Specified Dosages of Biochar Application Not Impact Native Organic Carbon but Promote a Positive Effect on Native Humic Acid in Humicryepts Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Soil, Design, and Soil Sampling
2.2. Biochar Production and Properties
2.3. Measurements of Labile Organic C
2.4. Extraction, Fractionation, and Purification of HSs
2.5. Organic C Measurement
2.6. Calculation in C Management Index
2.7. δ13C Analysis
2.8. FTIR Spectra Analysis
2.9. Statistics
3. Results
3.1. Total SOC, Labile Organic C and CMI
3.2. HSs Distributed in Soil Heavy Fractions
3.3. δ13C Analyses of SOC and HA
3.4. Elementary and FTIR Spectra Analyses on HA
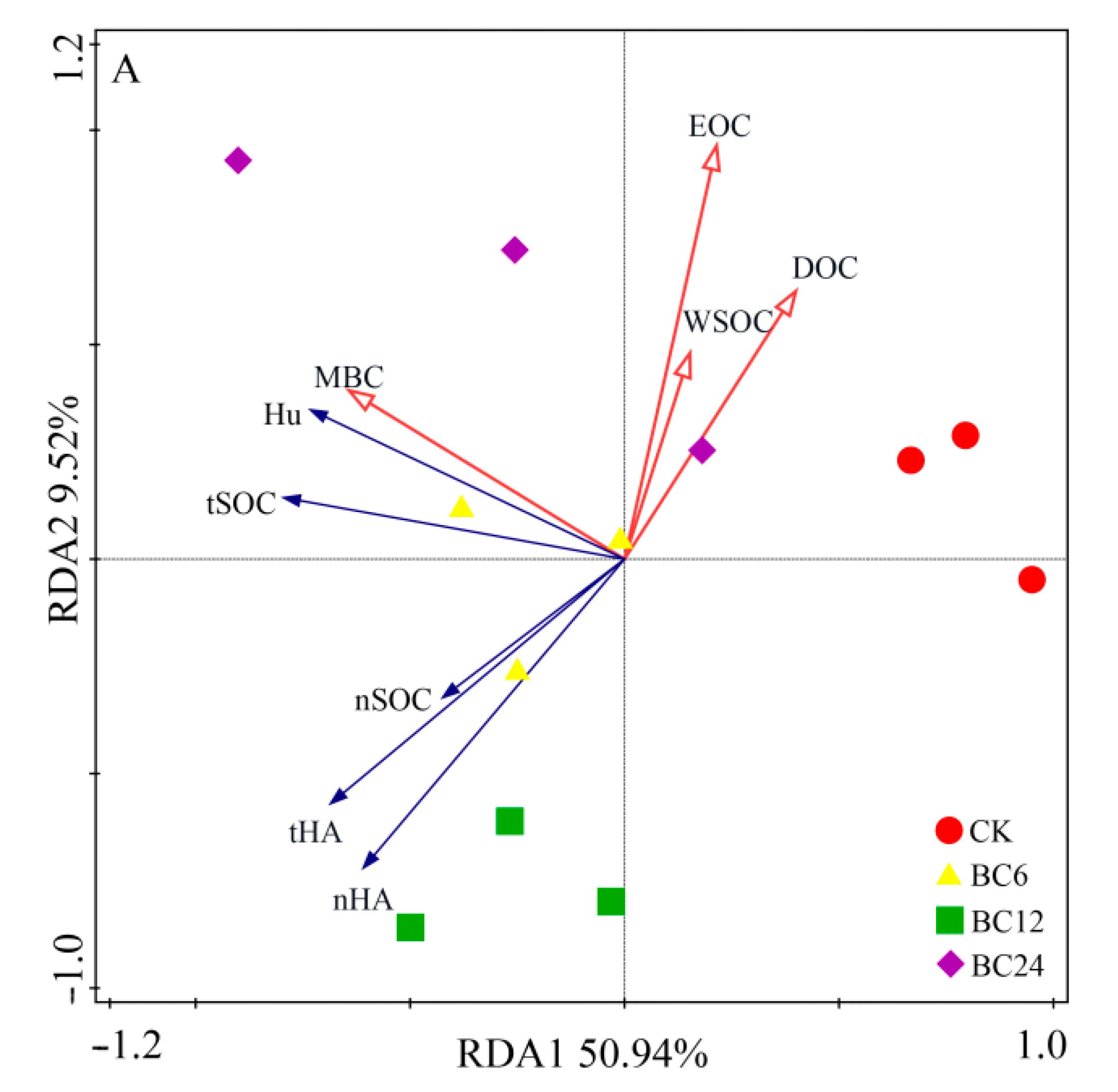
3.5. Redundancy Analyses of Stable- and Labile-Organic C
4. Discussion
4.1. Effects of Different Dosages of Biochar Application on the Total SOC and CMI
4.2. Effect of Different Dosages of Biochar Application on Native SOC
4.3. Effects of Different Dosages of Biochar Application on Biochar-Derived HA and Native HA
4.4. Differences in the Effects of Three Dosages of Biochar Application on SOC and HA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, R.; Zhang, Z.; Xiao, X.; Zhang, N.; Wang, X.; Yang, Z.; Liang, Y. Structural changes of soil organic matter and the linkage to rhizosphere bacterial communities with biochar amendment in manure fertilized soils. Sci. Total Environ. 2019, 692, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Luo, X.; Li, Y.; Liu, D.; Zhao, Y.; Deng, J. Modeling impacts of mulching and climate change on crop production and N2O emission in the Loess Plateau of China. Agric. For. Meteorol. 2019, 268, 86–97. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Q.; Wang, F.; Zhang, B. Is straw return-to-field always beneficial? Evidence from an integrated cost-benefit analysis. Energy 2019, 171, 393–402. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Hu, G.; Shao, S.; He, H.; Zhang, W.; Li, L. Dynamic contribution of microbial residues to soil organic matter accumulation influenced by maize straw mulching. Geoderma 2019, 333, 35–42. [Google Scholar] [CrossRef]
- Wang, W.; Akhtar, K.; Ren, G.; Yang, G.; Feng, Y.; Yuan, L. Impact of straw management on seasonal soil carbon dioxide emissions, soil water content, and temperature in a semi-arid region of China. Sci. Total Environ. 2019, 652, 471–482. [Google Scholar] [CrossRef]
