Abstract
Sustainable development is an integrated approach to tackle ongoing global challenges such as resource depletion, environmental degradation, and climate change. However, a paradigm shift from a fossil-based economy to a bio-based economy must accomplish the circularity principles in order to be sustainable as a solution. The exploration of new feedstock possibilities has potential to unlock the bio-based economy’s true potential, wherein a cascading approach would maximize value creation. Seaweed has distinctive chemical properties, a fast growth rate, and other promising benefits beyond its application as food, making it a suitable candidate to substitute fossil-based products. Economic and environmental aspects can make seaweed a lucrative business; however, seasonal variation, cultivation, harvesting, and product development challenges have yet not been considered. Therefore, a clear forward path is needed to consider all aspects, which would lead to the commercialization of financially viable seaweed-based bioproducts. In this article, seaweed’s capability and probable functionality to aid the bio-based economy are systematically discussed. The possible biorefinery approaches, along with its environmental and economic aspects of sustainability, are also dealt with. Ultimately, the developmental process, by-product promotion, financial assistance, and social acceptance approach are summarized, which is essential when considering seaweed-based products’ feasibility. Besides keeping feedstock and innovative technologies at the center of bio-economy transformation, it is imperative to follow sustainable-led management practices to meet sustainable development goals.
1. Highlights
- A systematic review on seaweed functionality;
- Seaweed characteristics and potential value chains;
- The exploitation of quantitative and qualitative analysis seaweed value chain benefits;
- Summary of the “PBFS” approach, essential for considering seaweed-based products’ feasibility.
2. Introduction
‘Sustainability’ is a central topic in today’s research, and is a crucial pillar in the socio-political landscape. The world’s population is expected to reach 9 billion by 2050, putting prodigious pressure on environmental resources [1]. Climate change, resource depletion, and toxicity potentials are the concerning threats to the society for which a new solution is urgently needed. Focusing on reducing our dependency on the fossil-based economy and shifting toward a bio-based economy could help in tackling these situations, as well as for achieving sustainable development goals (SDG’s) [2]. The accomplishment of a bio-based economy is entirely dependent on the utilization of renewable resources, especially biomass, to produce multi-functional applications, including food, animal feed, bio-based materials, energy, and pharmaceuticals. Recently, much focus has been given to either producing novel bio-based materials, or replacing the existing fossil-based products by the scientific community and start-up industries [3]. Moreover, the prevailing question arises: can sustainability and economic growth synchronize to lead the way forward? Policymakers and researchers must answer this question and set up a framework within the boundaries of the circular economy.
The circular economy is the most discussed and promoted concept, wherein the life cycle of materials, products, and resources are extended as much as possible to extract their economic benefits [1]. The bio-based economy can manage resources efficiently, thereby improving the life cycle of the system at every stage by minimizing waste and improving economic benefits [4]. The European Union (EU) has made considerable efforts in making the bio-economy sustainable by establishing a European bio-based industries-joint undertaking (BBI-JU) fund worth 3.7 billion euros via public-private partnership (PPP) [5]. Moreover, the EU has established initiatives to encourage and help the bio-based industry to interlink within new value chains. It is estimated [6] that the bio-based industry generated nearly 2.3 trillion euros worth of turnover in 2015, wherein, 50% was contributed by food and beverage related industries, ~17% was accompanied by the agricultural industry ~8% by the paper industry [7]. The bio-based economy is at its early stage, albeit having created more than 22 million job opportunities in 2012, with the numbers increasing yearly [8]. The United States (US) has also made a significant effort to boost the bio-based economy, as 4.2 million new employees were created with nearly a USD 400 billion contribution to the US economy in 2014 [9].
Camia et al., 2018 [10] reported that nearly 1400 Mt dry matter is produced in Europe, out of which approximately 950 Mt comes from the agricultural sector and 150 Mt from the forestry sector. It was also estimated that out of 1 billion tons of available biomass, the EU utilizes roughly 60% of it in the food sector, 20% in the bio-based energy sector, and 19% in the novel bio-based material sector [7]. The EU chemical sector used 77.7 Mt of organic raw material, with 10% of its share coming from renewable material [10]. The prospects for the bio-based economy look promising; however, the cost associated with the development of bio-based materials is significantly dependent upon the availability and efficacy of feedstock [1].
In the past few decades, the environmental outlook has changed from loss of biodiversity to resource depletion and climate change. As a result, seaweed has been getting exponential interest as an innovative feedstock for bio-based materials from different sectors as a sustainability target. From an industrial point of view, such biomass has potential application in various fields, including pharmaceutical, food, feed, cosmetics, bioenergy, etc. A sufficient equilibrium between social, environmental, and economic performances can set up a benchmark for future sustainable development, and newly established industries can benefit from these astonishing consequences. However, most of such industries are inceptive, and even institutional research is rudimentary. Therefore, it is valuable to cumulate the recent progress, coherent potential value chains, and subsequent sustainability impacts in a comprehensive overview.
According to Scopus, nearly 7000 articles related to seaweed applications were published in 2020 alone, wherein, a significant focus was placed upon agricultural and medicinal applications. Subsequently, >5000 reviews in total have been published so far; however, ~70% of such articles discuss a seaweed strain. The remaining review articles have focused on domain-specific applications. It is essential to understand that seaweed’s application suitability has not been evaluated in every case. Moreover, the economic, social, and environmental aspects of seaweed’s value chains are discussed collectively in just one article [11]; despite this, the entire value chain’s inclusiveness is lacking. The approach to gathering the information presented in this review is shown in Figure 1 below.
