sulphur uptake and efficiency in the mustard-moong-maize cropping system. Fertil. News 1990, 35, 49–55. [Google Scholar]
- Schnug, E.; Haneklaus, S.; Borchers, A.; Polle, A. Relations between sulphur supply and glutathione and ascorbate concentrations in Brassica napus. Z. Für Pflanz. Und Bodenkd. 1995, 158, 67–69. [Google Scholar] [CrossRef]
- Dubousset, L.; Etienne, P.; Avice, J.C. Is the remobilization of S and N reserves for seed filling of winter oilseed rape modulated by sulphate restrictions occurring at different growth stages? J. Exp. Bot. 2010, 61, 4313–4324. [Google Scholar] [CrossRef] [PubMed]
- Eppendorfer, W.H. Effects of S, N and P on amino acid composition of field beans (Vidafabd) and responses of the biological value of the seed protein to S-amino acid content. J. Sci. Food Agric. 1971, 22, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.S.; Pasricha, N.S.; Sahota, N.S. Nitrogen-sulphur relationship in brown sarson and Indian mustard. Indian J. Agric. Sci. 1977, 47, 249–253. [Google Scholar]
- Dev, G.; Kumar, V. Secondary nutrients. In Review of Soil Research in India; Indian Society of Soil Science: New Delhi, India, 1982; Volume 1, pp. 342–360. [Google Scholar]
- Janzen, H.H.; Bettany, J.R. Sulfur nutrition of rapeseed: I. Influence of fertilizer nitrogen and sulfur rates. Soil Sci. Soc. Am. J. 1984, 48, 100–107. [Google Scholar] [CrossRef]
- Dev, G.; Jaggi, R.C.; Aulakh, M.S. Study of nitrate-sulphate interaction on the growth and nutrient uptake by maize using 35S. J. Indian Soc. Soil Sci. 1979, 27, 302–307. [Google Scholar]
- Randall, P.J.; Spencer, K.; Freney, J.R. Sulfur and nitrogen fertilizer effects on wheat. I. Concentrations of sulfur and nitrogen and the nitrogen to sulfur ratio in grain, in relation to the yield response. Aust. J. Agric. Res. 1981, 32, 203–212. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Clarkson, D.T.; Cambridge, M.; Lambers, H.; Hawkesford, M.J. SO42-deprivation has an early effect on the content of ribulose-1, 5-bisphosphate carboxylase/oxygenase and photosynthesis in young leaves of wheat. Plant Physiol. 