- Cui, Y.; Meng, J.; Wang, Q.; Zhang, W.; Cheng, X.; Chen, W. Effects of straw and biochar addition on soil nitrogen, carbon, and super rice yield in cold waterlogged paddy soils of North China. J. Integr. Agric. 2017, 16, 1064–1074. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Hayes, M.H.B. Biochar from Biomass and Waste. Waste Biomass Valori. 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Sanchez, M.E.; Lindao, E.; Margaleff, D.; Martinez, O.; Moran, A. Pyrolysis of agricultural residues from rape and sunflower: Production and characterization of bio-fuels and biochar soil management. J. Anal. Appl. Pyrol. 2009, 85, 142–144. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrol. 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wyzińska, M.; Smreczak, B. Influence of type and rate of biochar on productivity of winter wheat. In Proceedings of the 2019 International Conference “Engineering for Rural Development”, Jelgava, Latvia, 22–24 May 2019; pp. 594–599. [Google Scholar] [CrossRef]
- Thers, H.; Djomo, S.N.; Elsgaard, L.; Knudsen, M.T. Biochar potentially mitigates greenhouse gas emissions from cultivation of oilseed rape for biodiesel. Sci. Total Environ. 2019, 671, 180–188. [Google Scholar] [CrossRef]
- Krasilnikov, P.V. Stable carbon compounds in soils: Their origin and functions. Eurasian Soil Sci. 2015, 48, 997–1008. [Google Scholar] [CrossRef]
- Meng, J.; He, T.; Sanganyado, E.; Lan, Y.; Zhang, W.; Han, X.; Chen, W. Development of the straw biochar returning concept in China. Biochar 2019, 1, 139–149. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Liu, S.; Liu, R.; Zhang, J.; Ren, J.; Cai, H.; Lin, X. Soil organic carbon associated with aggregate-size and density fractions in a Mollisol amended with charred and uncharred maize straw. J. Integr. Agric. 2019, 18, 1496–1507. [Google Scholar] [CrossRef]
- Cao, X.; Schmidt-Rohr, K. Abundant nonprotonated aromatic and oxygen-bonded carbons make humic substances distinct from biopolymers. Env. Sci. Technol. Lett. 2018, 5, 476–480. [Google Scholar] [CrossRef]
- Ikeya, K.; Maie, N.; Han, X.; Wang, G.; Watanabe, A. Comparison of carbon skeletal structures in black humic acids from different soil origins. Soil Sci. Plant Nutr. 2019, 65, 109–113. [Google Scholar] [CrossRef]
- Tikhova, V.D.; Deryabina, Y.M.; Vasilevich, R.S.; Lodygin, E.D. Structural features of tundra and taiga soil humic acids according to IR EXPERT analytical system data. J. Soil Sediment. 2019, 19, 2697–2707. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114–184. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Luo, Y.; Zang, H.; Yu, Z.; Chen, Z.; Gunina, A.; Kuzyakov, Y.; Brookes, P.C. Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol. Biochem. 2017, 106, 28–35. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, R.; Ding, Y.; Niggemann, J.; Vähätalo, A.V.; Stubbins, A.; Spencer, R.G.; Campbell, J.; Dittmar, T. Global charcoal mobilization from soils via dissolution and riverine transport to the oceans. Science 2013, 340, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Haumaier, L.; Zech, W. Black carbon-possible source of highly aromatic components of soil humic acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Velasco-Molina, M.; Berns, A.E.; Macías, F.; Knicker, H. Biochemically altered charcoal residues as an important source of soil organic matter in subsoils of fire-affected subtropical regions. Geoderma 2016, 262, 62–70. [Google Scholar] [CrossRef]
