1. Introduction
Natural organic matter (NOM) is generated through the breakdown of bacteria, algae and both terrestrial and aquatic plants, resulting in a complex mixture of organic substances. In freshwater bodies used as a raw water source for drinking water production NOM needs to be removed, representing a substantial challenge due to changes in seasonal NOM composition. During drinking water production, the percentage of removal of NOM affects the efficacy of drinking water treatment processes. If chlorine is used as a disinfectant, the presence of NOM will result in the generation of potentially carcinogenic disinfection byproducts [
1,
2]. A comprehensive review of the different methods for the characterization of NOM in relation to drinking water production was published by Matilainen et al. [
3].
Advanced technologies for the removal of NOM include enhanced coagulation, membrane filtration, and granulated activated carbon, among others [
4]. The different existing technologies for water treatment, including NOM removal, have been summarized elsewhere [
5,
6]. A wide variety of conventional and non-conventional adsorbents have been recently reviewed by Bhatnagar and Sillanpää [
7] for their potential in NOM removal from water. The authors showed that modified adsorbents, composite materials and a few nanomaterials have shown promising results for NOM removal from water. A brief overview of the state-of-the-art methods for NOM removal from supply waters was recently published by Callegari et al. [
8].
Among the different options, coagulation is one of the most widely used techniques for the removal of NOM in drinking water production [
9] because of its low cost and efficiency in removing both disinfection by-product precursors and turbidity [
10]. The most commonly used coagulants are based on trivalent ions such as aluminum sulphate, Al
2 (SO
4)
3 xH
2O (with x between 14 and 21), aluminum chloride and sodium aluminate. There are also iron-based coagulants such as ferric sulphate, ferrous sulphate, ferric chloride and ferric chloride sulphate [
11].
Coagulation operates by destabilizing the colloidal particles that comprise NOM and that remain in solution due to their natural negative charge. Of the four known methods to destabilize colloids—charge neutralization, double layer compression [
12], particle adsorption and bridging, and enmeshment in a precipitate [
13]—three of them can be addressed by using aluminum sulphate or ferric chloride, which makes these coagulants ideal choices for a wide variety of water compositions in different areas or that experience seasonal variations [
14]. It is simple to adjust the coagulant dosage and pH value for different raw water compositions, making the process easy to operate. When using aluminum, the pH range for coagulation was found to be between approximately 5.2 and 6.4, while in the case of using iron as a coagulant, the pH range is between 4.2 and 5.5 [
13]. The speciation and solubility are controlled by pH which is a key parameter to adjust during the coagulation process.
Different factors affect the coagulant’s effectiveness, such as raw water alkalinity, pH, turbidity, and mixing conditions. Other NOM properties such as molecular weight distribution, charge density, solubility and functional groups can also affect the coagulation process [
15]. The different methods used for the removal of NOM from drinking water, including coagulation, adsorption, oxidation, membrane, biological treatment processes and the combination of different treatment processes, have been reviewed elsewhere [
4]. The use of co-coagulants is a popular alternative to improve the efficiency of NOM removal and reduce cost.
At the time of this study, very little was published on CaCl
2 and its use in water treatment [
16] in comparison with other compounds used for similar purposes [
17,
18]. We know that CaCl
2 is more readily available than metal salts and does not have the same environmental impact. Studying CaCl
2 also suggests a potential economic impact for the industry if calcium can replace primary coagulants in drinking water production. The following economic benefit would result from the reduced requirement of Al
2(SO
4)
3: (a) CaCl
2 costs less than this primary coagulant, (b) the amount of sludge is reduced, (c) the amount of chemicals used for pH-adjustment is potentially decreased.
The reduction in primary coagulant in the sludge also eases sludge management in a broader sense. Primary coagulant often includes undesirable contaminants, such as manganese, which can increase to a significant concentration when they accumulate in the sludge. Handling the sludge with less Al (or Fe) and other contaminants is a significant benefit. However, these benefits still assume that the produced water quality is sufficient for its purpose.