1997, 115, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ambreen, H.; Variath, M.T.; Rao, A.R.; Agarwal, M.; Kumar, A.; Goel, S.; Jagannath, A. Utilization of molecular, phenotypic, and geographical diversity to develop compact composite core collection in the oilseed crop, safflower (Carthamus tinctorius L.) through maximization strategy. Front. Plant Sci. 2016, 7, 1554. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Tan, X.; Zhang, L.; Yuan, D.; Lin, J.; Liu, X.; Jiang, Y.; Zhou, B. Increasing branch and seed yield through heterologous expression of the novel rice S-acyl transferase gene OsPAT15 in Brassica napus L. Breed. Sci. 2018, 17126. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; MoradTalab, N.; Fathi Abd-Allah, E.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. Water Stress Crop. Plants A Sustain. Approach 2016, 2. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef]
- Li, F.; Chen, B.; Xu, K.; Gao, G.; Yan, G.; Qiao, J.; Li, J.; Li, H.; Li, L.; Xiao, X.; et al. A genome-wide association study of plant height and primary branch number in rapeseed (Brassica napus). Plant Sci. 2016, 242, 169–177. [Google Scholar] [CrossRef]
- Seepaul, R.; Marois, J.; Small, I.; George, S.; Wright, D. Carinata dry matter accumulation and nutrient uptake responses to nitrogen fertilization. Agron. J. 2019, 111, 2038–2046. [Google Scholar] [CrossRef]
- Seepaul, R.; Small, I.; Marois, J.; George, S.; Wright, D. Brassica carinata and Brassica napus growth, nitrogen use, seed, and oil productivity constrained by post-bolting nitrogen deficiency. Crop Sci. 2019, 59, 2720–2732. [Google Scholar] [CrossRef]
- Seepaul, R.; Mulvaney, M.J.; Small, I.M.; George, S.; Wright, D.L. Carinata growth, yield and chemical composition responses to nitrogen fertilizer management. Agron. J. 2020. [Google Scholar] [CrossRef]
- Ogunlela, V.B.; Kullmann, A.; Geisler, G. Nitrogen distribution and dry matter accumulation in oilseed rape (Brassica napus L.) as influenced by N nutrition. J. Agron. Crop Sci. 1990, 164, 321–333. [Google Scholar] [CrossRef]
