Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Upland Rice Fields
2.2. Soil Sampling
2.3. Soil Analyses
2.4. Biological Materials
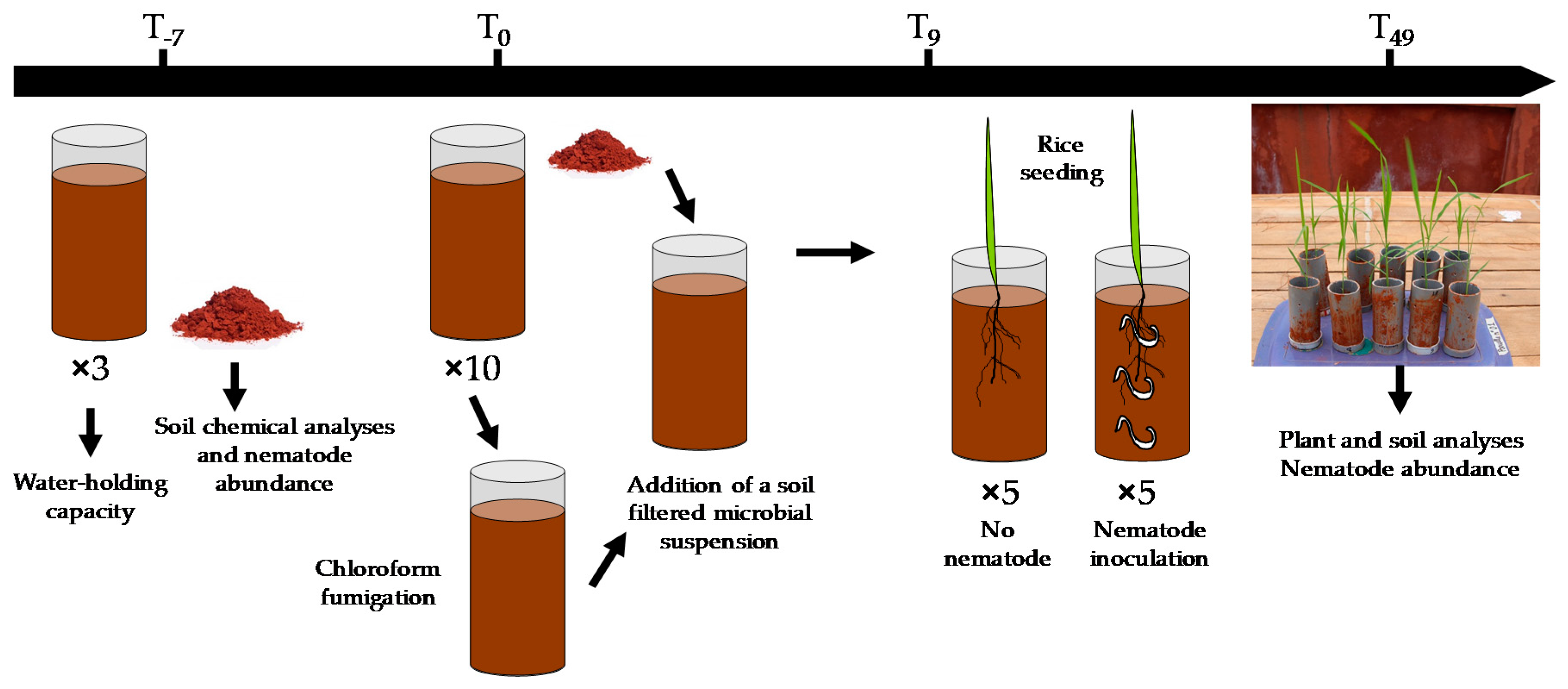
2.5. Soil Core Preparation for the Assay
2.6. Experimental Design and Setup
2.7. Soil and Plant Analyses at the End of the Assay
2.8. Statistical Analyses
3. Results
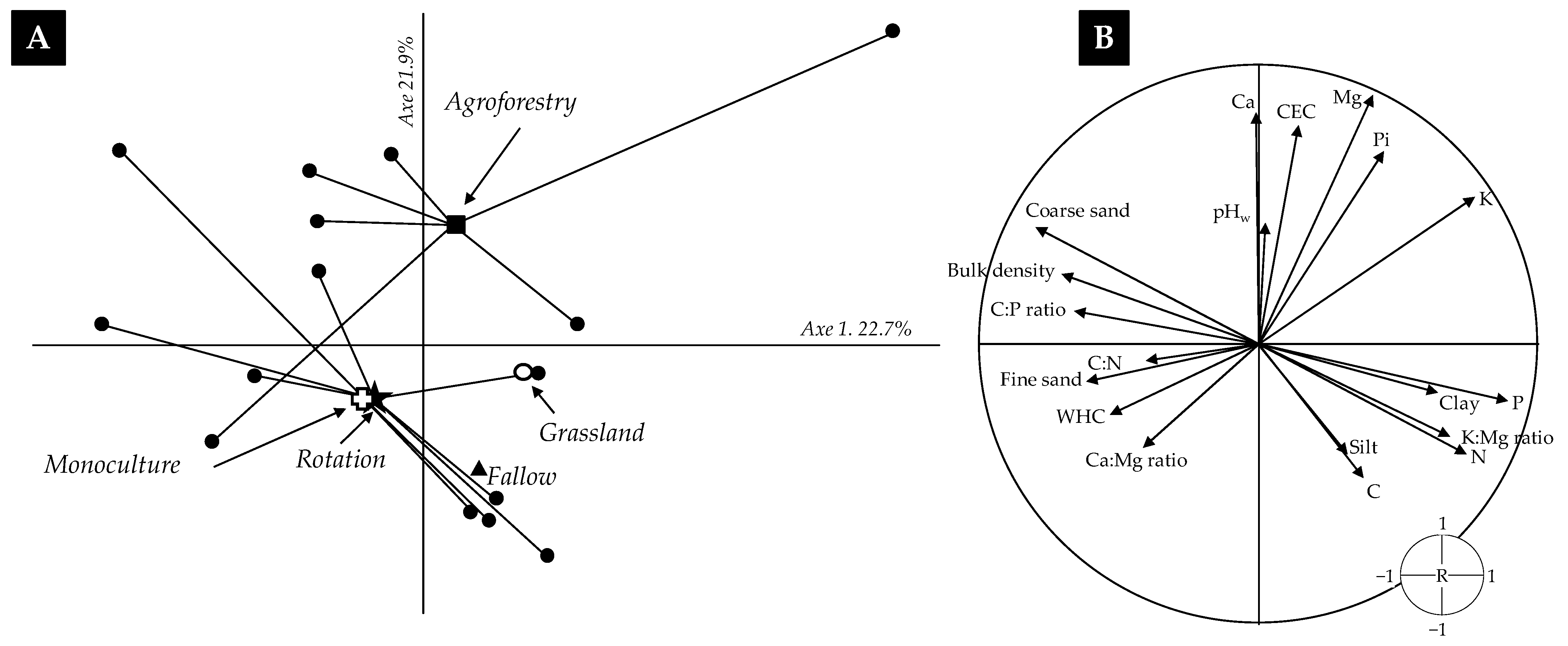
3.1. Soil Properties of the Selected Fields
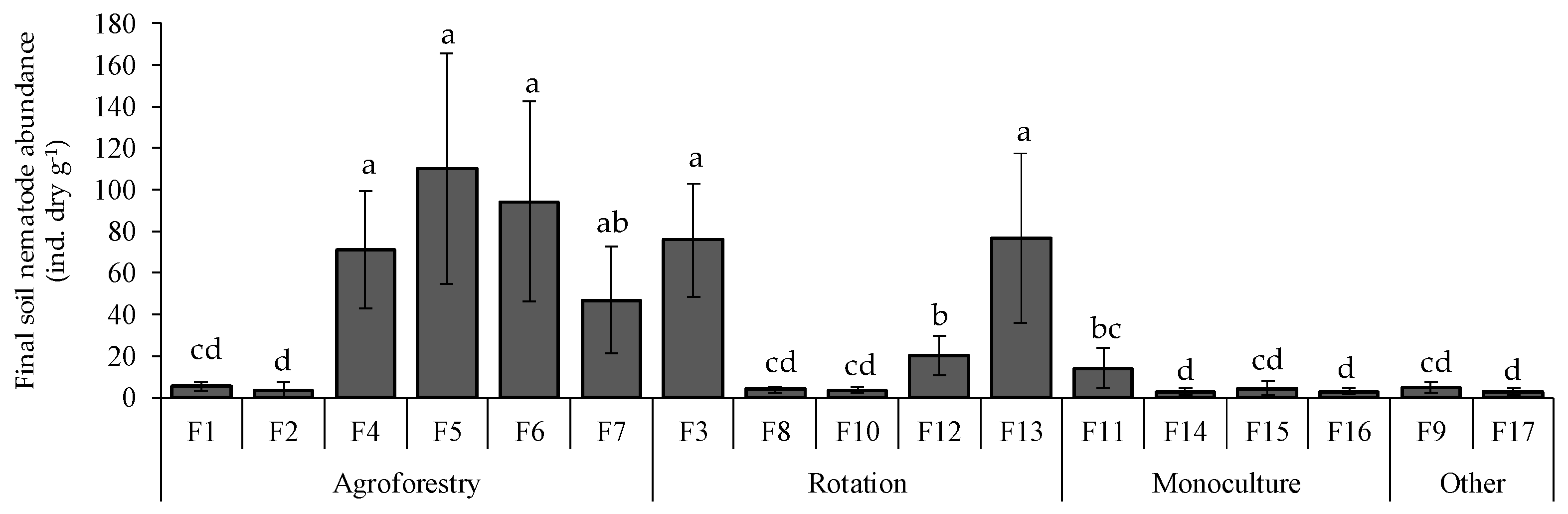
3.2. Final Soil Nematode Abundance
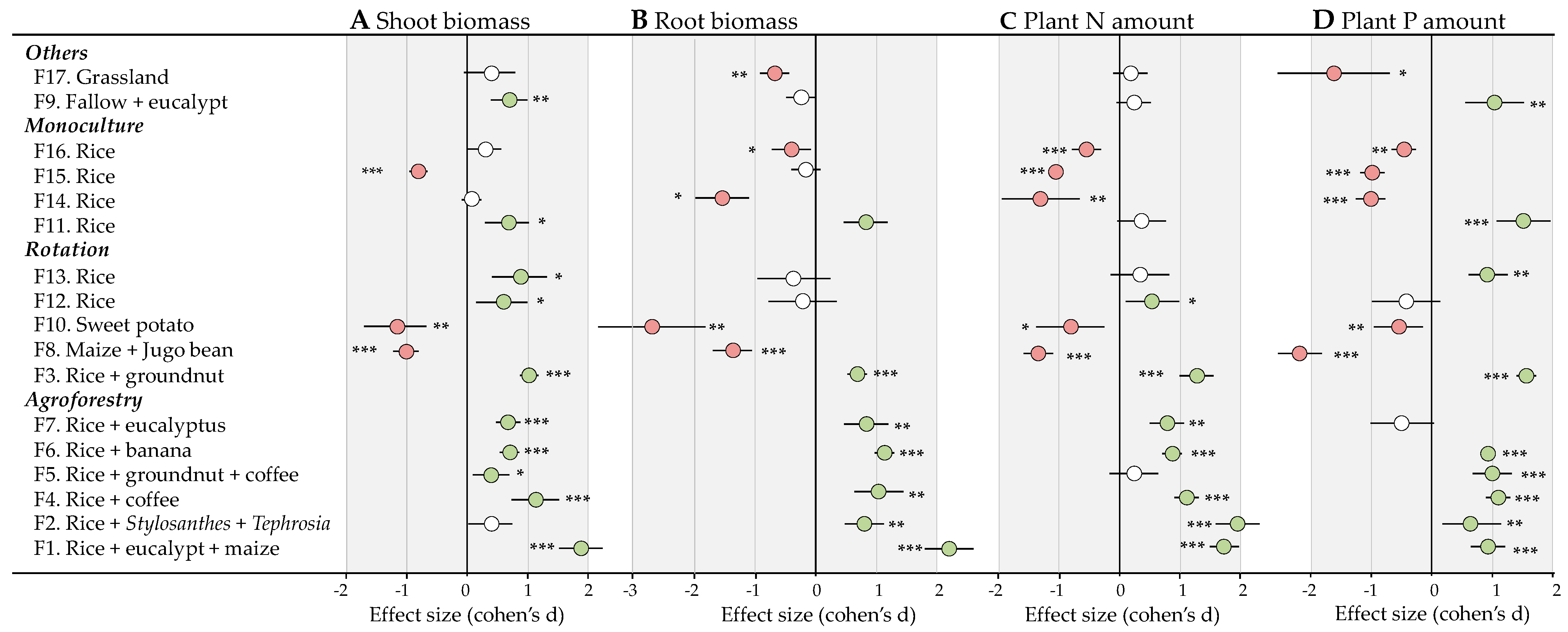
3.3. Effects of Bacterial-Feeding Nematodes on Plant Growth
3.4. Effects of Bacterial-Feeding Nematodes on Plant Nutrition
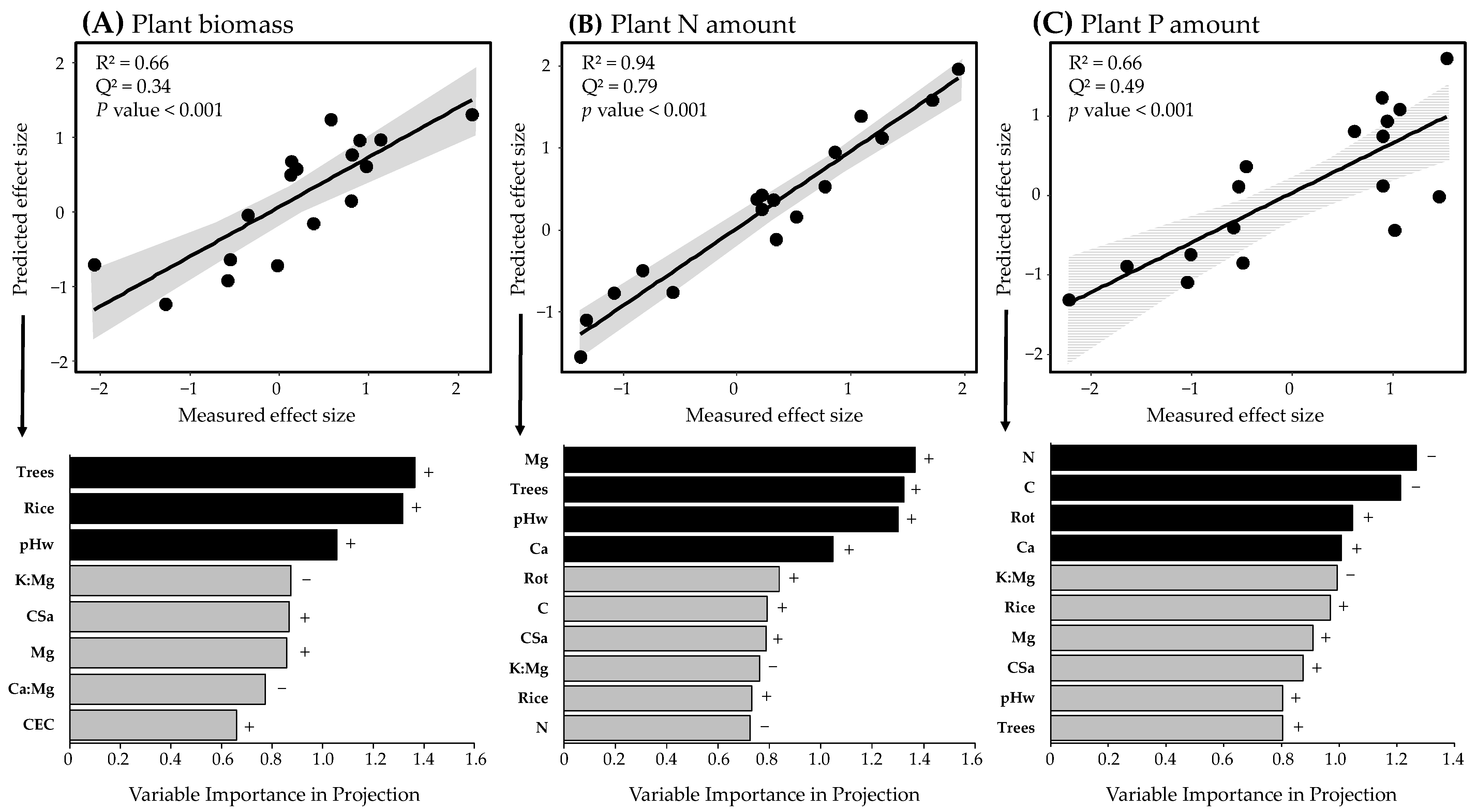
3.5. Agronomic and Soil Drivers of the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition
4. Discussion
4.1. Diversity of Practices in Upland Rainfed Rice Systems
4.2. Variable Effects of Bacterial-Feeding Nematodes on Plant Growth and Nutrition
4.3. Soil Drivers of the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition
4.4. Agronomic Drivers of the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition
5. Conclusions: Limits and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant Sci. 2020, 26, 237–247. [Google Scholar] [CrossRef]
- Freckman, D.W. Bacterivorous nematodes and organic-matter decomposition. Agric. Ecosyst. Environ. 1988, 24, 195–217. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63. [Google Scholar] [PubMed]
- Blanc, C.; Sy, M.; Djigal, D.; Brauman, A.; Normand, P.; Villenave, C. Nutrition on bacteria by bacterial-feeding nematodes and consequences on the structure of soil bacterial community. Eur. J. Soil Biol. 2006, 42, S70–S78. [Google Scholar] [CrossRef]
- Djigal, D.; Baudoin, E.; Philippot, L.; Brauman, A.; Villenave, C. Shifts in size, genetic structure and activity of the soil denitrifier community by nematode grazing. Eur. J. Soil Biol. 2010, 46, 112–118. [Google Scholar] [CrossRef]
