Terra Preta Properties in Northwestern Amazonia (Colombia)
Abstract
:1. Introduction
2. Materials and Methods
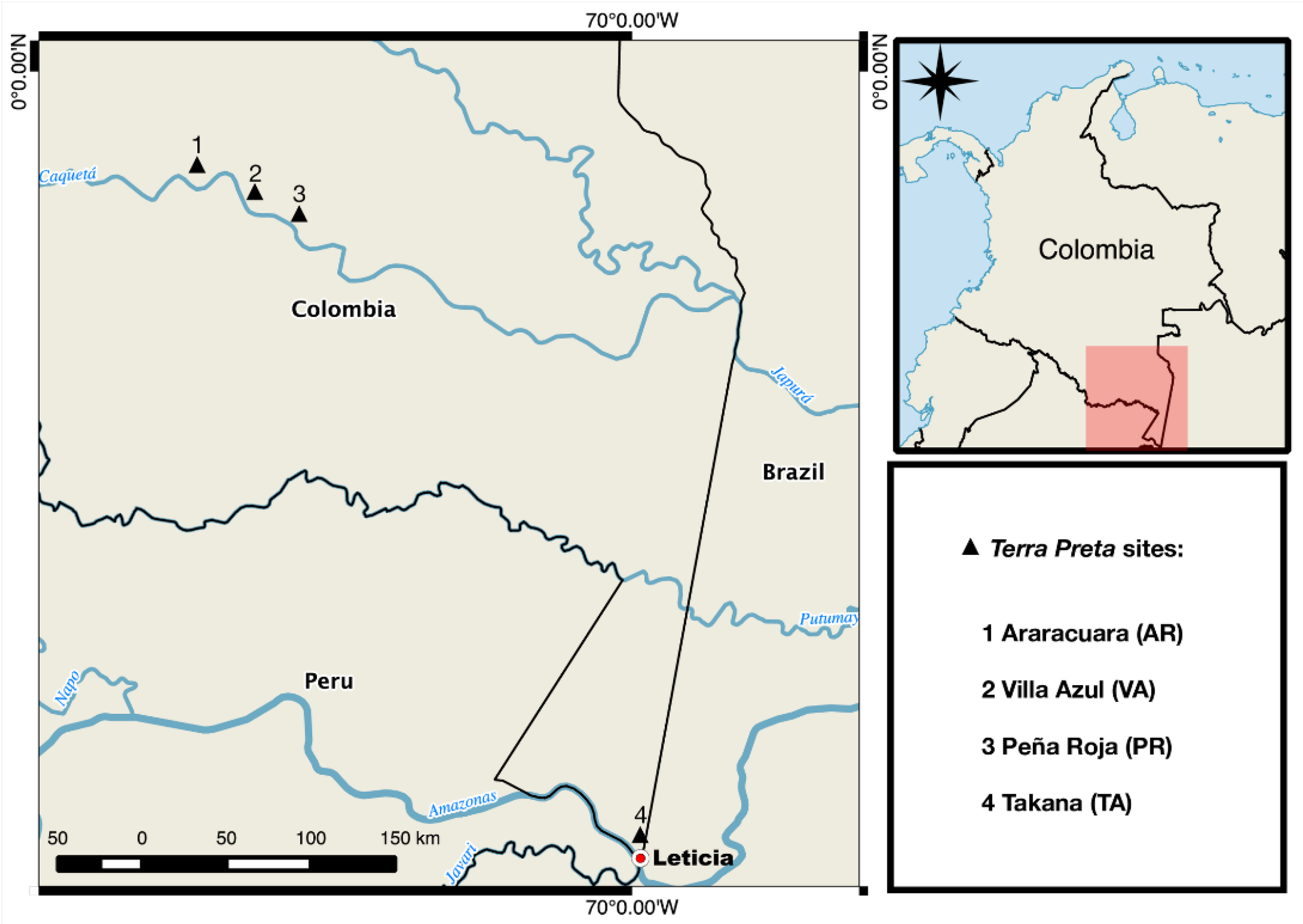
2.1. Sites and Sampling
- The absence of ceramic sherds;
- Thickness of the A horizon (not more than 10 cm);
2.2. Analytical Procedures
2.3. Calculations
3. Results
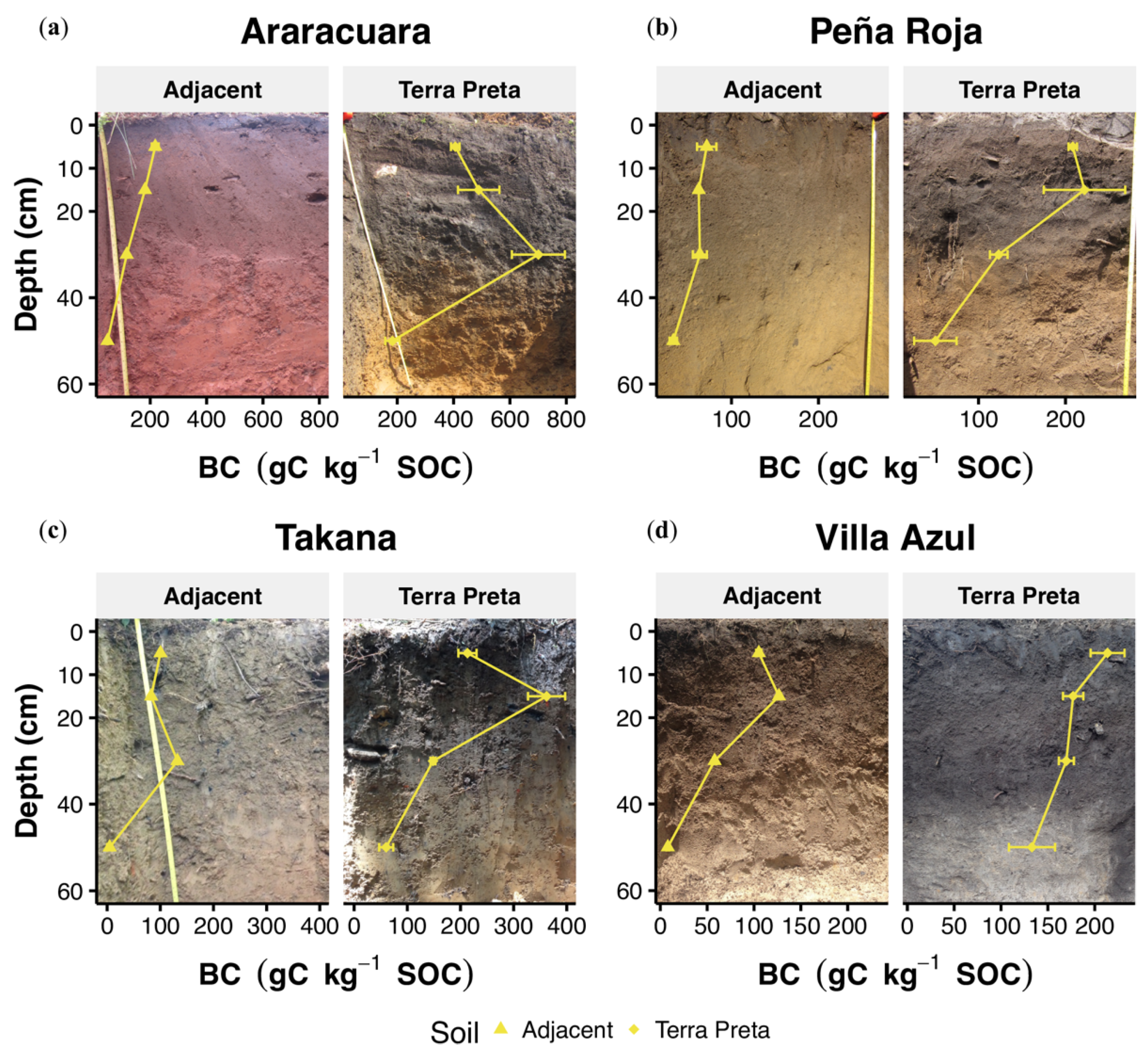
3.1. Basic Chemical and Physical Soil Parameters
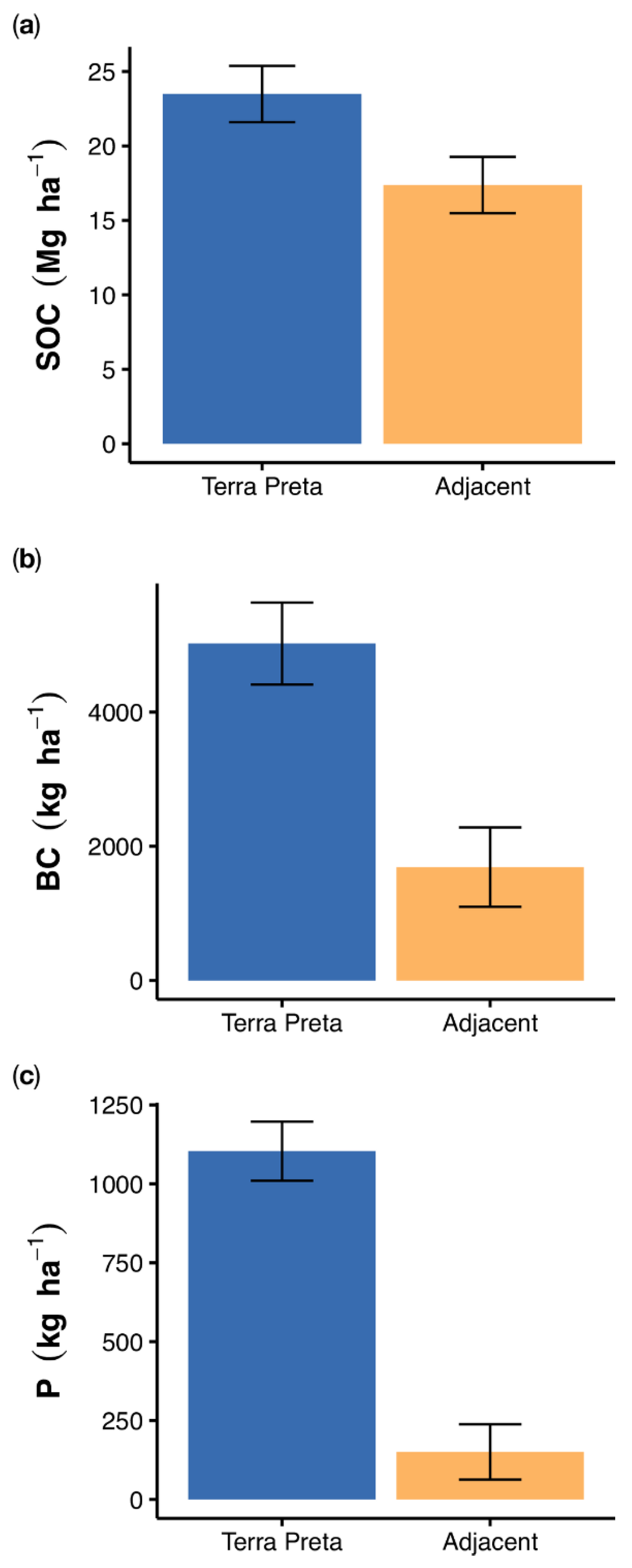
3.2. Element and Black Carbon Stocks
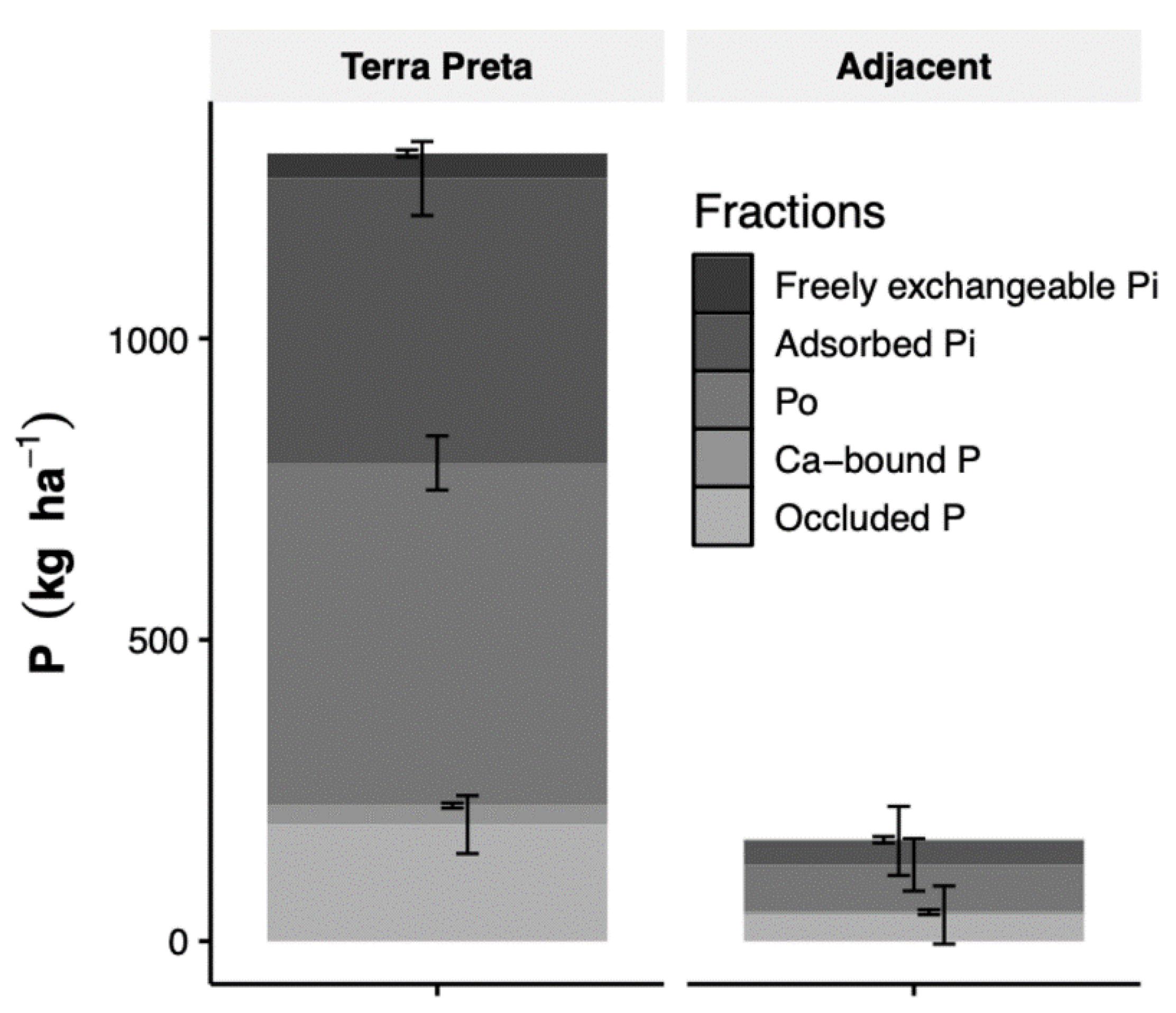
3.3. P Fractions
3.4. Amino Sugars
4. Discussion
4.1. Morphological Variation and pH Values in TP Profiles
4.2. Organic Matter, Black Carbon and Phosphorus Stocks in TP Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clement, C.R.; Denevan, W.M.; Heckenberger, M.J.; Junqueira, A.B.; Neves, E.G.; Teixeira, W.G.; Woods, W.I. The domestication of Amazonia before European conquest. Proc. R. Soc. B 2015, 282. [Google Scholar] [CrossRef] [Green Version]
- Watling, J.; Iriarte, J.; Mayle, F.E.; Schaan, D.P.; Pessenda, L.C.R.; Loader, N.J.; Street-Perrott, F.A.; Dickau, R.E.; Damasceno, A.; Ranzi, A. Impact of pre-Columbian “geoglyph” builders on Amazonian forests. Proc. Natl. Acad. Sci. USA 2017, 114, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, J.G.; Schaan, D.P.; Robinson, M.; Barbosa, A.D.; Aragão, L.E.O.C.; Marimon, B.H., Jr.; Marimon, B.S.; da Silva, I.B.; Khan, S.S.; Nakahara, F.R.; et al. Pre-Columbian earth-builders settled along the entire southern rim of the Amazon. Nat. Commun. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, B. Prehistorically modified soils of central Amazonia: A model for sustainable agriculture in the twenty-first century. Philos. Trans. R. Soc. B 2007, 362, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M. Amazonian Dark Earths: Pathways to sustainable development in tropical rainforests? Bol. Mus. Para. Emílio Goeldi Ciências Hum. 2013, 8, 11–38. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.J.H. Anthrosols and human carrying capacity in Amazonia. Ann. Assoc. Am. Geogr. 1980, 70, 553–566. [Google Scholar] [CrossRef]
- Schmidt, M.; Heckenberger, M.J. Amerindian anthrosols: Amazonian dark earth formation in the Upper Xingu. In Amazonian Dark Earths: Wim Sombroeks’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A.M.G.A., Rebellato, L., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2008; pp. 163–192. [Google Scholar]
- Myers, T.P.; Denevan, W.M.; WinklerPrins, A.M.G.A.; Porro, A. Historical perspectives on Amazonian Dark Earths. In Amazonian Dark Earths; Lehmann, J., Kern, D.C., Glaser, B., Woods, W.I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 15–28. [Google Scholar]