In this paper, we investigate the effects of using CaCl2 as a co-coagulant aid in the removal efficiency of NOM from raw water obtained from Lake Bolmen, the tenth largest lake in Sweden. The main coagulants used in this study were aluminum sulphate and ferric chloride, and the effect of adding calcium chloride was investigated.
2. Materials and Methods
2.1. Lake Bolmen
In this study, raw water samples were taken from Lake Bolmen, in southern Sweden. Lake Bolmen is an oligotrophic lake with high NOM and color content caused by a catchment area dominated by forest and iron-rich soil [
6,
19]. Water from Lake Bolmen is currently used as raw water for Ringsjö Water Works (WW), a surface water treatment plant (WTP). During this study, the treatment process included coagulation with FeCl
3, flocculation, lamella sedimentation, rapid and slow sand filtration, and disinfection through chlorination (Sydvatten AB 2016, Malmö, Sweden).
2.2. Jar Tests
The experiments were carried out using a flocculator with 6 glass beakers of 1 L each with programmable agitators (
Figure 1). Before each experiment, pH tests were conducted in 1 L raw water using NaOH or H
2SO
4 and primary coagulant. The test was designed to make sure the desired pH was achieved during the jar tests.
Each jar test began with the addition of CaCl2 to 1 L of raw water, followed by the pre-determined NaOH or H2SO4 amount. The program was started, and a 200 g/L aluminum sulphate (ALG) solution was added with 3 s remaining of the rapid stirring (400 rpm). The program continued with 20 min slow stirring (50 rpm), during which the resulting pH was measured for each beaker. Lastly, the stirring was stopped, allowing for sedimentation (0 rpm for 30 min). When the sedimentation was complete, samples were taken using a 50 mL plastic syringe approximately 3 cm below the surface.
2.3. Coagulants and Additivies
In this study, aluminum sulphate (ALG), Al2(SO4)3, and FeCl3 (PIX-311), a 40% by weight FeCl3 solution, were used as the primary coagulants. ALG was prepared by dissolving pellets in distilled water to a concentration of 200 g ALG/L solution. The CaCl2 used in the experiments was a 36% by weight food-grade quality solution provided by TETRA Chemicals AB. A NaOH (AkzoNobel, Angered, Sweden) and H2SO4 solution was used to control the resulting pH during the experiments.
2.4. Sample Analysis
Water samples were tested for pH and analyzed in a spectrophotometer (Hach DR 5000, Düsseldorf, Germany) where the water was tested for residues of NOM. For the NOM reduction, the absorption was measured at λ = 254 nm and λ = 436 nm with a 5 cm cuvette.
The pH was measured using a WTW pH-197 (Sigma-Aldrich Sweden AB, Stockholm, Sweden) pH meter, which contains a built-in thermometer that corrects for temperature. The program was started and when 3 s remained of the rapid mixing, coagulant was added. Once the rapid mixing was finished and slow mixing had started, the samples were pH-tested.
3. Results and Discussion
The raw water samples used in this work were taken from Lake Bolmen, located in southern Sweden (56°54′59.99″ N, 13°39′59.99″ E). The lake covers 184 km2, has a maximum depth of 37 m and is the tenth largest lake in Sweden. The lake supplies part of Skåne region with fresh water through an 80 km long tunnel (Bolmen water tunnel), followed by a 25 km long pipeline that ends in the municipality-owned drinking water production plant (Ringsjö Station) located in the municipality of Stehag. Samples were taken at the end point.
In
Table 1, we show the analysis of raw water from the raw water supplied by Lake Bolmen at Ringsjö Station. The table shows the chemical composition of the raw water, including salinity and conductivity as well as its high UV-VIS absorbance.
Iron and aluminum-based coagulants are commonly used for different tasks in the water and wastewater treatment industry. In particular, iron and aluminum coagulants have been used for odour and pathogen reductions and the enhancement of dewaterability in sludge digesters [
20]. Aluminum sulphate (Kemira
TM ALG) and ferric chloride (Kemira
TM PIX-311) are used as primary coagulants for NOM removal applied directly to the raw water.