- Ingham, R.E.; Trofymow, J.; Ingham, E.R.; Coleman, D.C. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; van der Meulen, H.R.; Lau, S.S. Nitrogen mineralization by bacterial-feeding nematodes: Verification and measurement. Plant Soil 1998, 203, 159–171. [Google Scholar] [CrossRef]
- Ranoarisoa, M.P.; Morel, C.; Andriamananjaraa, A.; Jourdan, C.; Bernard, L.; Becquer, T.; Rabeharisoa, L.; Rahajaharilazaa, K.; Plassard, C.; Blanchard, E.; et al. Effects of a bacterivorous nematode on rice 32P uptake and root architecture in a high P-sorbing ferrallitic soil. Soil Biol. Biochem. 2018, 122, 39–49. [Google Scholar] [CrossRef]
- Clarholm, M. Interactions of bacteria, protozoa and plants leading to mineralization of soil-nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Holford, I.C.R. Soil phosphorus: Its measurement, and its uptake by plants. Aust. J. Soil Res. 1997, 35, 227–239. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, D.C.; Cole, C.V.; Anderson, R.V.; Blaha, M.; Campion, M.K.; Clarholm, M.; Elliott, E.T.; Hunt, H.W.; Shaefer, B.; Sinclair, J. An analysis of rhizosphere-saprophage interactions in terrestrial ecosystems. Ecol. Bull. 1977, 25, 299–309. [Google Scholar]
- Darbyshire, J.F.; Davidson, M.S.; Chapman, S.J.; Ritchie, S. Excretion of nitrogen and phosphorus by the soil Ciliate Colpoda steinii when fed the soil bacterium Arthrobacter sp. Soil Biol. Biochem. 1994, 26, 1193–1199. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Buchan, D.; De Neve, S. Quantifying the influences of free-living nematodes on soil nitrogen and microbial biomass dynamics in bare and planted microcosms. Soil Biol. Biochem. 2014, 70, 131–141. [Google Scholar] [CrossRef]