- Neves, E.; Petersen, J.B.; Bartone, R.N.; da Silva, C.A. Historical and socio-cultural origins of Amazonian Dark Earths. In Amazonian Dark Earths: Origin, Properties, Management; Lehmann, J., Kern, D.C., Glaser, B., Woods, W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 29–50. [Google Scholar]
- Rebellato, L.; Woods, W.I.; Neves, E.G. Pre-columbian settlement dynamics in the central Amazon. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Neves, E.; Petersen, J. Political economy and pre-columbian landscape transformation in central Amazonia. In Time and Complexity in Historical Ecology: Studies in the Neotropical Lowlands; Balee, W., Erickson, C., Eds.; Columbia University Press: New York, NY, USA, 2006. [Google Scholar]
- Love, E. Nature and Culture in Prehistoric Amazonia: Using G.I.S. to Reconstruct Ancient Ethnogenetic Processes from Archaeology, Linguistics, Geography, and Ethnohistory; Lund University: Lund, Sweden, 2011. [Google Scholar]
- Kämpf, N.; Woods, W.; Kern, D.C.; Cunha, T.J.F. Classification of Amazonian Dark Earths and other ancient anthopic soils. In Amazonian Dark Earths: Origin, Properties, Management; Lehmann, J., Kern, D.C., Glaser, B., Woods, W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 77–104. [Google Scholar]
- Schmidt, M. Historical Landscapes in the Neotropics: A model for prehistoric anthrosol (terra preta) fromation in the Upper Xingu. In Archaeologia Amazonica II; Museu Paraense Emilio Goeldi: Belém, Brazil, 2010; pp. 341–366. [Google Scholar]
- Kern, D.; Lima, H.; da Costa, J.; de Lima, H.; Ribeiro, A.; Moraes, B.; Kämpf, N. Terras pretas: Approaches to formation processes in a new paradigm. Geoarchaeology 2017, 32, 694–706. [Google Scholar] [CrossRef]
- Fraser, J.; Teixeira, W.; Falcao, N.; Woods, W.; Lehmann, J.; Junqueira, A.B. Anthropogenic soils in the Central Amazon: From categories to a continuum. Area 2011, 43, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Kern, D.C.; Glaser, B.; Woods, W. Amazonian Dark Earths: Origin, Properties and Management; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W. Soil organic matter in major pedogenic soil groups. Geoderma 2020, 384, 114785. [Google Scholar] [CrossRef]
- Macedo, R.S.; Teixeira, W.G.; Corrêa, M.M.; Martins, G.C.; Vidal-Torrado, P. Pedogenetic processes in anthrosols with pretic horizon (Amazonian Dark Earth) in Central Amazon, Brazil. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eden, M.J.; Bray, W.; Herrera, L.; Mcewan, C. Terra-Preta Soils and Their Archaeological Context in the Caqueta Basin of Southeast Colombia. Am. Antiq. 1984, 49, 125–140. [Google Scholar] [CrossRef]