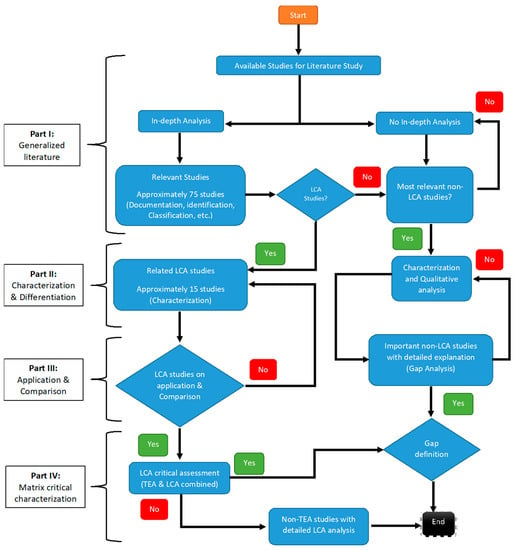
Figure 1.
Literature survey strategy.
The prerequisite of the seaweed value chain can provide a valuable addition to the actual delivery of the product through nutrient uptake and carbon sequestration. The primary aim of the present manuscript is to assess whether seaweed biomass can deal with ongoing sustainability issues, and to identify routes of potential improvement. First, the manuscript discusses the current seaweed market and seaweed characteristics. Next, it deals with the various types of seaweed cultivation and harvesting, as well as seaweed functionality and its application in various fields, in order to give a comprehensive overview to the instigators. Later, this manuscript discusses the aspects surrounding sustainability, including environmental and economic perspectives. Finally, a PBFS approach is discussed to develop a trajectory for future scenarios. In this review, the latest information on seaweed functionality and its sustainability proceedings is collected, which may help policymakers, industries, and researchers to further develop a bio-based economy.
3. What Is Seaweed?
Seaweed is a macroscopic alga, with its usual habitat at the bottom of shallow coastal waters. It typically grows at a depth of 180 m, and can be found on rocks, pebbles, shells, black water, and seawater plants. They are classified into green algae (Chlorophyceae), red algae (Rhodophyceae), and brown algae (Phaeophyceae) based on pigmentation. Approximately 624 algal species have been identified in India alone, wherein green seaweed contributes nearly 72%, red algae 27%, and brown algae nearly 1% [12]. It is essential to understand that seaweeds are the only species responsible for producing several phytochemicals, including agar-agar, align, and carrageen which has extensive additional applications [13]. Various consumptive and non-consumptive applications such as, fertilizers, animal feed, medicines, building materials, soups, sushi, salads, and other snacks are mentioned in the literature [14]. Seaweeds have been prominently used as a feedstock for thousands of years, and are mentioned in Greek texts extensively. It is also reported that during the scarcity of fodder, seaweeds were dried and fed to horses, sheep, and cattle until the early 1900s [15].
Recently, the EU and various countries’ interest have increased for seaweed’s cultivation and application due to its multi-dimensional functionality. However, historical aspects of seaweed functionality must be considered in order to gain a better understanding. The first commercial use of seaweed was reported in the 17th century, where it was used to replace wood ash in glass production, especially in France and Norway [14]. Norway followed a similar path; seaweed burning to produce potash was one of the significant incomes during the 18th century. It was reported that during 1913, 150,000 tons of Kelp (a brown seaweed) was dried and burned for the production and export of 6000 tons of potash [14]. In France, seaweed was also an essential ingredient in the production of iodine in 1823. The production of seaweed meals was first industrialized in 1937, produced in nine factories, all of which are still functional [14]. Traditionally, algae cultivation was a household activity with a focus on agricultural purposes only. However, the first commercial algal harvesting plant was established in 1947 in the western part of Ireland to harvest enough algae for feed and food applications. Though seaweed has comprehensive functionality, it was advised to focus on the techno-economic improvements in seaweed farming and cultivation, as knowledge and skills are not yet advanced enough to encourage stakeholders to invest.
4. Seaweed Market
The Food and Agricultural Organization (FAO) of the USA estimated that nearly 30 Mt of seaweed was utilized in 2014, wherein most of it was produced via aquaculture and nearly 6% was harvested through wild species. Seaweed usage has been increased by nearly 176% since 1995 due to the scientific and technical enhancement in harvesting practices worldwide [16]. The world seaweed product market is dominated by the Philippines, Indonesia, and China, whereas European countries and the USA are the latest emerging players. It is estimated that if a special economic zone can be implemented for the marine aquaculture program globally, the seaweed yield could be increased exponentially to 300–1120 Mt [17,18]. The conversion of at least half of this available seaweed biomass into valuable products such as biogas would neutralize the natural gas import in the USA. As seaweed’s product demand, production, and domestication increase, an improvement in seaweed biotechnology is ultimately encouraged [19].
Subsequently, research and innovation have increased exponentially in the past decade, resulting in many research publications and patents [20]. Although publications and patents do not necessarily indicate the market overview, it certainly shows a potential way forward [21]. A total of 15 countries have filed patents on seaweed and its application. Nearly 37% of the total scientific publications are within the food sector, followed by agricultural (19%) and human health applications (13%). Out of 795 seaweed species registered, 31 species were subjected to a patent application, and 138 species were discussed in publications [19]. The seaweed patent registration scenario in the EU is presented in Figure 2.
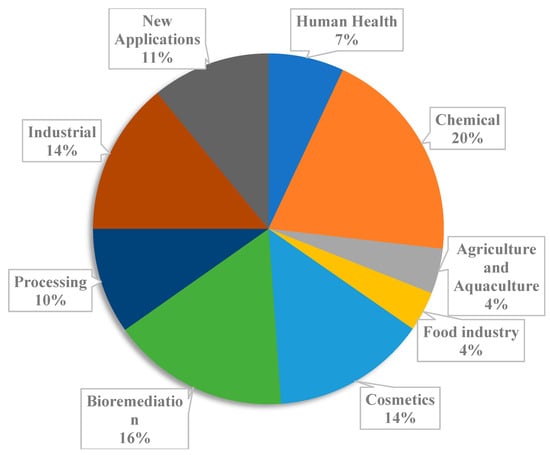
Figure 2.
Seaweed patent registration in Europe per application.