- Irshad, U.; Brauman, A.; Villenave, C.; Plassard, C. Phosphorus acquisition from phytate depends on efficient bacterial grazing, irrespective of the mycorrhizal status of Pinus Pinaster. Plant Soil 2012, 358, 148–161. [Google Scholar] [CrossRef]
- Griffiths, B.S. Enhanced nitrification in the presence of bacteriophagous protozoa. Soil Biol. Biochem. 1989, 21, 1045–1051. [Google Scholar] [CrossRef]
- Griffiths, B.S. Mineralization of nitrogen and phosphorus by mixed cultures of the Ciliate protozoan Colpoda steinii, the nematode Rhabditis sp and the bacterium Pseudomonas Fluoresc. Soil Biol. Biochem. 1986, 18, 637–641. [Google Scholar] [CrossRef]
- Taylor, W.D. The effect of grazing by a ciliated protozoan on phosphorus limitation of heterotrophic bacteria in batch culture. J. Protozool. 1986, 33, 47–52. [Google Scholar] [CrossRef]
- Rønn, R.M.; Griffiths, B.S.; Young, I.M. Protozoa, nematodes and N-mineralization across a prescribed soil textural gradient. Pedobiologia 2001, 45, 481–495. [Google Scholar] [CrossRef]
- Woods, L.; Cole, C.; Elliott, E.; Anderson, R.; Coleman, D. Nitrogen transformations in soil as affected by bacterial-microfaunal interactions. Soil Biol. Biochem. 1982, 14, 93–98. [Google Scholar] [CrossRef]
- Cole, C.V.; Elliott, E.T.; Hunt, H.W.; Coleman, D.C. Trophic interactions in soils as they affect energy and nutrient dynamics. Phosphorus transformations. Microb. Ecol. 1978, 4, 381–387. [Google Scholar] [CrossRef]
- Coleman, D.C.; Anderson, R.V.; Cole, C.V.; Elliott, E.T.; Woods, L.; Campion, M.K. Trophic interactions in soils as they affect energy and nutrient dynamics. Flows of metabolic and biomass carbon. Microb. Ecol. 1978, 4, 373–380. [Google Scholar] [CrossRef]
- Jentschke, G.; Bonkowski, M.; Godbold, D.L.; Scheu, S. Soil protozoa and forest tree growth—Non-nutritional effects and interaction with mycorrhizae. Biol. Fertil. Soils 1995, 20, 263–269. [Google Scholar] [CrossRef]