- Orlova, N.; Abakumov, E.; Orlova, E.; Yakkonen, K.; Shahnazarova, V. Soil organic matter alteration under biochar amendment: Study in the incubation experiment on the Podzol soils of the Leningrad region (Russia). J. Soil Sediment. 2019, 19, 2708–2716. [Google Scholar] [CrossRef]
- Hua, L.; Wang, Y.; Wang, T.; Ma, H. Effect of biochar on organic matter conservation and metabolic quotient of soil. Environ. Prog. Sustain. Energy. 2015, 34, 1467–1472. [Google Scholar]
- Zhao, S.; Ta, N.; Li, Z.; Yang, Y.; Zhang, X.; Liu, D.; Zhang, A.; Wang, X. Varying pyrolysis temperature impacts application effects of biochar on soil labile organic carbon and humic fractions. Appl. Soil Ecol. 2018, 123, 484–493. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Liu, J.; Yuan, J.; Liang, Y.; Ren, J.; Cai, H. Effects of maize straw and its biochar application on organic and humic carbon in water-stable aggregates of a Mollisol in Northeast China: A five-year field experiment. Soil Till. Res. 2019, 190, 1–9. [Google Scholar] [CrossRef]
- Song, X.; Yang, J.; Hussain, Q.; Liu, X.; Zhang, J.; Cui, D. Stable isotopes reveal the formation diversity of humic substances derived from different cotton straw-based materials. Sci. Total Environ. 2020, 740, 140202. [Google Scholar] [CrossRef]
- Fernández–Ugalde, O.; Gartzia–Bengoetxea, N.; Arostegi, J.; Moragues, L.; Arias–González, A. Storage and stability of biochar–derived carbon and total organic carbon in relation to minerals in an acid forest soil of the Spanish Atlantic area. Sci. Total Environ. 2017, 587–588, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Vedove, G.D.; Miglietta, F.; Tonon, G. Biochar mineralization and priming effect in a poplar short rotation coppice from a 3-year field experiment. Biol. Fertil Soils. 2019, 55, 67–78. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Bolan, N.S.; Baskaran, S.; Thiagarajan, S. An evaluation of the methods of measurement of dissolved organic carbon in soils, manures, sludges, and stream water. Commun. Soil Sci. Plan. 1996, 27, 2723–2737. [Google Scholar] [CrossRef]
- Liu, C.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and characterization of dissolved organic carbon from biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef]