5. Seaweed Characteristics
The most significant advantage with seaweed is that it does not contain distinct parts usually found in other terrestrial plants; therefore, the whole seaweed can be used as biomass [22]. Most of the green seaweeds, including Monostroma, Caulerpa, and Ulva species, are discussed in the literature for their capability to produce Ulva. Ulva is a complex acidic polysaccharide, which has cosmetic and medical applications [22,23]. In red seaweed, Palmaria palmata, Kapapaphysus, Pyropia Yezoensis, species are widely discussed to produce a large quantity of carrageenan, agar with applications in pharmaceutical, textile, paint, antibiotic, food, and biotechnology sectors [24]. Brown seaweed, Ascophyllum nodosum, and Laminaria are known for their capacity to produce various polysaccharides (alginate) with the primary application as a thickener, stabilizer, and gelling agent [22,25,26]. Typical seaweed characterization is presented in Table 1 below. Seaweed has a relatively similar composition to other bioenergy crops. Seaweed has excellent nutritional value and has a higher polysaccharide content, making it suitable for dried and fresh vegetables, as an ingredient for the commercial production of phycocolloids [27], and a perfect candidate in the changing fuel market [28].

Table 1.
Typical Seaweed Characterization.
Seaweeds are usually characterized by high ash content (13–30%), protein content (5–43%), fibers (5–53%), and a low amount of fats. Few macroalgae have high lipid content in their biomass, whereas some other macroalgae are considered to have high protein content. Their composition may vary according to the type, geographic condition, seasons, and cultivation method. Nevertheless, it is documented that green and red algal species have high carbohydrate content; moreover, brown algal species have a high protein and carbohydrate content [29].
5.1. Lipids
As noted previously, lipid content is relatively lower in seaweed than in other plants, including sunflower and soy. Moreover, its lipid quality is of great interest, as it contains essential fatty acids, including omega-3 and other fat-soluble vitamins [30]. It was observed that the lipid content in brown seaweed varies between 1 to 4.5 g/100 g of dry seaweed biomass; however, there have been many discussions in the literature about its specific lipid content [31]. Few publications discuss the phospholipid as a significant lipid constituent in seaweed [32], while others argue that glycolipids are the prominent constituents [33,34]. The seasonal variation has a crucial impact on lipid content as well as on seaweed growth. In one study, it was observed that Undaria, a green seaweed, reaches a maximum height of 4 m by the spring; however, it degenerates in summer [35]. The lipid content was observed to increase during winter, and this also declined in summer. The same observation was noted by Nelson et al., 2002 of Egregia menziesii, a brown seaweed, wherein the highest lipid content of 13.3 mg/g was observed in spring, and the lowest lipid content of 6.3 mg/g was observed in summer [36].
5.2. Carbohydrates
Carbohydrates are the primary constituent of seaweed, with these accounting for at least 40% of its mass. The majority (approximately 45%) of the ash-free volatile solids found in Sargassum, an all-season brown seaweed, are complex carbohydrates. Fucoidan, a sulfated polysaccharide, has been extracted from brown seaweed in recent years [37]. Ulva, the most discussed green seaweed, is observed to have a high content of monosaccharides, including rhamnose, glucose, uronic acid, etc., [38]. In the case of red seaweed, Gracilaria, galactose and glucose are found to have a dominant presence. The variation in carbohydrate content also originates from the season, temperature and is species-dependent [39].
5.3. Proteins
Protein content is an essential aspect of seaweed functionality. However, it varies according to species, geographical region, growth environment, and season. It has been observed that brown seaweed contains a low protein concentration (4–10%), and green seaweed contains a moderately high protein concentration (15–25%). The highest protein concentration was observed in red seaweed (8–40%), which has a high commercial importance [40]. In the case of the Laminaria digitata species of brown seaweed, a protein content of nearly 15% has been reported, whereas in the case of Undaria pinnatifida, a species of brown seaweed, a protein content of up to 24% has been reported [41]. In green seaweed, seasonal changes have been found to affect the protein content, as evidenced by the fact that the protein content of Ulva armoricana, a species of green seaweed was found to be 18% in October and 24% in February [42], respectively. In the case of Porphyra tenera, a species of red seaweed, protein content was reported to reach up to 47% during ideal circumstances [43]. It was hypothesized that the seasonal variation in seaweed protein content is catalyzed by the nitrogen nutrients found in the cultivation water. Salinity was also found to impact seaweed growth directly, as it was observed that the algal growth slows down in the rainy season due to a decrease in salinity, resulting in a decreased protein concentration [44]. The light intensity was also found to impact protein content significantly, as light-harvesting pigments bound by phycobiliprotein were produced by red seaweed [45].
5.4. Pigments
Seaweeds are also reported to be a source of pigments that are of commercial importance. As mentioned previously, phycobiliproteins are the primary light-harvesting pigments observed in red seaweed, which are the only water-soluble pigments [46] and contribute nearly 50% of its total protein content. It is commercially significant in various applications, including dairy products, wasabi, and gums. Brown seaweed dominates the Fucoxanthin, a Xanthophyll pigment responsible for the brown coloration. It has been reported that the Fucoxanthin is the most stable compound, which can get through the drying and storage process at room temperature [47]. Moreover, it has been reported that chlorophyll-a contributes nearly 1.5% of seaweed organic content [48].
5.5. Other Constituents
The algal phenolic compounds are called Phlorotannins and are derived from the Phloroglucinol unit, i.e., 1–3-trihydroxybenzene. The brown seaweed is found to have a rich concentration of Phlorotannins. Researchers have found immense interest in replacing synthetic antioxidants with natural seaweed-derived antioxidants in the last decade [47,48]. Apart from antioxidants, seaweeds are a rich source of vitamins, including A, B-complex, C, D, niacin, folic acid, etc. The brown seaweed named Ascophyllum and Fucus has a higher vitamin E concentration than any other seaweed species. As the seaweed is grown in marine environments, it has a high concentration of minerals, including Mg, Ca, Cu, Fe, as well as some rare minerals [49]. In recent studies, seaweed was also found to have bioactive compounds that can be used as secondary metabolites.