- Baath, E.; Lohm, U.; Lundgren, B.; Rosswall, T.; Soderstrom, B.; Sohlenius, B. Impact of microbial-feeding animals on total soil activity and nitrogen dynamics—A soil microcosm experiment. Oikos 1981, 37, 257–264. [Google Scholar] [CrossRef]
- Irshad, U.; Villenave, C.; Brauman, A.; Plassard, C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 2011, 43, 2121–2126. [Google Scholar] [CrossRef]
- Trap, J.; Ranoarisoa, P.M.; Irshad, U.; Plassard, C. Richness of rhizosphere organisms affects plant P nutrition according to P source and mobility. Agriculture 2021, 11, 157. [Google Scholar] [CrossRef]
- Alphei, J.; Bonkowski, M.; Scheu, S. Protozoa, Nematoda and Lumbricidae in the rhizosphere of Hordelymus europeaus (Poaceae): Faunal interactions, response of microorganisms and effects on plant growth. Oecologia 1996, 106, 111–126. [Google Scholar] [CrossRef]
- Djigal, D.; Brauman, A.; Diop, T.A.; Chotte, J.L.; Villenave, C. Influence of bacterial-feeding nematodes (Cephalobidae) on soil microbial communities during maize growth. Soil Biol. Biochem. 2004, 36, 323–331. [Google Scholar] [CrossRef]
- Bonkowski, M.; Clarholm, M. Stimulation of plant growth through interactions of bacteria and protozoa: Testing the auxiliary microbial loop hypothesis. Acta Protozool. 2012, 51, 237–247. [Google Scholar]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Trap, J.; Bernard, L.; Brauman, A.; Pablo, A.-L.; Plassard, C.; Ranoarisoa, M.P.; Blanchart, E. Plant roots increase bacterivorous nematode dispersion through nonuniform glass-bead media. J. Nematol. 2015, 47, 296. [Google Scholar] [PubMed]
- Sánchez-Moreno, S.; Nicola, N.L.; Ferris, H.; Zalom, F.G. Effects of agricultural management on nematode–mite assemblages: Soil food web indices as predictors of mite community composition. Appl. Soil Ecol. 2009, 41, 107–117. [Google Scholar] [CrossRef]