3.1. Conventional Coagulation
In the first stage, both ALG and PIX-311 were used as main coagulants upon raw water collected from Lake Bolmen at the optimal pH operational range recommended for those primary coagulants. The jar experiments described above were conducted between pH 6 and 7 and again between pH 4.6 and 5.6.
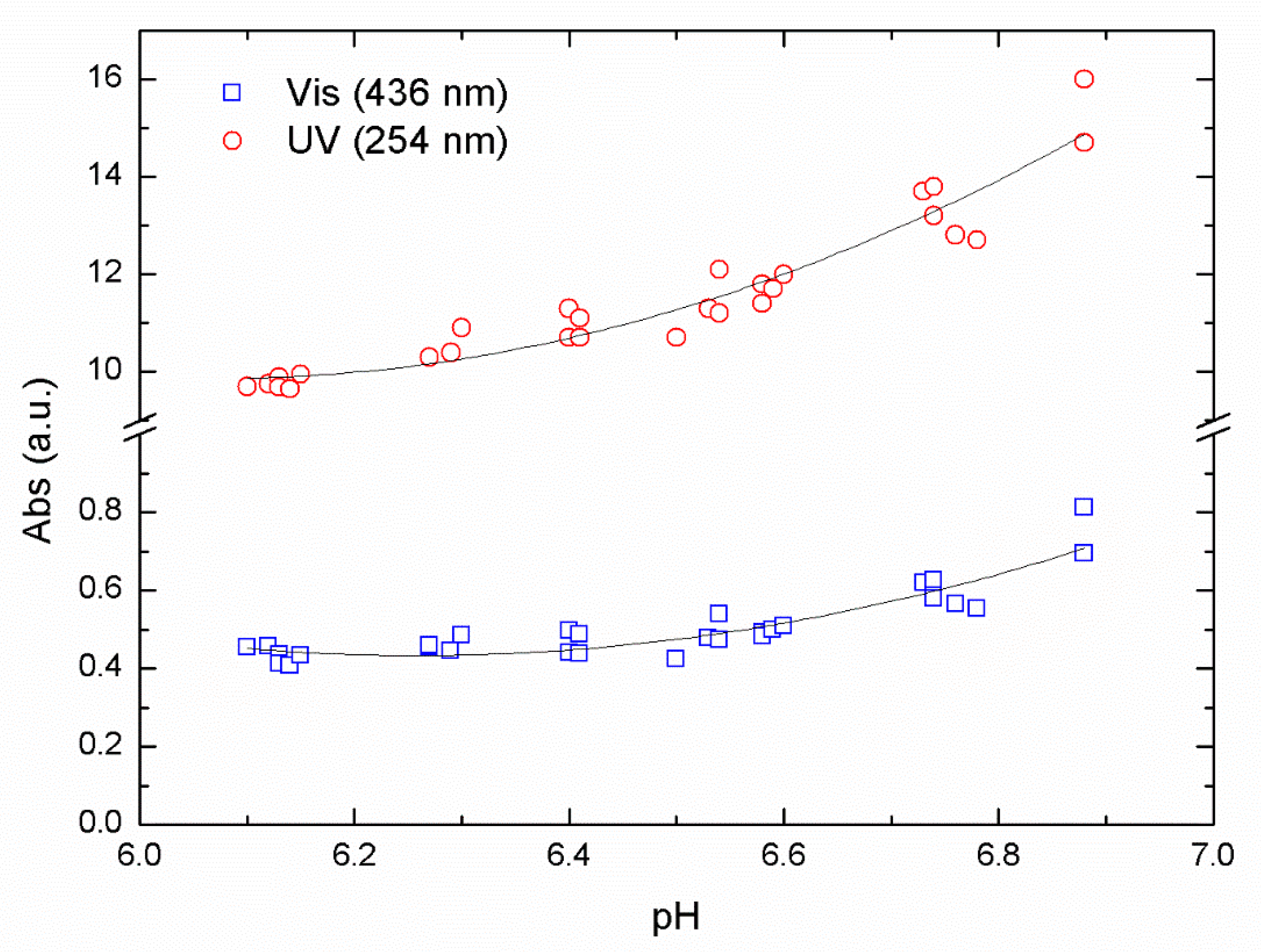
In
Figure 2, we show absorption values after flocculation and sedimentation for a series of samples at different pH using ALG as a primary coagulant.