- Dixit, A.K.; Rai, A.K.; Prasad, M.; Choudhary, M.; Kumar, S.; Srivastava, M.K.; Singh, H.V. Long–term fertilization effects on carbon pools and carbon management index of loamy soil under grass–forage legumes mixture in semi–arid environment. Arch. Agron. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Song, G.; Novotny, E.H.; Mao, J.; Hayes, M.H.B. Characterization of transformations of maize residues into soil organic matter. Sci. Total Environ. 2017, 579, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Karpukhina, E.; Mikheev, I.; Perminova, I.; Volkov, D.; Proskurnin, M. Rapid quantification of humic components in concentrated humate fertilizer solutions by FTIR spectroscopy. J. Soil Sediment. 2019, 19, 2729–2739. [Google Scholar] [CrossRef]
- DeCiucies, S.; Whitman, T.; Woolf, D.; Enders, A.; Lehmann, J. Priming mechanisms with additions of pyrogenic organic matter to soil. Geochim. Cosmochim. Ac. 2018, 238, 329–342. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Smebye, A.; Alling, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef]
- Bruun, S.; EL-Zehery, T. Biochar effect on the mineralization of soil organic matter. Pesqui. Agropecu. Bras. 2012, 47, 665–671. [Google Scholar] [CrossRef]
- Reed, E.Y.; Chadwick, D.R.; Hill, P.W.; Jones, D.L. Critical comparison of the impact of biochar and wood ash on soil organic matter cycling and grassland productivity. Soil Biol. Biochem. 2017, 110, 134–142. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Singh, B.P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef]
- Kerré, B.; Hernandez-Soriano, M.C.; Smolders, E. Partitioning of carbon sources among functional pools to investigate short-term priming effects of biochar in soil: A 13C study. Sci. Total Environ. 2016, 547, 30–38. [Google Scholar] [CrossRef]
- Weng, Z.; Zwieten, L.V.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat Clim. Chang. 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar application constrained native soil organic carbon accumulation from wheat residue inputs in a long-term wheat-maize cropping system. Agric. Ecosyst. Environ. 2018, 252, 200–207. [Google Scholar] [CrossRef]
- Fischer, D.; Erben, G.; Dunst, G.; Glaser, B. Dynamics of labile and stable carbon and priming effects during composting of sludge and lop mixtures amended with low and high amounts of biochar. Waste Manag. 2018, 78, 880–893. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Nazaries, L.; Keith, A.; Tavakkoli, E.; Wilson, N.; Singh, B. Interactive carbon priming, microbial response and biochar persistence in a Vertisol with varied inputs of biochar and labile organic matter. Eur. J. Soil Sci. 2019, 70, 960–974. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 2014, 7, 577–590. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.; Bolan, N.; OK, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Cheng, C.; Lin, T.; Lehmann, J.; Fang, L.; Yang, Y.; Menyailo, O.V.; Chang, K.; Lai, J. Sorption properties for black carbon (wood char) after long term exposure in soils. Org. Geochem. 2014, 70, 53–61. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma 2015, 245–246, 56–64. [Google Scholar] [CrossRef]