- Senapati, B. Biotic interactions between soil nematodes and earthworms. Soil Biol. Biochem. 1992, 24, 1441–1444. [Google Scholar] [CrossRef]
- Yeates, G. Influence of earthworms on soil nematode populations. J. Nematol. 1980, 12, 242. [Google Scholar]
- Sayre, R. Bacterial diseases of nematodes and their role in controlling nematode populations. Agric. Ecosyst. Environ. 1988, 24, 263–279. [Google Scholar] [CrossRef]
- Raminoarison, M.; Razafimbelo, T.; Rakotoson, T.; Becquer, T.; Blanchart, E.; Trap, J. Multiple-nutrient limitation of upland rainfed rice in ferralsols: A greenhouse nutrient-omission trial. J. Plant Nutr. 2020, 43, 270–284. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef] [Green Version]
- Sommers, L.; Nelson, D. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Amer, F.; Bouldin, D.; Black, C.; Duke, F. Characterization of soil phosphorus by anion exchange resin adsorption and P 32-equilibration. Plant Soil 1955, 6, 391–408. [Google Scholar] [CrossRef]
- Rao, A.S.; Reddy, K.S.; Takkar, P. Malachite green method compared to ascorbic acid for estimating small amounts of phosphorus in water, 0.01 M calcium chloride, and Olsen soil extracts. Commun. Soil Sci. Plant Anal. 1997, 28, 589–601. [Google Scholar] [CrossRef]
- Seinhorst, J. Modifications of the elutriation method for extracting nematodes from soil. Nematologica 1962, 8, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Raboin, L.M.; Ramanantsoanirina, A.; Dzido, J.L.; Frouin, J.; Radanielina, T.; Tharreau, D.; Dusserre, J.; Ahmadi, N. Création variétale pour la riziculture pluviale d’altitude à Madagascar: Bilan de 25 années de sélection. Cah. Agric. 2013, 22, 450–458. [Google Scholar]