In
Figure 2, the reduction in the absorbance value passes through a minimum that represents the optimal reduction for the removal of NOM in this pH range. The optimal pH value was found between pH 6.3 and 6.1, resulting from the data collected using absorbance values of 254 nm (UV) and 436 nm (Vis) from the same samples. Using a second-order polynomial fit over our data, we obtained an R
2 of 0.83 and 0.92 for UV and Vis data respectively.
It has been reported that iron coagulants have the advantage of producing a denser floc than that produced by aluminum sulphate, resulting in improved settlement characteristics but at the expense of an approximately 40% increase in the weight of hydroxide sludge when compared to aluminum coagulants [
5]. As many iron coagulants contain approximately 2–6 g of manganese per kg of iron as an impurity depending on the product specification, this might be an aspect of sludge management that needs to be taken into consideration.
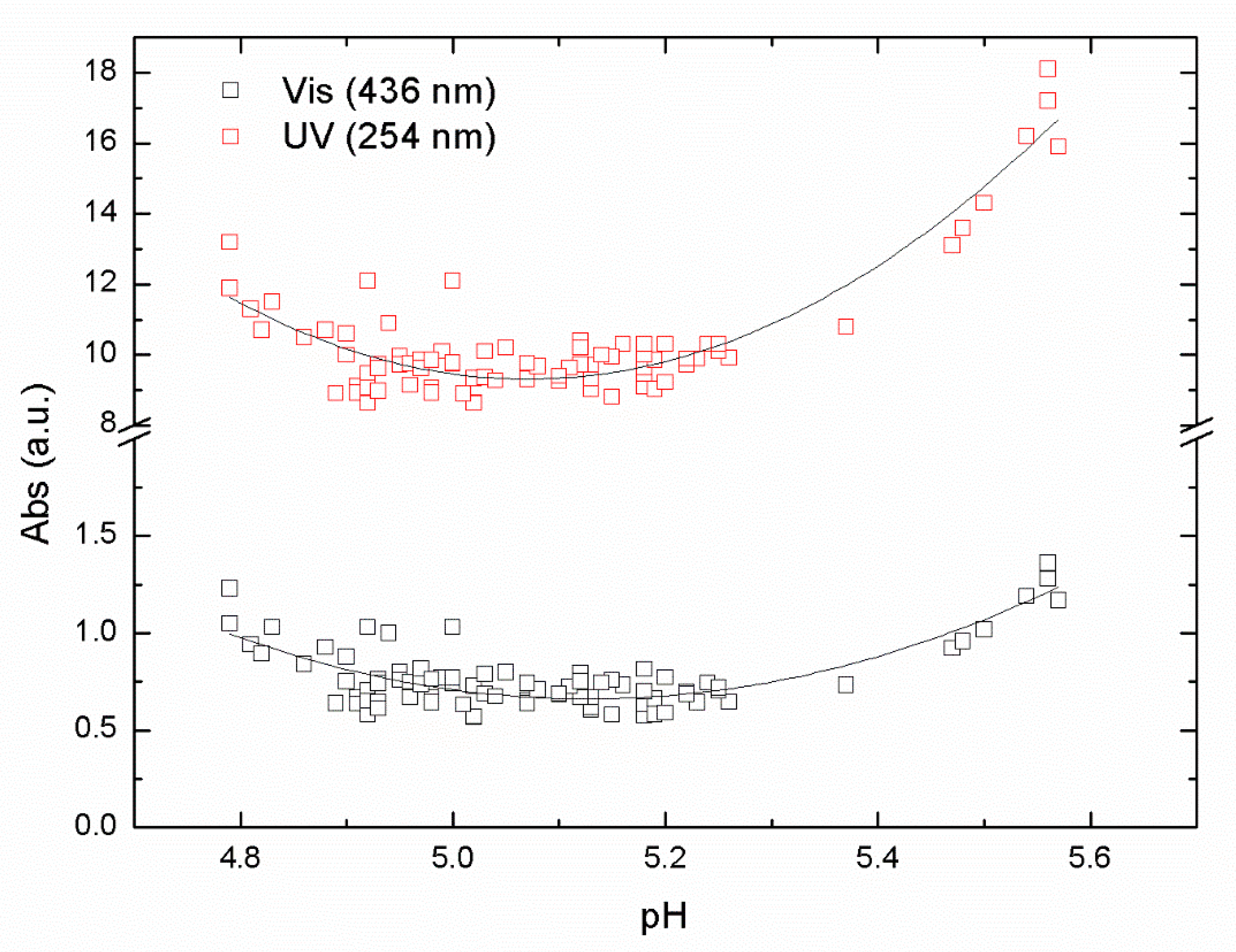
Experiments with iron-based coagulant were performed with the same raw water to compare with the aluminum-based coagulant ALG used in this study. A series of jar samples were prepared using PIX-311 as a primary coagulant using raw water from Lake Bolmen, within the pH range of from 4.5 to 6. The results of absorbance in the UV and Vis ranges are shown in
Figure 3.
The results in
Figure 3 again show a clear U-shape behavior with a minimum around pH 5.1 from the absorbance data at the UV and Vis wavelengths used in our study. Those pH minimum values represent the optimal pH for the removal of NOM from raw water taken from Lake Bolmen. The R