- Dong, X.; Li, G.; Lin, Q.; Zhao, X. Quantity and quality changes of biochar aged for 5 years in soil under field conditions. Catena 2017, 159, 136–143. [Google Scholar] [CrossRef]
- Jiang, X.; Tan, X.; Cheng, J.; Haddix, M.L.; Cotrufo, M.F. Interactions between aged biochar, fresh low molecular weight carbon and soil organic carbon after 3.5 years soil-biochar incubations. Geoderma 2019, 333, 99–107. [Google Scholar] [CrossRef]
- Kramer, R.W.; Kujawinski, E.B.; Hatcher, P.G. Identification of black carbon derived structures in a volcanic ash soil humic acid by Fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2004, 38, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Placido, J.; Capareda, S.; Karthikeyan, R. Production of humic substances from cotton stalks biochar by fungal treatment with Ceriporiopsis subvermispora. Sustain. Energy. Technol. Assess. 2016, 13, 31–37. [Google Scholar]
- Wang, C.; Tu, Q.; Dong, D.; Strong, P.J.; Wang, H.; Sun, B.; Wu, W. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard. Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Hu, Z.; Xue, J.; Lu, Y.; Chen, X.; Griffiths, B.S.; Whalen, J.K.; Liu, M. Biochar exerts negative effects on soil fauna across multiple trophic levels in a cultivated acidic soil. Biol. Fertil. Soils 2020. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Gao, W.; Chen, Y.; Pan, W.; Lee, X.; Tang, Y. Effects of biochar on Cd and Pb mobility and microbial community composition in a calcareous soil planted with tobacco. Biol. Fertil. Soils 2018, 54, 373–383. [Google Scholar] [CrossRef]
- Cheng, H.; Jones, D.L.; Hill, P.; Bastami, M.S.; Tu, C. Influence of biochar produced from different pyrolysis temperature on nutrient retention and leaching. Arch. Agron. Soil Sci. 2017, 64, 850–859. [Google Scholar] [CrossRef]
- Yang, X.; Chang, K.; Kim, Y.J.; Zhang, J.; Yoo, G. Effects of different biochar amendments on carbon loss and leachate characterization from an agricultural soil. Chemosphere 2019, 226, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dou, S.; Ndzelu, B.S.; Guan, X.; Zhang, B.; Bai, Y. Effects of different corn straw amendments on humus composition and structural characteristics of humic acid in black soil. Commun. Soil Sci. Plan. 2020, 51, 107–117. [Google Scholar] [CrossRef]