- Raboin, L.M.; Randriambololona, T.; Radanielina, T.; Ramanantsoanirina, A.; Ahmadi, N.; Dusserre, J. Upland rice varieties for smallholder farming in the cold conditions in Madagascar’s tropical highlands. Field Crop. Res. 2014, 169, 11–20. [Google Scholar] [CrossRef]
- Villenave, C.; Rabary, B.; Chotte, J.L.; Blanchart, E.; Djigal, D. Impact of direct seeding mulch-based cropping systems on soil nematodes in a long-term experiment in Madagascar. Pesqui. Agropecu. Bras. 2009, 44, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Paliy, O.; Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenhaus, M. La Régression PLS; Editions Technip: Paris, France, 1998; p. 254. [Google Scholar]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Rakotomalala, R. TANAGRA: Un logiciel gratuit pour l’enseignement et la recherche. Actes De EGC RNTI-E3 2005, 2, 697–702. [Google Scholar]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Ranoarisoa, M.P.; Blanchart, E.; Vom Brocke, K.; Ramanantsoanirina, A.; Sester, M.; Plassard, C.; Cournac, L.; Trap, J. Attractancy of bacterivorous nematodes to root-adhering soils differs according to rice cultivars. Rhizosphere 2017, 3, 128–131. [Google Scholar] [CrossRef]
- Sundin, P.; Valeur, A.; Olsson, S.; Odham, G. Interactions between bacteria-feeding nematodes and bacteria in the rape rhizosphere—Effects on root exudation and distribution of bacteria. FEMS Microbiol. Ecol. 1990, 73, 13–22. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, H.; Venette, R.C.; Lau, S.S. Population energetics of bacterial-feeding nematodes: Carbon and nitrogen budgets. Soil Biol. Biochem. 1997, 29, 1183–1194. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Calba, H.; Cazevieille, P.; Jaillard, B. Modelling of the dynamics of Al and protons in the rhizosphere of maize cultivated in acid substrate. Plant Soil 1999, 209, 57–69. [Google Scholar] [CrossRef]