2 values from the polynomial fitting were 0.65 and 0.84 for UV and Vis data, respectively. The minimum value of the absorbance reached the point of maximum NOM removal efficiency. The pH optimal values were around 5.1 for both sets of data, UV and Vis. Interestingly, the optimal pH value for Vis was slightly higher than the optimal pH for UVA. This is likely due to differences in the organic matter, resulting in differences in requirements for charge neutralization, i.e., aromatic compounds that absorb UV light, requiring more positive charges.
3.2. Enhance Coagulation
Because conventional coagulation with aluminum sulphate or iron chloride can sometimes be unable to sufficiently reduce the presence of NOM in raw water for drinking water production, the use of additional co-coagulants or coagulant aid compounds can be an alternative enhanced coagulation treatment. The reduction in the amount of conventional coagulant needed in the NOM removal process can be of additional economic interest for water utilities if the co-coagulant is cheaper than the primary coagulant conventionally used. In the current study, we investigated the effect of CaCl2 as a co-coagulant on NOM removal efficiency when using ALG and PIX-311 as primary coagulants.
As a first step, we sought to understand the effect of adding CaCl2 directly as a supplement to the primary coagulant. As the FeCl3 dosage of coagulant changes daily at the WTP (around 56.5 g PIX-311/m3 or 140 mmol Fe3+/m3), the actual dosages of primary coagulant used during the experiments were defined as 100% dosage. For ALG dosages, 100% is equivalent to about 145 mmol Al3*/m3. The percentage (by mol %) of CaCl2 added was based on the primary coagulant dosage.