- Sun, K.; Han, L.; Yang, Y.; Xia, X.; Yang, Z.; Wu, F.; Li, F.; Feng, Y.; Xing, B. Application of hydrochar altered soil microbial community composition and the molecular structure of native soil organic carbon in a Paddy Soil. Env. Sci. Technol. 2020, 54, 2715–2725. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Shen, J.; Yuan, Q.; Fan, F.; Wei, W.; Li, Y.; Wu, J. Biochar alters soil microbial communities and potential functions 3–4 years after amendment in a double rice cropping system. Agric. Ecosyst. Environ. 2021, 311, 107291. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Doyle, R.B.; Bound, S.A.; Bowman, J.P. Assessment of bacterial community composition, methanotrophic and nitrogen-cycling bacteria in three soils with different biochar application rates. J. Soil Sediment. 2018, 18, 148–158. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Singh, H.; Raghubanshi, A.S. Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar 2019, 1, 229–235. [Google Scholar] [CrossRef]
- Safari, S.; von Gunten, K.; Alam, M.S.; Hubmann, M.; Blewett, T.A.; Chi, Z.; Alessi, D.S. Biochar colloids and their use in contaminants removal. Biochar 2019, 1, 151–162. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Shan, J.; Xia, Y.; Lin, J.; Yan, X. A 2-year study on the effect of biochar on methane and nitrous oxide emissions in an intensive rice–wheat cropping system. Biochar 2019, 1, 177–186. [Google Scholar] [CrossRef]
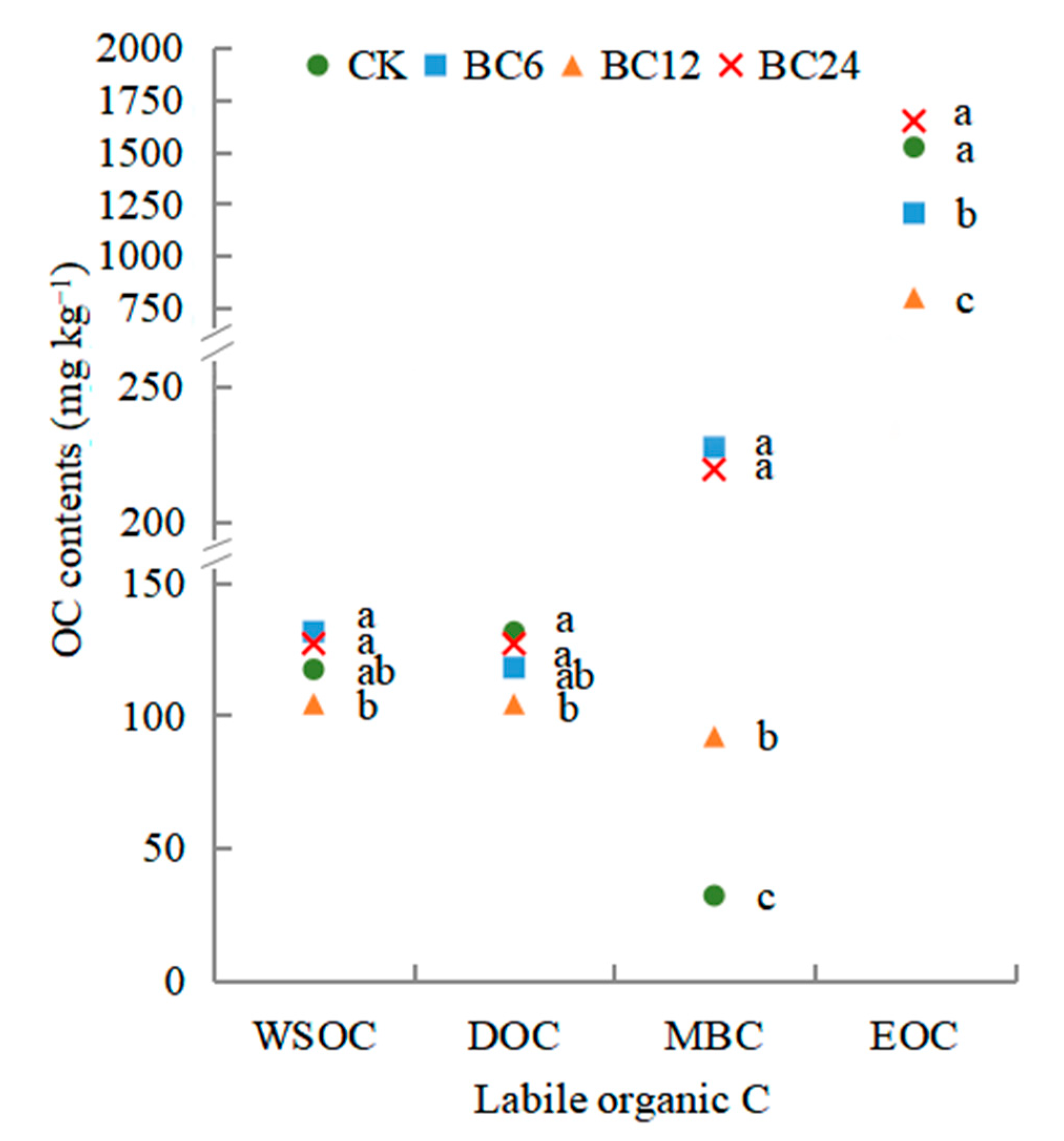


| Treatment | C Lability | C Lability Index | C Pool Index | C Management Index (%) |
|---|---|---|---|---|
| CK | 0.081 ± 0.007 a | 1.00 ± 0.00 a | 1.00 ± 0.00 c | 100.00 ± 0.00 a |
| BC6 | 0.053 ± 0.008 b | 0.66 ± 0.10 b | 1.17 ± 0.07 b | 77.21 ± 12.39 b |
| BC12 | 0.033 ± 0.006 c | 0.41 ± 0.08 c | 1.23 ± 0.07 ab | 50.27 ± 10.90 c |
| BC24 | 0.065 ± 0.007 b | 0.81 ± 0.09 b | 1.33 ± 0.08 a | 106.96 ± 16.56 a |
| Treatment | tSOC (g kg−1) | Soil δ13C (‰) | f (%) | 1 − f (%) | Biochar-C (g kg−1) | Native SOC (g kg−1) |
|---|---|---|---|---|---|---|
| CK | 20.43 ± 1.03 c | −24.11 ± 0.17 d | / | 100 a | / | 20.43 ± 1.03 a |
| BC6 | 23.80 ± 1.61 b | −23.42 ± 0.03 c | 6.54 ± 0.29 c | 93.46 ± 0.29 b | 1.55 ± 0.04 c | 22.25 ± 1.41 a |
| BC12 | 25.07 ± 1.24 b | −22.90 ± 0.13 b | 11.56 ± 1.22 b | 88.44 ± 1.22 c | 2.91 ± 0.46 b | 22.16 ± 0.94 a |
| BC24 | 27.05 ± 1.46 a | −21.60 ± 0.43 a | 23.89 ± 4.12 a | 76.11 ± 4.12 d | 6.42 ± 0.73 a | 20.63 ± 2.28 a |
| Treatment | δ13C of HA (‰) | f (%) | 1 − f (%) | Biochar-C (g kg−1) | Native HA (g kg−1) | EHA (%) | ΔRHA (%) |
|---|---|---|---|---|---|---|---|
| CK | −25.31 ± 0.11 c | / | 100 a | / | 3.90 ± 0.31 d | / | / |
| BC6 | −25.08 ± 0.08 b | 1.96 ± 0.55 b | 98.04 ± 0.55 b | 0.11 ± 0.02 c | 5.30 ± 0.03 b | 6.43 ± 1.82 a | 36.49 ± 11.04 a |
| BC12 | −24.94 ± 0.12 b | 3.13 ± 1.06 b | 96.88 ± 1.06 b | 0.20 ± 0.06 b | 6.06 ± 0.07 a | 5.95 ± 1.51 a | 55.95 ± 10.53 a |