- Girondé, A.; Dubousset, L.; Trouverie, J.; Etienne, P.; Avice, J.C. The impact of sulfate restriction on seed yield and quality of winter oilseed rape depends on the ability to remobilize sulfate from vegetative tissues to reproductive organs. Front. Plant Sci. 2014, 5, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noquet, C.; Avice, J.C.; Rossato, L.; Beauclair, P.; Henry, M.P.; Ourry, A. Effects of altered source–sink relationships on N allocation and vegetative storage protein accumulation in Brassica napus L. Plant Sci. 2004, 166, 1007–1018. [Google Scholar] [CrossRef]
- Zhao, F.; Evans, E.J.; Bilsborrow, P.E.; Syers, J.K. Influence of sulphur and nitrogen on seed yield and quality of low glucosinolate oilseed rape (Brassica napus L.). J. Sci. Food Agric. 1993, 63, 29–37. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, S.; Murphy, D. Impact of sulphur fertilization on fertilizer nitrogen efficiency. Sulphur Agric. 1993, 17, 12. [Google Scholar]
- Kopriva, S.; Rennenberg, H. Control of sulphate assimilation and glutathione synthesis: Interaction with N and C metabolism. J. Exp. Bot. 2004, 55, 1831–1842. [Google Scholar] [CrossRef]
- Rossato, L.; Lainé, P.; Ourry, A. Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: Nitrogen fluxes within the plant and changes in soluble protein patterns. J. Exp. Bot. 2001, 52, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiancai, G.; Wei, F.; Huijie, Z.; Guodian, X.; Huacen, W.; Yonghua, W.; Zhanjun, Y. Photosynthetic characteristics of flag leaves and nitrogen effects in two winter wheat cultivars with different spike type. Zuo Wu Xue Bao 2004, 30, 115–121. [Google Scholar]
- Zhou, W.; Zhao, D.; Lin, X. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J. Plant Growth Regul. 1997, 16, 47–53. [Google Scholar] [CrossRef]
- Men, S.; Chen, H.; Chen, S.; Zheng, S.; Shen, X.; Wang, C.; Yang, Z.; Liu, D. Effects of supplemental nitrogen application on physiological characteristics, dry matter and nitrogen accumulation of winter rapeseed (Brassica napus L.) under waterlogging stress. Sci. Rep. 2020, 10, 10201. [Google Scholar] [CrossRef] [PubMed]
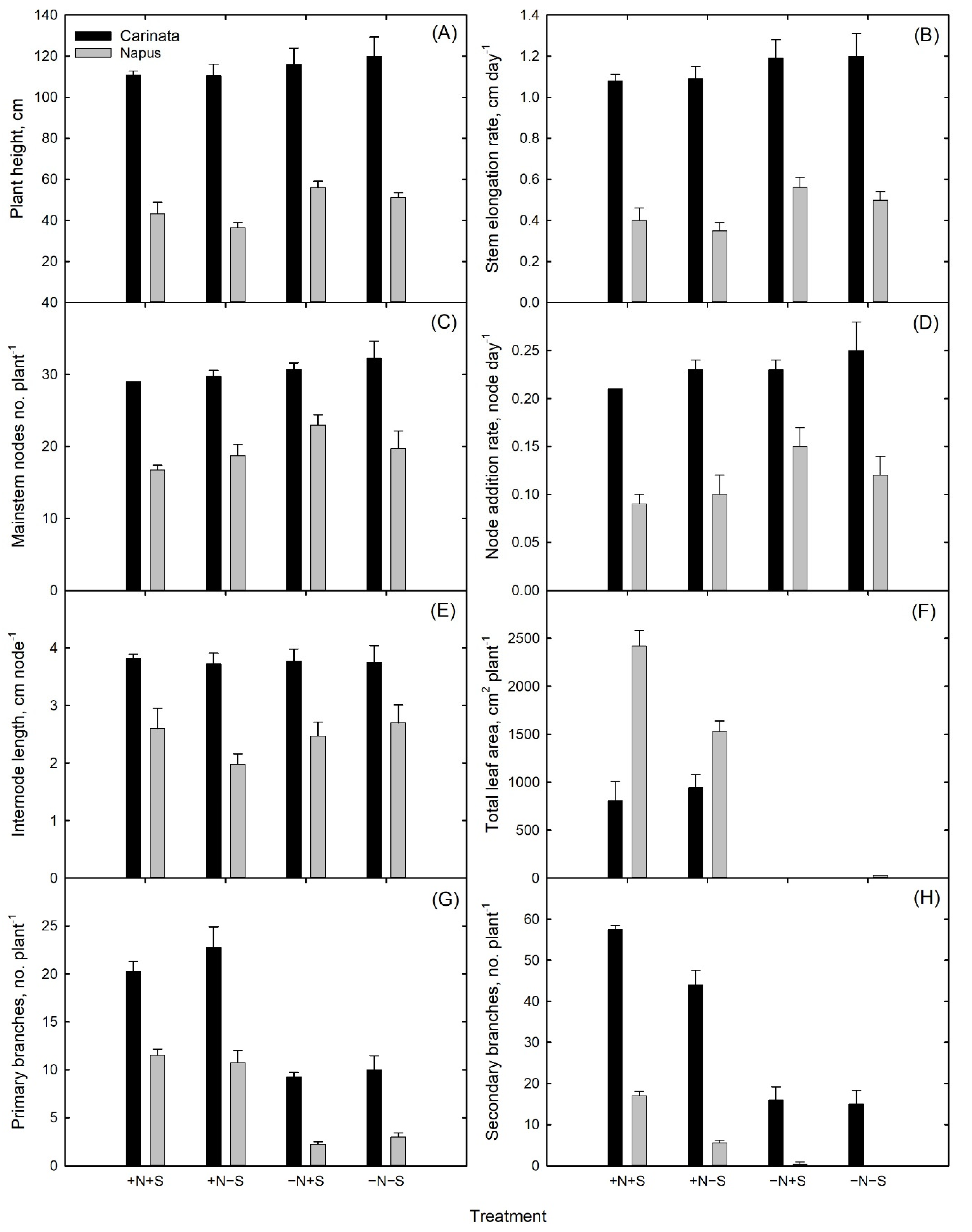
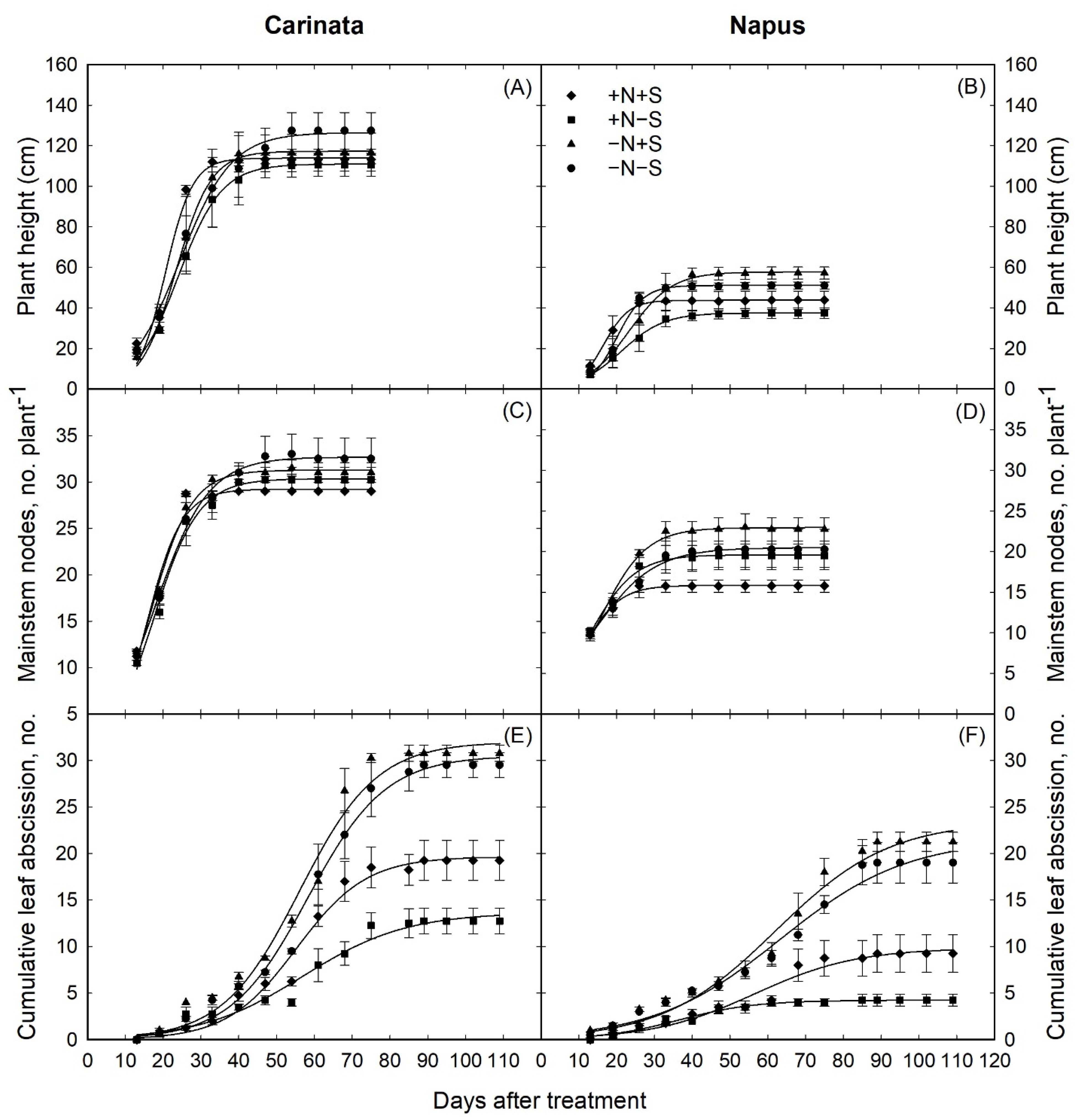
| Species | Treatment * | Plant Height | Stem Elongation Rate | Mainstem Nodes | Node Addition Rate | Internode Length | Primary Branches | Secondary Branches | Total Leaf Area |
|---|---|---|---|---|---|---|---|---|---|