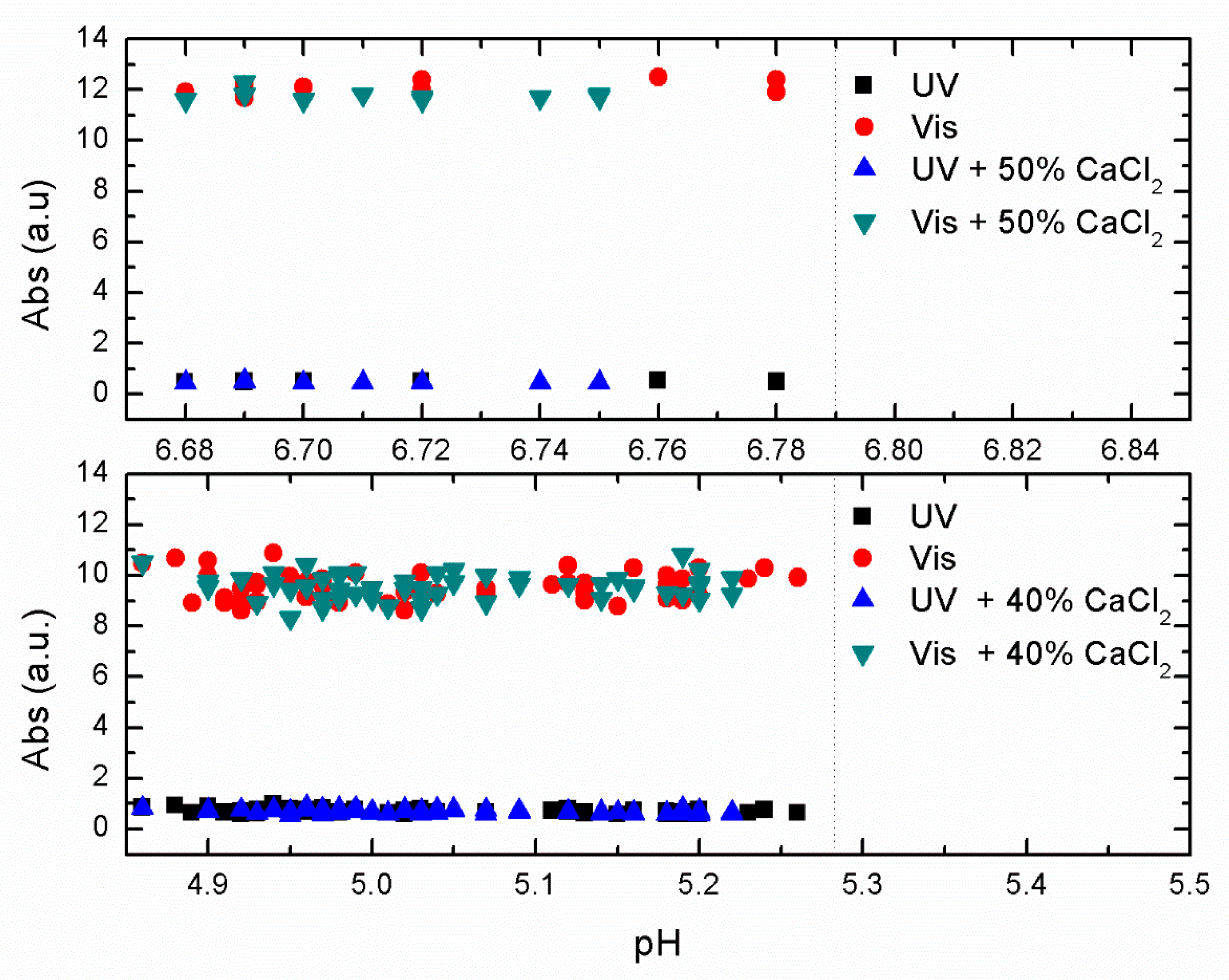
In
Figure 4, we show the effect of adding 40% (bottom) and 50% (top) of CaCl
2 to the primary coagulants at their optimal concentration efficiency.
In
Figure 4, where a limited set of jar experiments were done over a narrow range of pH around the optimal pH removal, we saw no significant improvement in removal efficiency with the addition of CaCl
2. This indicates no improvement when adding CaCl
2 to the primary coagulant under optimal conditions.
The experiments at 100% and 150% of CaCl
2 with optimal ALG concentration as well as 80% of CaCl
2 and optimal PIX-311 concentration, not shown in
Figure 4, resulted in a similar lack of appreciable improvement in the removal efficiency of NOM. We can partially conclude that the direct addition of CaCl
2 as a co-coagulant to the optimal concentration of primary coagulant ALG or PIX-311 does not add any substantial or appreciable improvement in removal efficiency.