- Grzebisz, W.; Przygocka-Cyna, K.; Szczepaniak, W.; Diatta, J.; Potarzycki, J. Magnesium as a nutritional tool of nitrogen efficient management-plant production and environment. J. Elem. 2010, 15, 771–788. [Google Scholar] [CrossRef] [Green Version]
- Ettema, C.H. Soil nematode diversity: Species coexistence and ecosystem function. J. Nematol. 1998, 30, 159. [Google Scholar]
- Jiang, Y.; Liu, M.; Zhang, J.; Chen, Y.; Chen, X.; Chen, L.; Li, H.; Zhang, X.X.; Sun, B. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J. 2017, 11, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.V.; Coleman, D.C. Population development and interactions between 2 species of bacteriophagic nematodes. Nematologica 1981, 27, 6–19. [Google Scholar] [CrossRef]
- Noble, A.; Zenneck, I.; Randall, P. Leaf litter ash alkalinity and neutralisation of soil acidity. Plant Soil 1996, 179, 293–302. [Google Scholar] [CrossRef]
| Practices | Fields | Crops | Fertilization | Tillage | SY | |
|---|---|---|---|---|---|---|
| Sampling Year (SY) | Previous Year (PY) | SY | SY | |||
| Grassland | F17 | Grassland | Grassland | No | No | 2016 |
| Fallow | F9 | Fallow with eucalypt | Rice with eucalypt | No | No | 2016 |
| Monoculture | F16 | Rice | Grassland | Manure with ashes and rice chaff | Yes | 2016 |
| F15 | Rice | Grassland | Compost | Yes | 2016 | |
| F14 | Rice | Grassland | Manure | Yes | 2016 | |
| F11 | Rice | Rice | Compost with ashes | Yes | 2017 | |
| Rotation | F13 | Rice | Sweet potato | Ashes with crop residues | Yes | 2017 |
| F12 | Rice | Jugo bean | Compost with ashes | Yes | 2017 | |
| F10 | Sweet potato (Ipomoea batatas) | French beans | Rice straw | Yes | 2016 | |
| F8 | Maise with Jugo bean (Vigna subterranea) | Rice | Compost | Yes | 2016 | |
| F3 | Rice with groundnut (Arachis hypogaea) | Soybean (Glycine max) | Compost with manure and crop residues | Yes | 2017 | |