| cm | cm Day−1 | No. | Node Day−1 | cm Node−1 | No. | No. | cm2 Plant−1 | ||
| Carinata | +N+S | 110.8 a | 1.08 a | 32.3 a | 0.21 a | 3.8 a | 20.3 a | 57.5 a | 805.6 c |
| +N−S | 110.5 a | 1.09 a | 30.8 a | 0.23 a | 3.7 a | 22.8 a | 44.0 b | 943.3 c | |
| −N+S | 116.0 a | 1.20 a | 29.8 a | 0.23 a | 3.8 a | 9.3 b | 16.0 c | 0 d | |
| −N−S | 120.0 a | 1.20 a | 29.0 a | 0.25 a | 3.8 a | 10.0 b | 15.0 c | 0 d | |
| Napus | +N+S | 43.3 bc | 0.40 bc | 16.8 c | 0.09 c | 2.6 bc | 11.5 b | 17.0 c | 2419.6 a |
| +N−S | 36.5 c | 0.35 c | 18.8 bc | 0.10 c | 2.0 c | 10.8 b | 5.5 d | 1528.7 b | |
| −N+S | 56.0 b | 0.56 b | 23.0 b | 0.15 b | 2.5 bc | 2.3 c | 0.5 d | 0 d | |
| −N−S | 51.3 bc | 0.50 bc | 19.8 bc | 0.12 bc | 2.7 bc | 3.0 c | 0 d | 29.2 d | |
| ANOVA | Species | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | 0.068 | 0.0539 | 0.0563 | 0.0574 | 0.394 | <0.0001 | <0.0001 | <0.0001 | |
| Species * Treatment | 0.6376 | 0.8748 | 0.3773 | 0.3123 | 0.537 | 0.1218 | <0.0001 | <0.0001 |
| Species | Treatment * | Biomass Production | Biomass Allocation | Root Shoot Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Reproductive | Total | Roots | Stems | Leaves | Reproductive | |||
| Structures | Structures | ||||||||||
| gm Plant−1 | % | ||||||||||
| Carinata | +N+S | 14.9 b | 31.6 a | 4.2 b | 53.6 ab | 104.3 ab | 14 de | 31 bc | 4 d | 51 a | 0.17 d |
| +N−S | 16.7 ab | 26.6 b | 5.9 b | 48.7 b | 97.9 b | 17 cde | 27 cd | 6 c | 50 a | 0.20 cd | |
| −N+S | 6.9 c | 13.9 de | - | 11.8 c | 32.5 c | 21 abc | 42 a | - | 36 b | 0.27 ab | |
| −N−S | 7.2 c | 17.2 cd | - | 13.0 c | 37.4 c | 19 bc | 46 a | - | 35 b | 0.24 bc | |
| Napus | +N+S | 16.4 ab | 29.8 ab | 10.2 a | 58.7 a | 115.1 a | 14 e | 26 cd | 9 b | 51 a | 0.17 d |
| +N−S | 19.4 a | 20.6 c | 11.5 a | 51.3 ab | 102.9 b | 19 bc | 20 d | 11 a | 50 a | 0.23 bc | |
| −N+S | 6.4 c | 9.9 e | - | 12.3 c | 28.6 c | 22 ab | 35 b | - | 43 ab | 0.29 ab | |
| −N−S | 6.8 c | 9.0 e | - | 12.0 c | 27.8 c | 24 a | 32 bc | - | 44 ab | 0.32 a | |
| ANOVA | Species | 0.389 | 0 | <0.0001 | 0 | 0.8491 | 0.083 | 0.0001 | 0.083 | 0.119 | 0.0815 |
| Treatment | <0.0001 | <0.0001 | 0 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | 0.0001 | 0.0014 | 0.0002 | |