- Herrera, L.; Cavelier, I.; Rodriguez, C.; Mora, S. The technical transformation of an agricultural system in the colombian Amazon. World Archaeol. 1992, 24, 98–113. [Google Scholar] [CrossRef]
- Costa, M.; Morcote, G.; Carvalho, M.; Jholy, G.; Molano-Valdes, U. Mineralogy and chemistry of archaeological ceramic fragments from Archaeological Dark Earth Site in Colombian Amazon. Geosciences 2011, 64, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Peña-Venegas, C.P.; Kuyper, T.W.; Davison, J.; Jairus, T.; Vasar, M.; Stomph, T.J.; Struik, P.C.; Öpik, M. Distinct arbuscular mycorrhizal fungal communities associate with different manioc landraces and Amazonian soils. Mycorrhiza 2019, 29, 263–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etter, A.; McAlpine, C.; Wilson, K.; Phinn, S.; Possingham, H.P. Regional patterns of agricultural land use and deforestation in Colombia. Agr. Ecosyst. Environ. 2006, 114, 369–386. [Google Scholar] [CrossRef]
- Morcote-Rios, G.H.; Sicard, T.L. Las Terras Pretas del Igarapé Takana. Un Sistema de Cultivo Precolombino en Leticia-Amazonas, Colombia; Universidad Nacional de Colombia: Bogotá, Colombia, 2012. [Google Scholar]
- Peña-Venegas, C.P.; Verschoor, G.; Stomph, T.J.; Struik, P.C. Challenging current knowledge on Amazonian Dark Earths: Indigenous manioc cultivation on different soils of the Colombian Amazon. Cult. Agric. Food Environ. 2017, 39, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Morcote-Rios, G.H. Terras Pretas de Índio of the Caquetá-Japurá River (Colombian Amazonia). Tipití J. Soc. Anthropol. Lowl. South Am. 2013, 11, 30–39. [Google Scholar]
- Moraes, C.d.P. O determinismo agrícola na arqueologia amazônica. Estud. Avançados 2015, 29, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Macedo, R.; Teixeira, W.G.; Lima, H.N.; Souza, C.; Silva, F.; Encinas, O.C.; Neves, E.G. Amazonian dark earths in the fertile floodplains of the Amazon River, Brazil: An example of non-intentional formation of anthropic soils in the Central Amazon region. Bol. Mus. Para. Emílio Goeldi 2019, 14, 207–227. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Py-Daniel, A.R.; Moraes, C.D.; Valle, R.B.M.; Caromano, C.F.; Texeira, W.G.; Barbosa, C.A.; Fonseca, J.A.; Magalhaes, M.P.; Santos, D.S.D.; et al. Dark earths and the human built landscape in Amazonia: A widespread pattern of anthrosol formation. J. Archaeol. Sci. 2014, 42, 152–165. [Google Scholar] [CrossRef]