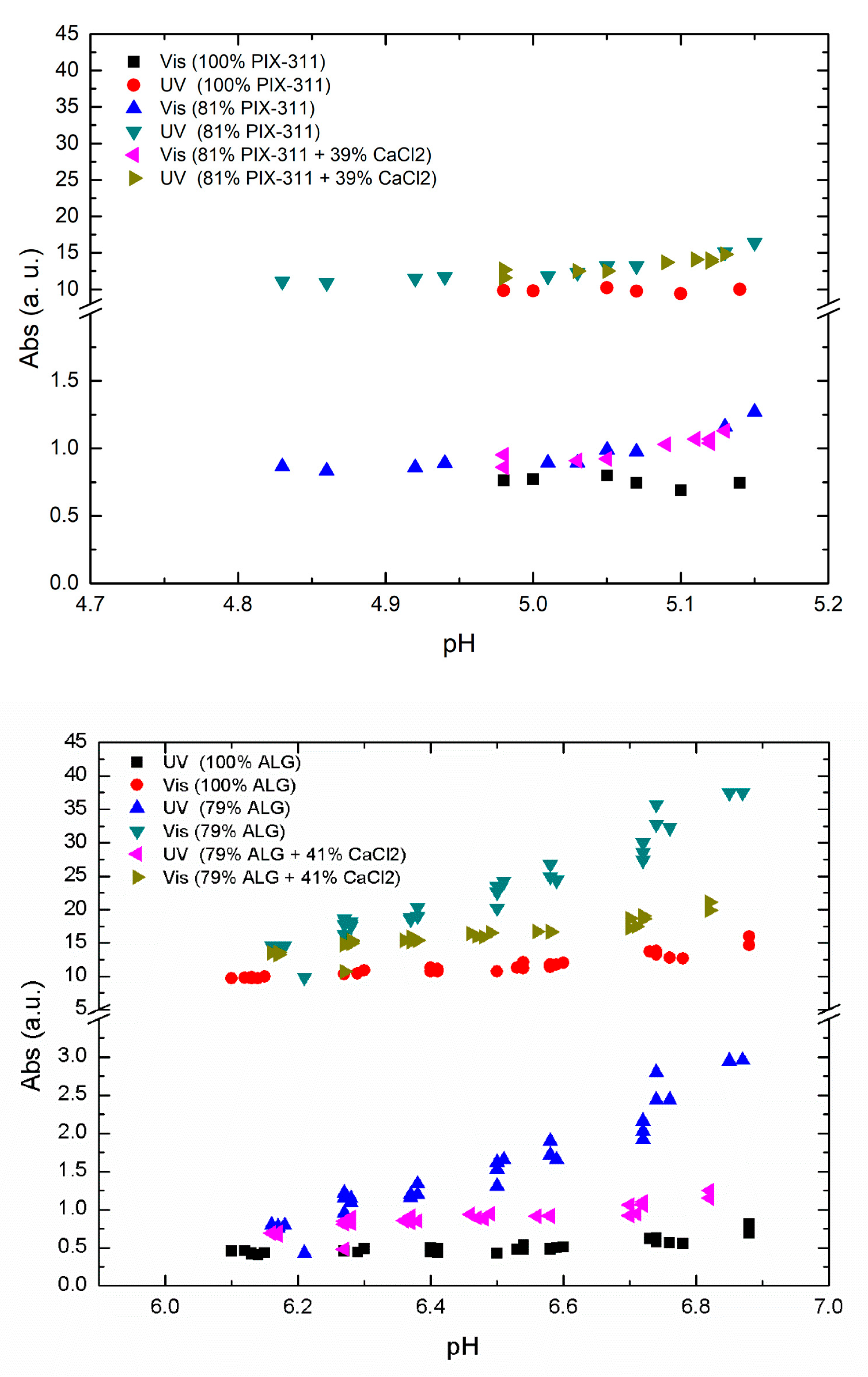
As a next step, we investigated the effect of adding CaCl
2 in combination with a lower concentration of primary coagulant to see the effect of replacing the primary coagulant with co-coagulant. In
Figure 5, we show the results of optimal ALG and PIX-311 concentration compared to decreased concentration with and without the addition of CaCl
2 as a co-coagulant.