6. Seaweed Cultivation
Seaweed cultivation is not frequently discussed in the literature, despite the fact that seaweed has a rich history of applications. Globally, however, few seaweed species are commercially cultivated. Cultivation methods are traditional in most cases, and the cultivation areas are significantly smaller in size than other vegetables. In the last decade, the focus has been on seaweed cultivation at the molecular level after realizing that seaweed has substantial economic potential. The commercial cultivation of seaweed is carried out at a sizable scale throughout the world. The whole process is mainly distinguished into juvenile plant (seed stock) cultivation, adult plant cultivation, harvesting, and final processing [50]. Seed stock cultivation is the primary step that determines the success of the whole seaweed cultivation process. It depends upon the quality of the crop and seasonal variations. The actual cultivation process starts with adult plant cultivation, wherein the seaweed grows to the full extent and is divided into microscopic and macroscopic processes. The macroscopic process starts with algal fragments, and only two life cycle stages are under control during the cultivation process. The microscopic cultivation process is a relatively new method that starts with the seaweed’s microscopic pores, and the entire life cycle of seaweed is under control during the cultivation process. Based on the cultivation environment, the cultivation process is performed in five different setups:
- Land-based—indoor cultivation;
- Land-based—outdoor pond, open-air cultivation;
- Shallow sea—semi-floating or pole system;
- Shallow sea—U-type floating system (in a subtidal zone);
- Shallow sea fixed support system (in an intertidal zone).
Harvesting is the ultimate purpose of seaweed cultivation, and the seaweed is harvested using different advanced techniques mentioned elsewhere [51]. However, the processing is the final, partially dependent stage in seaweed cultivation, carried out to produce the intended product [50].
7. Sustainability in Seaweed Cultivation
As seaweed cultivation and its application are still at the development stage, environmental sustainability may encourage future establishments. Environmental sustainability can be assessed using life cycle assessment (LCA), which evaluates both the benefits and burdens associated with the whole life cycle of seaweed, from seaweed production to the application and the end-of-life stages. In general, LCA appears to be one of the essential techniques to quantify various environmental impacts associated with a product, process, system, or service from cradle-to-grave, based on the ISO 14040 series guidelines [52,53]. The LCA methodology considers the life cycle starting from extraction of raw materials, manufacturing a product, transport, distribution, use, and end-of-life, including waste collection, segregation, treatment, recycling, and disposal [54]. However, very few studies are available in the literature that solely focuses on the seaweed cultivation’s life cycle assessment. Most of the available literature focuses on the application and uses of seaweed. The seaweed applications in the different sectors and the related LCA studies are discussed in the latter part of this paper; however, the use of algal biomass in biorefinery as third-generation feedstock has gained a lot of interest worldwide. The value chain of seaweed cultivation is presented in Figure 3 below.
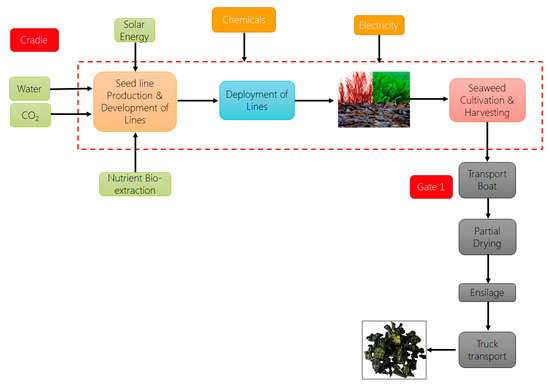
Figure 3.
Seaweed Cultivation & Harvesting System Boundary.
The life cycle for seaweed cultivation started with the seed line production and development of lines, which is the juvenile plant cultivation process mentioned previously. The development of lines and seaweed harvesting is considered inside the system boundary by most available studies [54,55]. The inventories required for developing the LCA model of seaweed cultivation processes include electricity, water, seashore land required, nutrients, such as phosphorus (P), magnesium (Mg), zinc (Zn), nitrogen (N). Researchers have found that carbon dioxide is required to grow juvenile seaweed, which means that seaweed utilizes the oceanic carbon from the water column directly for their growth, and reduces oceanic carbon content [55].
At present, most seaweed cultivation activities are carried out in limited coastal locations; therefore, it is estimated that a maximum of 2.48 million tons of carbon have been extracted from the ocean, which is nearly 0.4% of the total expected oceanic carbon. The agricultural sector is expected to produce nearly 30% of the total global warming gases; seaweed has a great potential to reduce these emissions due to its efficient carbon sequestration. After the cultivation and harvesting process, seaweed is partially dried and transported to the next facility to produce intermediates and products. The seaweed cultivation output usually contains emissions such as liquid waste, solid waste, and seaweed as a product itself. The starch and lipid content of the seaweed can also be considered in the output based on the intended application of the sustainability studies [56]. The available literature focusing on the sustainability assessment of seaweed cultivation is presented in Table 2.

Table 2.
Life Cycle Assessment of Seaweed Cultivation.