- Peña-Venegas, C.P.; Stomph, T.J.; Verschoor, G.; Echeverri, J.A.; Struik, P.C. Classification and use of natural and anthropogenic soils by indigenous communities of the upper Amazon Region of Colombia. Hum. Ecol. 2016, 44, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Campos, C.V.; Vasconselos de Macêdo, J.L.; German, L. Sequential P fractionation of relict anthropogenic dark Earths of Amazonia. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 113–123. [Google Scholar]
- Souza, K.W.d.; Lima, H.N.; Schaefer, C.E.G.R.; Teixeira, W.G.; Pulrolnik, K.; Corrêa, G.R. Phosphorous forms in cultivated indian black earth (anthrosols) of varying texture in the brazilian Amazon. Rev. Bras. Cienc. Solo 2009, 33, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, C.E.G.R.; Lima, H.N.; Gilkes, R.J.; Mello, J.W.V. Micromorphology and electron microprobe analysis of phosphorus and potassium forms of an Indian Black Earth (IBE) Anthrosol from Western Amazonia. Aust. J. Soil Res. 2004, 42, 401–409. [Google Scholar] [CrossRef]
- Sombroek, W. Amazon. Soils: A Reconnaissance of the Soils of the Brazilian Amazon Region; Centre for Agricultural Publications and Documentation: Wageningen, The Netherlands, 1966. [Google Scholar]
- Sato, S.; Neves, E.G.; Solomon, D.; Liang, B.Q.; Lehmann, J. Biogenic calcium phosphate transformation in soils over millennial time scales. J. Soil Sediments 2009, 9, 194–205. [Google Scholar] [CrossRef]
- Chamberlain, P.M.; Bull, I.D.; Black, H.I.J.; Ineson, P.; Evershed, R.P. Fatty acid composition and change in Collembola fed differing diets: Identification of trophic biomarkers. Soil Biol. Biochem. 2005, 37, 1608–1624. [Google Scholar] [CrossRef]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Combining Biomarker with Stable Isotope Analyses for Assessing the Transformation and Turnover of Soil Organic Matter; Sparks, D.L., Ed.; Elsevier: Burlington, MA, USA, 2008; Volume 100. [Google Scholar]
- Mckey, D.; Rostain, S.; Iriarte, J.; Glaser, B.; Birk, J.J.; Holst, I.; Renard, D. Pre-Columbian agricultural landscapes, ecosystem engineers, and self-organized patchiness in Amazonia. Proc. Natl. Acad. Sci. USA 2010, 107, 7823–7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birk, J.J.; Teixeira, W.G.; Neves, E.G.; Glaser, B. Faeces deposition on Amazonian Anthrosols as assessed from 5 beta-stanols. J. Archaeol. Sci. 2011, 38, 1209–1220. [Google Scholar] [CrossRef]