| Agroforestry | F7 | Rice with eucalypt | Eucalypt | Compost with manure | Yes | 2017 |
| F6 | Rice with banana (Musa sp.) | Banana with Taro | Compost | Yes | 2017 | |
| F5 | Rice with groundnut and coffee trees | Coffee tree with Taro (Colocasia esculenta) | Compost | Yes | 2017 | |
| F4 | Rice with coffee (C. arabica) trees | French beans (Phaseolus vulgaris) with coffee tree | Crop residues | Yes | 2017 | |
| F2 | Rice with Stylosanthes guianensis and Tephrosia vogelii | Stylosanthes with Tephrosia | No | No | 2016 | |
| F1 | Rice$ with Eucalyptus robusta and maize (Zea mays) | Grassland with eucalypt | Compost with green manure | No | 2016 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trap, J.; Ranoarisoa, M.P.; Raharijaona, S.; Rabeharisoa, L.; Plassard, C.; Mayad, E.H.; Bernard, L.; Becquer, T.; Blanchart, E. Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique. Sustainability 2021, 13, 7181. https://doi.org/10.3390/su13137181
Trap J, Ranoarisoa MP, Raharijaona S, Rabeharisoa L, Plassard C, Mayad EH, Bernard L, Becquer T, Blanchart E. Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique. Sustainability. 2021; 13(13):7181. https://doi.org/10.3390/su13137181
Chicago/Turabian StyleTrap, Jean, Mahafaka Patricia Ranoarisoa, Sariaka Raharijaona, Lilia Rabeharisoa, Claude Plassard, El Hassan Mayad, Laetitia Bernard, Thierry Becquer, and Eric Blanchart. 2021. "Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique" Sustainability 13, no. 13: 7181. https://doi.org/10.3390/su13137181
APA StyleTrap, J., Ranoarisoa, M. P., Raharijaona, S., Rabeharisoa, L., Plassard, C., Mayad, E. H., Bernard, L., Becquer, T., & Blanchart, E. (2021). Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique. Sustainability, 13(13), 7181. https://doi.org/10.3390/su13137181