Few LCA studies were published on the seaweed’s cultivation (Cradle-to-Gate-1) to its application (Cradle-to-Gate-2). Most LCA studies have focused on cultivation and energy application. Several flaws were identified using the life cycle assessment model after analyzing the seaweed cultivation system listed in Table 2. It has been consistently observed that the electricity required throughout the cultivation system has contributed to significant impacts in all the impact categories and resulted in resource depletion, global warming potential, and increased toxicity potential. The use of renewable energy to replace traditional energy may improve the overall environmental performance of the system. The design of seaweed lines has caused an increase in many of the impacts. Interestingly, it has been reported that as the distance between the rope increases from 2 m to 5 m, the resource demand was found to be increased, resulting in a nearly 4% increase in its environmental impacts [55]. Similarly, the alternate light floating material for the raft system was discussed in the literature, as the high-density polyethylene (HDPE) increases the environmental impacts [63,64]. The distance between the cultivation area and processing unit has also been found to have a notable impact from an environmental point of view. The cultivation of only economically feasible seaweed species, such as Palmaria palmate, requires fewer nutrients and a high carbon sequestration rate. This approach may also improve the environmental performance at the later stages, and is therefore encouraged in some techno-economic analysis studies [65,66,67,68]. The impact of raw materials consumed, including chemicals and minerals, is not discussed extensively in the literature; however, the carbon absorption has reportedly affected the environment by reducing the overall impacts, especially in terms of global warming. Moreover, the literature suggested that the traditional seaweed cultivation system needs to be technologically upgraded, which can mitigate the shortcomings related to large-scale handling [59].
8. Seaweed Applications
Seaweed has wide applications in biofuels, food and animal feed, energy, pharmacy, cosmetic, and other chemical industries. Interestingly, all the seaweed species have little to no lignin content, making them directly suitable for producing third-generation biofuels. After harvesting, seaweed traditionally goes through enzymatic hydrolysis and/or saccharification to separate the polysaccharides. The extracted C-5 or C-6 sugar is then used for the fermentation process to obtain various biofuels. In the past few decades, seaweed has only been considered for producing a single product, like alginate, or bioethanol, which stretched the economic, social, and environmental considerations. The cascading approach has been discussed in the literature for the past few years [69] as a means to fully explore seaweed’s functionality. The cascading approach promotes the production/application of high-valued material first, followed by the next high-valued materials in descending order.
Cascading the seaweed valorization has a great potential in yielding various by-products and other chemicals, which could ultimately reduce its environmental burden and improve the socio-economic potential. Provided that food and energy are the primary concerns in the 21st century, a strategy can be made to process the seaweed to utilize food, animal feed, and energy. In contrast, the technology can be improvised to use the leftover biomass [70] for other applications. In this functional order, the seaweed biorefinery concept has recently been upgraded to utilize the excess seaweed biomass to produce biofuels and biogas. In this manner, the seaweed biomass value can be increased to multiple folds, which would benefit the supply chain, and promote the circular economy.
8.1. Seaweed in Food Applications
Food safety is of prime concern all over the world. The United Nation’s SDGs prioritize food safety and encourage stakeholders to develop the policy around it. According to estimates, only half of the total 9 billion people would have access to nutritional food by 2050 [69]. Seaweed is well-known for its nutritional value to humans and animals, as it can provide minerals, vitamins, calories, and essential antioxidants [71,72]. The most significant benefit of seaweed cultivation is that it does not compete with other terrestrial plants. The total consumption of dried seaweed is estimated to be 2,000,000 tons, including sea vegetables, as a direct source and other applications, like phycocolloids. Asian countries, including China, Korea, Japan, Vietnam, the Philippines, and Thailand, are intensively involved in seaweed production. Moreover, some European countries, including France, Norway, Ireland, United Kingdom, Spain, and other countries, including Canada, New Zealand, and Chile, are active in seaweed production. Seaweeds are varied according to geographical location; however, L. japonica, P. yezeonsis, U. pinnatifida are the most consumed seaweeds. Seaweed is usually dried before any further processing, followed by salting, mixing, rolling, and slicing according to requirement. The link between the seaweed species and preparing seaweed food must be highlighted to get the customer’s approval [73]. Moreover, the prominent challenge in using seaweed as food is that it has to be accepted by a wider global community.
Apart from traditional use, seaweed has other commercial importance as well. The alginic acid obtained from the seaweed has stabilizing and thickening properties, making it suitable for syrups, ice-creams, sauces, juices, shakes, desserts, and bakery products [74]. There are seven different forms of carrageenas observed in seaweed with the sulfated galactose unit, out of which three forms of carrageenas are commercially used. The carrageenas is a roughly thickening agent and is preferred to prepare pizzas, desserts, gels, canned foods, etc. It is also reportedly used as a preservation or additive agent, and the carrageenas additives are usually marked as E407 nomenclature. Seaweed is composed mainly of agaropectin and agarose, which are the polymers of galactopyranose. These can be used as a gelling agent, primarily in canned products, desserts, pie fillings, etc., under the nomenclature of E406 in the food industry [75].
Seaweeds are nutritious, with many health benefits associated; however, they are prone to accumulate undesirable heavy metals. Therefore, it is always advised to check the organic and inorganic components present in the seaweed before any further processing. Seaweed’s daily consumption has not been prescribed as of now; moreover, Asian diets contain nearly 5–8 g of seaweed per day; therefore, it is safe to consider 5–8 g of seaweed (nearly 30 g of wet seaweed) consumption daily [49,75].
8.2. Seaweed into Furfural
Furfural is becoming an essential chemical that can be obtained after biomass conversion. The US Department of Energy (USDOE) has listed furfural as a platform chemical, and it is expected to increase its market potential [73] steadily. Furfural can be used as a base chemical to produce different derivatives, including various polymer units, liquid fuels, and organic solvents [76,77,78]. Furfural is produced from the extracted sugars of lignocellulosic biomass waste after catalytic conversion. The economic conversion of extracted biomass into furfural depends on various factors, including the availability of diversified feedstock, type, nature of the catalyst, and reacting conditions [79]. Park et al., 2016 have extensively researched the production of furfural from seaweed. The commercial H2SO4, H2PW12O40, and Ambelyst15 catalysts were tested for their efficiency, and it was observed that the H2PW12O40 catalyst exhibited significant catalytic activity [80]. Similarly, the tetrahydrofuran was an efficient reactive medium compared to water in the conversion of seaweed-derived alginate into the furfural. The seaweed extract was used to produce the alginic acid first, followed by alginic acid hydrolysis to the monomers. Later, the monomer was converted into the furfural through the series of decarboxylation-dehydration reactions.