- Birk, J.J.; Dippold, M.; Wiesenberg, G.L.; Glaser, B. Combined quantification of faecal sterols, stanols, stanones and bile acids in soils and terrestrial sediments by gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1242, 1–10. [Google Scholar] [CrossRef]
- Lombardo, U.; Szabo, K.; Capriles, J.M.; May, J.H.; Amelung, W.; Hutterer, R.; Lehndorff, E.; Plotzki, A.; Veit, H. Early and middle holocene hunter-gatherer occupations in western Amazonia: The hidden shell middens. PLoS ONE 2013, 8, e72746. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Indio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in Terra Preta and Oxisols of the Brazilian Amazon as estimated by benzenecarboxylic acids as specific markers. Abstr. Pap. Am. Chem. Soc. 1999, 217, U829. [Google Scholar]
- Glaser, B.; Balashov, E.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in density fractions of anthropogenic soils of the Brazilian Amazon region. Org. Geochem. 2000, 31, 669–678. [Google Scholar] [CrossRef]
- Novotny, E.H.; Hayes, M.H.B.; Madari, B.E.; Bonagamba, T.J.; deAzevedo, E.R.; de Souza, A.A.; Song, G.X.; Nogueira, C.M.; Mangrich, A.S. Lessons from the Terra Preta de Indios of the Amazon Region for the utilisation of charcoal for soil amendment. J. Braz. Chem. Soc. 2009, 20, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819. [Google Scholar] [CrossRef]
- Brodowski, S.; Rodionov, A.; Haumaier, L.; Glaser, B.; Amelung, W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem. 2005, 36, 1299–1310. [Google Scholar] [CrossRef]
- Kappenberg, A.; Blasing, M.; Lehndorff, E.; Amelung, W. Black carbon assessment using benzene polycarboxylic acids: Limitations for organic-rich matrices. Org. Geochem. 2016, 94, 47–51. [Google Scholar] [CrossRef]
- Schneider, M.P.W.; Lehmann, J.; Schmidt, M.W.I. Charcoal quality does not change over a century in a tropical agro-ecosystem. Soil Biol. Biochem. 2011, 43, 1992–1994. [Google Scholar] [CrossRef]
- Wolf, M.; Lehndorff, E.; Wiesenberg, G.L.B.; Stockhausen, M.; Schwark, L.; Amelung, W. Towards reconstruction of past fire regimes from geochemical analysis of charcoal. Org. Geochem. 2013, 55, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B.; Woods, W.I. Amazonian Dark Earths: Explorations in Space and Time; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Parsons, J.W. Chemistry and distribution of amino sugars in soils and soil organisms. In Soil Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Marcel Dekker: New York, NY, USA, 1981; Volume 5, pp. 197–227. [Google Scholar]