As shown in
Figure 5 we observed a nearly zero effect from the addition of CaCl
2 in the case of PIX-311, while in the case of ALG there was an improvement in NOM removal when 79% of the optimal ALG concentration was combined with 41% of CaCl
2. However, the efficiency presented by the optimum concentration of ALG was not matched by the combination of 71% ALG + 41% CaCl
2. As the positive effects of calcium addition with lower ALG dosages only occurred at a higher pH, we believe that the positive effect of adding CaCl
2 is due to calcium’s ability to assist in charge neutralization. As seen in
Figure 5, the positive effect of calcium chloride decreased with decreasing pH. This could mean that the positive effect of adding calcium chloride only occurs at higher pH values, and may explain why no positive effect was observed during Fe-assisted flocculation, which takes place at a lower pH. Another aspect is the natural calcium content in the raw water. In Lake Bolmen, the calcium content is very low, which could mean that the positive effects of calcium addition are reduced in other raw waters with higher calcium content. Despite the difference, the values reported by this combination could justify the use of CaCl
2 as a co-coagulant for economic purposes by reducing the overall chemical expenditures of drinking water utilities in some situations. This could be relevant in cases where the water quality demand is lower, e.g., as a pretreatment step before primary treatment. Irrespective of the primary coagulant used for natural organic matter, acidic pH values are favorable for their removal through the charge neutralization mechanism, as reported by Naceradska et al. [
21].
The use of CaCl
2 in the tested water allowed for a significant reduction in the amount of added ALG coagulant. The research results presented here are representative of acidic waters. We observed that the efficiency of using CaCl
2 as a co-coagulant increased with increasing pH. These results are consistent with the results of research conducted by Kowalski and Turkiewicz [
16], which showed that, in surface waters with pH = 7.3–7.9, the addition of calcium chloride in amounts of 10 g/m
3 and 20 g/m
3 to aluminum sulphate (ALG) allowed for an almost twofold increase in the efficiency of organic matter removal.
4. Conclusions
The use of aluminum sulphate and ferric chloride as a primary coagulant for the removal of NOM from raw water extracted from Lake Bolmen was investigated at approximately the optimal pH range. The results showed a U-shape behavior with an optimal minimum around pH 6.1 for ALG and pH 5.1 for PIX-311. A sustainable use of coagulation chemicals and co-coagulants requires the minimization (reduction) of the coagulant concentration.
The effect of adding CaCl2 as a co-coagulant was investigated to understand the possible positive effect in the removal of NOM. The direct addition of CaCl2 at different concentrations, with the optimal concentration of primary coagulant, resulted in no positive effect, while the addition of CaCl2 at approximately 40% to a reduced primary coagulant concentration of approximately 70% of the optimal value resulted in no positive effect for PIX-311 and a positive reduction in NOM in the case of ALG.
We conclude that CaCl2 could be used under certain conditions at higher pH (above 6) as a co-coagulant aid for the reduction in NOM when aluminum sulphate is used as a primary coagulant. This could result in some economic advantage by reducing the concentration of ALG needed for the coagulation process, and potentially reduce residual aluminum caused by high flocculation. Overall, the use of CaCl2 as a co-coagulant needs to be tested in each specific case because the characteristics of the raw water will affect the NOM removal efficiency. Hence, its use in other water utilities with different characteristic raw water compositions needs to be tested to properly assess the suitability of primary coagulant concentration reduction. Our results are in agreement with those reported by Kowalski and Turkiewicz showing that the addition of calcium chloride allows for an increase in the efficiency of organic matter removal. Another potential concern is that reducing the amount of primary coagulant could lessen the need for polishing steps after primary treatment, such as increasing the pH after flocculation. In addition, allowing flocculation to occur at a higher pH has a less corrosive effect on the flocculation basins.