8.3. Seaweed into Hydrogen as a Fuel Application
The monomers obtained from the biomass’s carbohydrate content play an essential role in producing fuels [81,82]. It has been discussed in the literature that hydrogen produced from the fermentation of simple carbohydrates, such as glucose and xylose, has great potential to dominate the fuel sector [83,84,85]. Mannitol is a simple carbohydrate with high water solubility, which is observed at nearly 20–30% in the dried brown seaweed [86]. It has been reported the fermentative production of hydrogen yields 5 moles of hydrogen per mole of mannitol consumed for the acetic acid pathway, 1 mole of hydrogen per mole of mannitol consumed for the ethanol or lactic acid pathway, and 3 moles of hydrogen per mole of mannitol consumed for the butyric acid pathway [87]. The heat pre-treatment, mannitol concentration, and pH were reported to be the rate-determining factor in hydrogen production. The anaerobic digestion process yields nearly 1.8 moles of hydrogen per mole of mannitol used; however, maintaining the fermentation conditions is a challenging task [88].
8.4. Seaweed into Biofuel
Production of bioethanol from seaweed can reduce agricultural land, freshwater consumption, and chemical fertilizer usage, ultimately reducing the environmental impacts throughout the lifecycle. The pulp, which remains after seaweed processing, creates an unnecessary burden if not utilized. The pulp contains many carbohydrates, which can be used for bioethanol production, and the leftovers from the fermentative bioethanol production can be used as a fertilizer [89]. Statistically speaking, Kumar S. et al., 2013 [90] have reported that nearly 25% (w/w) of the pulp can be obtained after the seaweed biomass processing, out of which the cellulosic material constitutes 40%, and the hemicellulosic material constitutes nearly 20% of the overall leftover pulp (w/w). On the successive enzymatic hydrolysis process, almost 88% of the cellulosic material is converted into simple sugars and utilized further in the fermentation process with 86% of bioethanol yield efficiency. Moreover, it has been assumed that the CO2 absorption in the algal biomass is nearly seven times higher than that of terrestrial woody biomass [91]. Therefore, the ultimate carbon sequestration can further reduce the environmental impacts associated with the lifecycle of bioethanol production. Several researchers have extensively worked on bioethanol production from the seaweed pulp, and several seaweed species have been reported in the literature in the same context. Yeon et al., 2016 have reported getting 0.386 g of bioethanol per g of Sargassum sagamianum seaweed pulp [92]. In contrast, Yanagisawa et al., 2011 have used a cluster of species, including U. pertussa, G. elegans, and A. crassifolia, to get the yield of 0.381 g, 0.376 g, and 0.281 g of bioethanol, respectively [93]. Kumar S. et al., 2013 have extensively collected the literature from the past decades to compare the bioethanol yield from various biomass [90], and it was reported that the highest bioethanol yield of 0.46 g of bioethanol was obtained per g pulp of G. verrucosa seaweed. Kim et al. (2015) have reported that red seaweed, especially Gelidium amansii, is a great potential source for bioethanol [94]. This red seaweed species has a high carbohydrate content, which can easily be converted into simple sugars such as galactose or glucose via hydrolysis, and produce bioethanol after fermentation. Approximately 11 million tons of red seaweed were produced in 2011, while there has been a constant increment reported in the production [94].
The production of bioethanol from seaweed has many technological barriers. The processes involved in bioethanol production, including seaweed harvesting, pulp pre-treatment, enzymatic hydrolysis, and the fermentation process, require techno-economic upgrades for achieving a higher yield. The adaptation of the new saccharification methods, i.e., simultaneous and combined saccharification process, and enzymatic hydrolysis, have reportedly increased the bioethanol yield by the 20%. The method was effective in most cases with low or single polysaccharide content in the seaweed [67].
8.5. Seaweed as an Animal Feed
The traditional raw materials used for animal feed include oats, soybean, wheat, barley, and sorghum. The critical setback in using the traditional feedstock is that, apart from being a healthy source of the human diet, they are seasonal crops, and they require a considerable amount of time to grow. The use of such resources throughout the year can create a feud among the supply value chain and lead to inflation in the food prices [95]. Seaweed possesses many advantages over seasonal crops as they can be harvested in any season, proliferates, and do not require terrestrial agricultural land [96]. Seaweed contains several bioactive compounds and healthy nutrients, which can serve the purpose and provide dietary benefits. The seaweed extract contains soluble fibers, including fucoidan, alginate, and ulvan, which have good antimicrobial properties. Gardiner et al., 2008 and Reilly et al., 2008 have mentioned the use of seaweed extract in pig diets as some feed antibiotics [97,98]. Kolb et al., 2004 mentioned using seaweed extract in the cattle’s diet as a dietary supplement [99]. They also mentioned that the addition of seaweed extract improves animal health and increases milk quality. It has been reported that seaweed can replace nearly 15–20% of the traditional poultry diets based on the type of seaweed and the poultry animal [100].
8.6. Seaweed in Pharmaceuticals
In the past few decades, extensive research has been carried out on seaweed extracts in various medical and pharmaceutical products. Cancer prevention, tumor-suppressing, and health recovery treatments using seaweed extract are documented in the literature. Funahashi et al., 1999 documented a series of seaweed types and their effectiveness in preventing and suppressing cancer. The administration of seaweed extract at 1.6 g/kg body weight of the patient for 28 days reportedly inhibited 46–70% of the Ehrlich carcinoma [101]. The brown seaweeds such as Scytosiphon lomentaria, Laminaria japonica, Lessonia nigrescens, and Sargassum ringgoldianum used in the study showed 70%, 58%, 60%, and 46% of inhibition, respectively. Apart from brown seaweed, some of the red seaweeds, like Eucheuma geltinae, Porphyra yezoensis, and green seaweeds, such as Enteromorpha prolifera, have shown nearly 52% of inhibition. Various biomolecules obtained from seaweeds such as fucoids, glycolipids, and phospholipids have shown great potential against the MethA fibrosarcoma and have an antimetastatic effect on A549 lung cancer [102].