- Amelung, W. Methods using amino sugars as markers for microbial residues in soil. In Assessment Methods for Soil Carbon; Lewis Publishers: Boca Raton, FL, USA, 2001; Volume 100, pp. 233–270. [Google Scholar]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Denevan, W.M. A bluff model of riverine settlement in prehistoric Amazonia. Ann. Assoc. Am. Geogr. 1996, 86, 654–681. [Google Scholar] [CrossRef]
- Kern, D.C.; da Costa, M.L.; Frazão, F.J.L. Evolution of the scientific knowledge regarding archaeological black Earths of Amazonia. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Lips, J.M.; Duivenvoorden, J.F. Regional patterns of well drained upland soil differentiation in the middle Caquetá basin of Colombian Amazonia. Geoderma 1996, 72, 219–257. [Google Scholar] [CrossRef]
- Duivenvoorden, J.F. Vascular plant species counts in the rain forests of the middle Caquetá area, Colombian Amazonia. Biodivers. Conserv. 1994, 3, 685–715. [Google Scholar] [CrossRef] [Green Version]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. Can. Soc. Soil Sci. 1993, 7, 75–86. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Kappenberg, A.; Lehndorff, E.; Pickarski, N.; Litt, T.; Amelung, W. Solar controls of fire events during the past 600,000 years. Quat. Sci. Rev. 2019, 208, 97–104. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Amelung, W.; Lobe, I.; Du Preez, C.C. Fate of microbial residues in sandy soils of the South African Highveld as influenced by prolonged arable cropping. Eur. J. Soil Sci. 2002, 53, 29–35. [Google Scholar] [CrossRef]
- Kunz, S. Stickstoff-Pools Tropischer Böden: Eine Vergleichende Studie von Oxisol und Terra Preta do Indio (Indianerschwarzerden); Martin Luther University Halle-Wittenberg: Halle, Germany, 1996. [Google Scholar]
- Glaser, B.; Zech, W.; Woods, W.I. History, current knowledge and future perspectives of geoecological research concerning the origin of Amazonian anthropogenic Dark Earths (Terra Preta). In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 9–18. [Google Scholar]
- Lima, H.N.; Schaefer, C.E.R.; Mello, J.W.V.; Gilkes, R.J.; Ker, J.C. Pedogenesis and pre-Colombian land use of “Terra Preta Anthrosols” (“Indian black earth”) of Western Amazonia. Geoderma 2002, 110, 1–17. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [Green Version]
- Falcão, N.P.S.; Clement, C.R.; Tsai, S.M.; Comerford, N.B. Pedology, fertility, and biology of central Amazonian Dark Earths. In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., Winkler Prins, A., Rebellato, L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 213–228. [Google Scholar]