| Species * Treatment | 0.53 | 0 | 1 | 1 | 0.0756 | 0.3739 | 0.3292 | 0.3739 | 0.4133 | 0.3249 | |
| Species | Treatment * | Nitrogen Uptake | Sulfur Uptake | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Reproductive Structures | Weighted Average | Roots | Stems | Leaves | Reproductive Structures | Weighted Average | ||
| gm | |||||||||||
| Carinata | +N+S | 0.22 a | 0.36 a | 0.30 ab | 3.37 a | 2.37 ab | 0.050 a | 0.11 a | 0.07 b | 0.89 a | 0.62 a |
| +N−S | 0.21 a | 0.33 ab | 0.26 b | 1.93 b | 1.25 c | 0.022 b | 0.01 d | 0.02 cd | 0.16 c | 0.10 b | |
| −N+S | 0.06 b | 0.08 c | 0.01 c | 0.40 c | 0.29 d | 0.024 b | 0.05 c | 0.01 cd | 0.14 c | 0.10 b | |
| −N−S | 0.06 b | 0.08 c | 0.02 c | 0.38 c | 0.25 d | 0.020 b | 0.04 c | 0.01 d | 0.14 c | 0.08 b | |
| Napus | +N+S | 0.20 a | 0.34 ab | 0.35 a | 3.75 a | 2.83 a | 0.046 a | 0.09 b | 0.15 a | 0.72 b | 0.54 a |
| +N−S | 0.19 a | 0.28 b | 0.26 b | 2.39 b | 1.81 bc | 0.020 b | 0.02 d | 0.02 cd | 0.17 c | 0.13 b | |
| −N+S | 0.06 b | 0.06 c | 0.02 c | 0.36 c | 0.28 d | 0.022 b | 0.03 d | 0.03 c | 0.13 c | 0.09 b | |
| −N−S | 0.07 b | 0.04 c | 0.01 c | 0.48 c | 0.40 d | 0.020 b | 0.02 d | 0.01 d | 0.15 c | 0.12 b | |
| ANOVA | Species | 0.5325 | 0.0933 | 0.3876 | 0.2021 | 0.068 | 0.351 | <0.0001 | <0.0001 | 0.123 | 0.8626 |
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Species * Treatment | 0.8645 | 0.9167 | 0.6551 | 0.6813 | 0.5339 | 0.9543 | 0.0466 | <0.0001 | 0.0348 | 0.3865 | |
| Species | Treatment * | Chla | Chlb | Chla + b | Carotenoids | SPAD |
|---|---|---|---|---|---|---|
| µg cm−2 | ||||||
| Carinata | +N+S | 18.5 a | 3.7 a | 22.4 a | 5.1 a | 47.7 b |
| +N−S | 17.0 a | 3.3 ab | 20.5 a | 4.3 b | 50.5 b | |
| −N+S | 10.7 c | 1.7 d | 13.2 c | 3.3 d | 41.3 cd | |
| −N−S | 11.6 c | 2.7 bc | 14.5 bc | 3.7 d | 38.2 e | |
| Napus | +N+S | 18.6 a | 3.4 ab | 22.2 a | 4.6 b | 55.0 a |
| +N−S | 17.9 a | 3.3 ab | 21.4 a | 4.2 bc | 57.6 a | |
| −N+S | 13.9 b | 2.3 c | 16.4 b | 3.4 d | 43.6 c | |
| −N−S | 13.6 b | 2.2 c | 16.0 b | 3.5 d | 39.9 de | |
| ANOVA | Species | 0.0028 | 0.9017 | 0.0179 | 0.1207 | <0.0001 |
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Species * Treatment | 0.1087 | 0.1732 | 0.2017 | 0.4288 | 0.0105 | |