Some of the biomolecules extracted from the brown seaweed have excellent dietary fiber content, which plays a significant role in cancer prevention. Brown seaweeds such as Undaria pinnatifida and Hijikia fusiformis were found to have a high concentration of Fucoxanthin, and are frequently used as a daily dietary supplement in Asian countries. Other brown seaweeds such as Ecklonia kurome and Laminaria japonica were found to have laminarin as an active ingredient. Laminarin is a water-soluble polysaccharide with β-(1–3)-glucan linkages [103]. Moreover, brown seaweeds are bountiful with Carrageenans and Phlorotannins, linear galactans with antioxidative properties [104]. Some of the red seaweeds, including Gracilaria arcuate and Actinotrichia fragilis, have 3-amino-1-propanesulfonic acid, which is commercially known as alzhemed. The alzhemed drug treatment at stage III clinical trials is effective in Alzheimer’s disease treatment [105]. The clinical trials have reported that the absorption of 500 mg of brown seaweed like Fucus vesiculosus can lead to an 8% increment in insulin sensitivity index than that of placebo in diabetic patients [106]. Alginic acid is one of the important biomolecules obtained from brown seaweeds, and has wide biomaterial applications to prepare nanoparticles or gel for targeted drug delivery [107].
8.7. Seaweed in Cosmetics
The cosmetic industry has been booming for the past few decades with approximately EUR 400 billion trade worldwide. Europe is the leading cosmetic market with a EUR 72 billion business, followed by the USA (~EUR 38 billion) and Japan (~EUR 30 billion). France has a substantial cosmetic market with nearly EUR 25 billion trade with roughly EUR 8 billion trade surplus [108]. The cosmetic industry is always hunting for new constituents for economic and social purposes. Bio-based materials have significant demand in the cosmetic industry, and the marine algal source has been considered extensively in cosmetics product development [109]. Seaweeds are the apparent option to consider in the cosmetic industry, as they contain various biomolecules, named phycocosmetics [108]. The brown seaweed Macrocystis pyrifera belongs to the laminariaceae family, which contains hyaluronic acid. Hyaluronic acid is the prominent constituent of the extracellular matrix and hence used as a treatment aid for burn victims since 1968 [110]. In many other cases, plastic surgeons use hyaluronic acid for treating volume loss and face wrinkles [108]. Fucoxanthin and Astaxanthin are major biomolecules constituted in the brown seaweeds. These biomolecules have excellent antioxidant properties, and therefore, have applications as an anti-aging ingredient in the cosmetic industry [111]. Micosporine-like amino acids (MAAs), such as shinorine and usujirene, are found in most seaweed algae. These molecules are responsible for absorbing most of the UV radiation between 310–360 nm and hence protect the seaweed algae. These MAAs have been exploited on an industrial scale for their application in sunscreen lotion [112]. Many brands such as Daniel Jouvance (LA Gacilly, France), Science & Mer (Paris, France), Phytomer (Brittany, France), Algotherm (Brittany, France), and Gelyma (Marseille, France) are working on commercializing the phycocosmetics, and many others are exploiting the future possibilities.
9. Sustainable Seaweed Applications
Researchers are exploiting the various ways through which seaweed can be utilized maximally. However, even though world seaweed production has been increased three times in the past 50 years, the sustainability of seaweed functionality is still a challenging concern [113]. Sustainability is a relative concept, especially in seaweed cultivation, and depends on the production region. Many reports on seaweed functionality have reported that the time of harvest, as discussed earlier, has a significant effect on its applicability. For example, Adam et al., 2011 reported a 30% higher biomethane yield when the Laminaria digitate seaweed is cultivated and harvested in June, compared to winter or spring [114]. Similarly, Jard et al., 2013 observed a 25% higher biomethane yield when Saccharina latissimi seaweed was cultivated and harvested in August, compared to the winter or summer [26]. Therefore, seaweed sustainability must be discussed and assessed to identify the sustainability benefits and drawbacks in relation to seaweed functionality.
Conversion of seaweed biomass has typically been presented as having strong potential for biorefinement in the present literature, wherein its composition, treatment technologies, and value chains are discussed, similar to that of microalgae. Few published reports have focused on the characteristics of seaweed and its application in bioenergy. The rest of the published research has noted its carbon capture capacity and viewed it as a solution to the ‘food vs. fuel’ debate. Moreover, the sustainability and thorough analysis of a value chain in the biorefinery process is still lacking in the literature. Sustainability is often a vaguely used term in literature on seaweed cultivation, focusing on ecology and the environment. Nonetheless, with an increase in the number of reports on seaweed functionality, it is necessary to implement sustainability assessments comprehensively, which could also support finding improved seaweed economics and social acceptance. In the present manuscript, available literature on environmental and techno-economical aspects of seaweed’s value chain has been collected and described. The value chain of seaweed is presented in Figure 4 below.
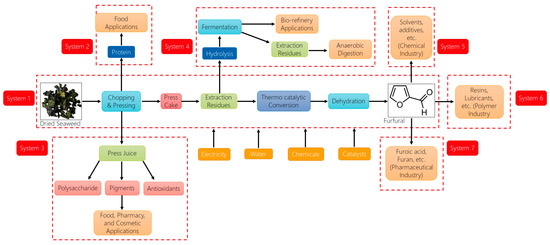
Figure 4.
Seaweed biorefinery.