- Novotny, E.H.; Hayes, M.H.B.; deAzevedo, E.R.; Bonagamba, T.J. Characterisation of black carbon-rich samples by C-13 solid-state nuclear magnetic resonance. Naturwissenschaften 2006, 93, 447–450. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L.; Condron, L.M.; Richardson, S.J.; Peltzer, D.A.; Allison, V.J. Soil organic phosphorus transformations during pedogenesis. Ecosystems 2007, 10, 1166–1181. [Google Scholar] [CrossRef]
- Zhang, Q.; Bol, R.; Amelung, W.; Missong, A.; Siemens, J.; Mulder, I.; Willbold, S.; Müller, C.; Westphal Muniz, A.; Klumpp, E. Water dispersible colloids and related nutrient availability in Amazonian Terra Preta soils. Geoderma 2020, 97, 115103. [Google Scholar]
- Lang, F.; Pohlmeier, A.; Kaupenjohann, M. Mechanism of molybdenum sorption to iron oxides using pressure-jump relaxation. J. Plant Nutr. Soil Sci. 2000, 163, 571–575. [Google Scholar] [CrossRef]
- Alho, C.F.B.V. Long-Term Persistence of Soil Organic Matter in Amazonian Dark Earth. Ph.D. Thesis, Embrapa Solos-Tese/Dissertação (ALICE). 2019. Available online: https://www.alice.cnptia.embrapa.br/handle/doc/1114989 (accessed on 23 June 2021).
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
| Coordinates | Munsell Color/ Chroma | Texture (WRB) 1 | Depth (cm) | Dry Density (ton m−3) | pH(H20) | SOC (g kg−1) | Ntotal (g kg−1) | Ptotal (mg kg−1) | SUM BPCA 2 [g C Kg−1] | B5CA: B6CA |
|---|---|---|---|---|---|---|---|---|---|---|
| TP Araracuara 00°36′36′6″ S 72°23′26′9″ W | 10YR 3/2 | SL | 0–10 | 1.04 | 4.7 ± 0.3 | 19.1 | 3.0 | 1016.0 | 179.1 | 0.79 |
| 10YR 4/2 | SL | 10–20 | 1.06 | 4.67 ± 0.4 | 26.8 | 2.1 | 865.2 | 215.7 | 0.79 | |
| 10YR 4/2 | SL | 20–40 | 1.11 | 4.52 ± 0.1 | 30.1 | 2.1 | 934.0 | 309.1 | 0.88 | |
| 10YR 5/4 | SCL | 40–60 | 1.38 | 4.07 ± 0.2 | 21.4 | 0.9 | 1269.2 | 80.9 | 0.84 | |
| ADJ Araracuara 00°36′27″ S 72°22′52′2″ W | 7.5YR 5/4 | SCL | 0–10 | 1.15 | 4.61 ± 0.3 | 16.9 | 2.3 | 221.5 | 96.0 | 0.87 |
| 7.5YR 4/4 | SCL | 10–20 | 1.16 | 4.42 ± 0.4 | 18.1 | 1.5 | 190.4 | 80.0 | 0.82 | |
| 7.5YR 4/6 | SCL | 20–40 | 1.26 | 4.75 ± 0.4 | 17.8 | 0.9 | 172.2 | 51.4 | 0.79 | |
| 7.5YR 6/6 | CL | 40–60 | 1.28 | 4.28 ± 0.3 | 14.5 | 0.6 | 174.4 | 21.3 | 0.75 | |
| TP Villa Azul 00°34′31′5″ S 072°06′52′4″ W | 2.5Y 41 | SL | 0–10 | 1.62 | 4.95 ± 0.1 | 1.8 | 0.11 | 302.0 | 94.2 | 0.88 |
| 2.5Y 4/2 | SL | 10–20 | 1.47 | 4.52 ± 0.2 | 1.3 | 0.08 | 118.3 | 78.0 | 0.85 | |
| 2.5Y 5/2 | SL | 20–40 | 1.53 | 4.64 ± 0.2 | 1.4 | 0.08 | 143.0 | 74.8 | 0.83 | |
| 2.5Y 7/1 | SL | 40–60 | 1.47 | 4.67 ± 0.1 | 0.8 | 0.04 | 71.4 | 58.7 | 0.85 | |
| ADJ Villa Azul 00°34′26.8″ S 072°06′48.5″ W | 5Y 4/1 | SL | 0–10 | 1.25 | 4.41 ± 02 | 0.8 | 0.05 | 36.7 | 46.4 | 0.88 |