| Plant Height | Node Number | Biomass | Nitrogen | Sulfur | Chla | Chlb | Carotenoids | SPAD | Photosynthesis | Chlorophyll Fluorescence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | - | ||||||||||
| Node number | 0.89 | - | |||||||||
| Biomass | −0.15 | −0.21 | - | ||||||||
| Nitrogen | −0.2 | −0.29 | 0.9 | - | |||||||
| Sulfur | −0.26 | −0.37 | 0.85 | 0.87 | - | ||||||
| Chla | −0.33 | −0.35 | 0.82 | 0.85 | 0.85 | - | |||||
| Chlb | −0.14 | −0.17 | 0.71 | 0.74 | 0.7 | 0.78 | - | ||||
| Carotenoids | 0.03 | −0.06 | 0.77 | 0.81 | 0.8 | 0.87 | 0.82 | - | |||
| SPAD | −0.45 | −0.49 | 0.78 | 0.88 | 0.76 | 0.79 | 0.62 | 0.59 | - | ||
| Photosynthesis | −0.34 | −0.36 | 0.81 | 0.82 | 0.81 | 0.72 | 0.51 | 0.63 | 0.78 | - | |
| Chlorophyll Fluorescence | −0.36 | −0.41 | 0.76 | 0.81 | 0.7 | 0.74 | 0.49 | 0.56 | 0.83 | 0.91 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Seepaul, R.; Small, I.M.; George, S.; O’Brien, G.K.; Marois, J.J.; Wright, D.L. Interactive Effects of Nitrogen and Sulfur Nutrition on Growth, Development, and Physiology of Brassica carinata A. Braun and Brassica napus L. Sustainability 2021, 13, 7355. https://doi.org/10.3390/su13137355
Kumar S, Seepaul R, Small IM, George S, O’Brien GK, Marois JJ, Wright DL. Interactive Effects of Nitrogen and Sulfur Nutrition on Growth, Development, and Physiology of Brassica carinata A. Braun and Brassica napus L. Sustainability. 2021; 13(13):7355. https://doi.org/10.3390/su13137355
Chicago/Turabian StyleKumar, Shivendra, Ramdeo Seepaul, Ian M. Small, Sheeja George, George Kelly O’Brien, James J. Marois, and David L. Wright. 2021. "Interactive Effects of Nitrogen and Sulfur Nutrition on Growth, Development, and Physiology of Brassica carinata A. Braun and Brassica napus L." Sustainability 13, no. 13: 7355. https://doi.org/10.3390/su13137355
APA StyleKumar, S., Seepaul, R., Small, I. M., George, S., O’Brien, G. K., Marois, J. J., & Wright, D. L. (2021). Interactive Effects of Nitrogen and Sulfur Nutrition on Growth, Development, and Physiology of Brassica carinata A. Braun and Brassica napus L. Sustainability, 13(13), 7355. https://doi.org/10.3390/su13137355