| BC24 | −24.65 ± 0.05 a | 5.61 ± 0.37 a | 94.39 ± 0.37 c | 0.27 ± 0.01 a | 4.52 ± 0.02 c | 4.08 ± 0.27 b | 16.30 ± 8.46 b |
| Treatment | Element Content (g·kg−1) | Molar Ratio | ||||
|---|---|---|---|---|---|---|
| C | H | O | N | O/C | H/C | |
| CK | 571.10 ± 4.23 a | 42.94 ± 1.23 b | 355.23 ± 6.47 a | 30.76 ± 1.52 a | 0.47 ± 0.012 a | 0.90 ± 0.02 b |
| BC6 | 565.57 ± 1.24 a | 44.63 ± 0.64 ab | 357.23 ± 1.60 a | 32.60 ± 0.41 a | 0.47 ± 0.003 a | 0.95 ± 0.01 a |
| BC12 | 572.40 ± 1.22 a | 45.40 ± 1.37 a | 351.03 ± 2.26 a | 31.17 ± 1.90 a | 0.46 ± 0.002 a | 0.95 ± 0.03 a |
| BC24 | 569.37 ± 5.55 a | 45.25 ± 0.96 a | 354.53 ± 6.11 a | 30.85 ± 0.64 a | 0.47 ± 0.013 a | 0.95 ± 0.02 a |
| Treatment | Relative Intensity (%) | I2920/I1720 | I2920/I1620 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3400 cm−1 | 2920 cm−1 | 2850 cm−1 | 1720 cm−1 | 1620 cm−1 | 1400 cm−1 | 1230 cm−1 | |||
| CK | 3.21 ± 0.16 a | 4.37 ± 0.06 c | 0.73 ± 0.08 b | 16.15 ± 0.20 b | 12.74 ± 0.23 b | 8.87 ± 0.71 b | 25.43 ± 0.58 b | 0.32 ± 0.00 b | 0.40 ± 0.01 b |
| BC6 | 3.63 ± 0.21 a | 6.24 ± 0.09 b | 0.82 ± 0.09 ab | 18.36 ± 0.69 a | 14.03 ± 0.55 a | 10.49 ± 0.69 ab | 27.51 ± 0.31 ab | 0.38 ± 0.01 a | 0.50 ± 0.02 a |
| BC12 | 3.88 ± 0.37 a | 6.50 ± 0.11 a | 0.86 ± 0.06 ab | 19.71 ± 0.75 a | 14.38 ± 0.64 a | 10.82 ± 0.43 ab | 29.20 ± 1.01 a | 0.37 ± 0.02 a | 0.51 ± 0.02 a |
| BC24 | 4.01 ± 0.89 a | 6.52 ± 0.04 a | 1.02 ± 0.11 a | 20.15 ± 0.91 a | 14.80 ± 0.99 a | 10.96 ± 0.97 a | 28.62 ± 1.76 a | 0.37 ± 0.02 a | 0.51 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, S.; Gao, S.; Zhou, X.; Liu, R.; Guan, S.; Dou, S. Specified Dosages of Biochar Application Not Impact Native Organic Carbon but Promote a Positive Effect on Native Humic Acid in Humicryepts Soil. Sustainability 2021, 13, 6392. https://doi.org/10.3390/su13116392
Li Q, Liu S, Gao S, Zhou X, Liu R, Guan S, Dou S. Specified Dosages of Biochar Application Not Impact Native Organic Carbon but Promote a Positive Effect on Native Humic Acid in Humicryepts Soil. Sustainability. 2021; 13(11):6392. https://doi.org/10.3390/su13116392
Chicago/Turabian StyleLi, Qiao, Songjian Liu, Shangzhi Gao, Xin Zhou, Riyue Liu, Song Guan, and Sen Dou. 2021. "Specified Dosages of Biochar Application Not Impact Native Organic Carbon but Promote a Positive Effect on Native Humic Acid in Humicryepts Soil" Sustainability 13, no. 11: 6392. https://doi.org/10.3390/su13116392
APA StyleLi, Q., Liu, S., Gao, S., Zhou, X., Liu, R., Guan, S., & Dou, S. (2021). Specified Dosages of Biochar Application Not Impact Native Organic Carbon but Promote a Positive Effect on Native Humic Acid in Humicryepts Soil. Sustainability, 13(11), 6392. https://doi.org/10.3390/su13116392