| 5Y 6/1 | SCL | 10–20 | 1.46 | 4.42 ± 0.1 | 0.9 | 0.05 | 35.7 | 55.8 | 0.88 | |
| 5Y 7/1 | SCL | 20–40 | 1.46 | 4.33 ± 0.3 | 0.7 | 0.04 | 30.9 | 25.5 | 0.82 | |
| 5Y 8/1 | SCL | 40–60 | 1.45 | 3.98 ± 0.4 | 0.2 | 0.03 | 22.7 | 3.5 | 1.00 | |
| TP Peña Roja 00°39′37′4″ S 72°05′00′00″ W | 10YR 3/1 | LS | 0–10 | 1.42 | 4.34 ± 0.1 | 1.4 | 0.07 | 1329.7 | 91.9 | 0.86 |
| 10YR 4/2 | LS | 10–20 | 1.41 | 4.11 ± 0.2 | 1.3 | 0.06 | 1256.0 | 97.8 | 0.84 | |
| 10YR 5/4 | LS | 20–40 | 1.44 | 4.57 ± 0.2 | 0.6 | 0.03 | 919.0 | 54.4 | 0.82 | |
| 10YR 7/4 | LS | 40–60 | 1.45 | 4.52 ± 0.3 | 0.4 | 0.02 | 1588.2 | 22.2 | 0.82 | |
| ADJ Peña Roja 00°39′25″ S 72°05′00′6″ W | 10YR 5/4 | LS | 0–10 | 1.18 | 4.9 ± 0.2 | 0.8 | 0.05 | 53.0 | 31.5 | 0.79 |
| 10YR 6/3 | LS | 10–20 | 1.27 | 4.59 ± 0.3 | 1.0 | 0.07 | 67.1 | 27.5 | 0.74 | |
| 10YR 6/3 | SL | 20–40 | 1.29 | 4.43 ± 0.1 | 0.9 | 0.05 | 57.8 | 28.1 | 0.93 | |
| 10YR 7/8 | SL | 40–60 | 1.28 | 4.15 ± 0.4 | 0.5 | 0.02 | 54.6 | 14.6 | 0.67 | |
| TP Takana 04°07′08′8″ S 069°55′15′7″ W | 7.5YR 4/4 | SL | 0–10 | 1.06 | 4.66 ± 0.2 | 1.6 | 0.14 | 441.9 | 213.2 | 0.89 |
| 7.5YR 5/4 | SL | 10–20 | 1.10 | 4.68 ± 0.2 | 1.5 | 0.11 | 470.1 | 362.0 | 0.92 | |
| 7.5YR 5/6 | SCL | 20–40 | 1.22 | 4.2 ± 0.1 | 0.8 | 0.07 | 514.6 | 148.4 | 0.98 | |
| 7.5YR 7/6 | SCL | 40–60 | 1.45 | 4.64 ± 0.4 | 0.5 | 0.07 | 301.0 | 60.3 | 0.98 | |
| ADJ Takana 04°06′59′3″ S 069°55′16′7″ W | 7.5YR 4/3 | SCL | 0–10 | 0.94 | 4.24 ± 0.3 | 2.3 | 0.22 | 97.6 | 100.3 | 0.70 |
| 7.5YR 6/6 | SCL | 10–20 | 1.13 | 4.34 ± 0.2 | 1.8 | 0.19 | 82.9 | 82.8 | 0.67 | |
| 7.5YR 7/3 | SCL | 20–40 | 1.16 | 4.39 ± 0.1 | 1.5 | 0.15 | 64.9 | 132.2 | 1.03 | |
| 7.5YR 7/6 | CL | 40–60 | 1.20 | 4.14 ± 0.3 | 0.5 | 0.10 | 51.2 | 4.7 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Ortiz, J.M.; Peña-Venegas, C.P.; Bauke, S.L.; Borgemeister, C.; Mörchen, R.; Lehndorff, E.; Amelung, W. Terra Preta Properties in Northwestern Amazonia (Colombia). Sustainability 2021, 13, 7088. https://doi.org/10.3390/su13137088
Orozco-Ortiz JM, Peña-Venegas CP, Bauke SL, Borgemeister C, Mörchen R, Lehndorff E, Amelung W. Terra Preta Properties in Northwestern Amazonia (Colombia). Sustainability. 2021; 13(13):7088. https://doi.org/10.3390/su13137088
Chicago/Turabian StyleOrozco-Ortiz, Juan Manuel, Clara Patricia Peña-Venegas, Sara Louise Bauke, Christian Borgemeister, Ramona Mörchen, Eva Lehndorff, and Wulf Amelung. 2021. "Terra Preta Properties in Northwestern Amazonia (Colombia)" Sustainability 13, no. 13: 7088. https://doi.org/10.3390/su13137088