In 2007, the Biorefinery International Energy Agency (BIEA) identified different tasks that need to be achieved in order to develop a low-carbon sustainable economy. Task 42 aimed to implement sustainable biorefineries with a zero-waste value chain and the production of both bio-based food and non-food-based value chains [115]. The biomass from terrestrial origins is still the predominant feedstock; however, aquatic biomass is considered for the biorefinery approach in recent years. A seaweed biorefinery process could provide an essential boost to the seaweed market, thereby closing the loops in the circular economy. With additional economic value given to seaweed processing, several technologies have increased along with interest in this field, as reported by Laurens et al., 2017 [116]. Since seaweed is still an expensive feedstock (~USD 50–80/ton), the biorefinery approach allows full use of available biomass, including high-value applications, giving more socio-economic and environmental benefits. Figure 4 depicts the potential biorefinery approaches in seaweed processing through different systems. System 1 illustrates the traditional methpd of utilizing seaweed for the production of furfural. System 1 is the most discussed pathway in the literature, wherein seaweed is processed via physical and thermochemical technologies to extract the complex carbohydrate content (such as xylose, alginate, levulinate, etc.), thereby converting it into furfural. This system’s implementation is mostly at an early stage of development and at pilot scale in some cases. Furfural is a starting material for various products and a key intermediate in many bio-based value chains. Furfural derived from bio-based sources can be converted to solvents and chemicals used in the polymer and pharmaceutical industry, as projected in Systems 5, 6, and 7.
System 2 is the obvious choice for seaweed processing, wherein the proteins and other parts are separated for a variety of food applications. This system has been established in Asian countries, where seaweed is consumed as a food in various cuisines, including Furikake, Jerky, Sea-chi (kimchi), pickle, salsa, tea, etc. Seaweed-based food companies such as Cargill, Acadian seaplants, DuPont de Nemours, Irish seaweed, Mara seaweed, and Beijing Leili had nearly USD 5 billion/year collective trade in the last decade [117]. System 3 depicts another biorefinery route for seaweed processing, wherein the seaweed extracts are used as active biological ingredients in food, pharma, and cosmetics industries, as discussed previously. Carrageenan, alginate, and agar were found to have antiviral, anti-inflammatory, antibacterial, and antioxidant properties, attracting interest from various pharmaceutical companies [118]. Institutes such as IOTA pharmaceuticals, Oncology SME, University of York, and the University of Cambridge have collaborated to develop a seaweed-based drug system to fight cancer. According to a recent public report, scientists at Rensselaer Polytechnic Institute have claimed that the seaweed extract (RPI-27) could outperform the well-known COVID-19 drug Remdesivir to trap the virus before it can infect the human body. In addition, several cosmetic industries, such as, Earthrise Nutritionals (California, USA), Cyanotech Corporation (Kailua-Kona, Hawaii), BlueBioTech Int. GmbH (Kaltenkirchen, Germany), Bluetec Naturals Co., Ltd. (Erdos, Inner Mongolia, China), and Tianjin Norland Biotech Co., Ltd. (Tianjin, China), etc., have been developing an entire range of cosmetic products based on seaweed extracts. System 4 presents one possible biofuel concept from seaweed extract, which is widely discussed in the literature [93]. There are also potential economic benefits in producing other value-added products, such as bio-butanol and biogas, for implementing this route. First- and second-generation biomass, used for fuel production, has limitations in terms of its usability and sustainability. However, macroalgae’s current developments can overcome these hurdles and provide a sustainable low-carbon economy by exploiting the output of value-added products, apart from bioenergy [119].
The LCA’s quantitative and qualitative analysis can exploit the benefits of the seaweed value chain. The literature on the LCA of the seaweed value chain is limited, with most papers focusing on biofuels. The European Directives embraced the LCA methodology in 2009 to evaluate the environmental impacts generated by biofuels during their entire life cycle, and created an objective to reduce the GHG emissions by 50% in the next decade [120]. The available literature on LCA of seaweed applications is compiled in Table 3 below.

Table 3.
Life Cycle Assessment of Seaweed Algae Applications.
After evaluating available LCA studies, it was observed that most of the studies focus on the cradle-to-gate approach. Discussion around the application or the end-of-life section is missing from most of these studies; wherein, the end-of-life section is only considered in biofuel applications (combustion of the fuel). Energy consumption and seaweed cultivation were major impact contributors in fuel applications. In the non-fuel applications of seaweed, LCA studies indicate that seaweed cultivation plays a significant role in imposing environmental impacts throughout the cradle-to-gate scenario. Traditionally, infrastructure is excluded from LCA studies; however, seaweed cultivation requires specific infrastructure, different from terrestrial ones, to facilitate higher growth. Therefore, impacts associated with infrastructure development have to be considered to get a realistic overview; however, this aspect has been neglected in available studies. Technology usage was different in every study, starting from the seaweed cultivation until the intended application production. Therefore, it is not easy to compare all studies on the potential seaweed-based product and their commercially available counterpart. Due to the presence of reactive nitrogen in nutrients and the anoxic conditions that occurred during seaweed cultivation, nitrogen emission (N2O, NH3, etc.) most likely occurs, which leads to acidification and GHG emissions [137,138]. Provided that N2O has a GWP of 250 times the GWP of CO2, more emphasis must be put on such emissions, and the emission factors have to be considered while interpreting the environmental impacts. Similarly, the CO2 fixation at the cultivation step and CO2 emission during the user phase (especially combustion in biofuel applications) need to be considered. Most studies have omitted this part and considered the entire process as a CO2-neutral process. This approach may significantly affect the GWP impact, and therefore, the greenhouse gas flow must be considered. Additionally, these studies have considered various functional units and impact assessment methodologies, making it challenging to compare seaweed-based products on an environmental or economic basis. As mentioned previously, the LCA studies have been conducted for the high-end seaweed-based products only, whereas most low-end products and their feasibility are not discussed. Moreover, the discussion about co-products or by-products generated during the seaweed’s processing is missing, which would improve the seaweed value chain. Feasibility studies, including techno-socioeconomic assessment, should be carried out to promote the seaweed-based products and support the selection of economically viable and environmentally sustainable value chains.
10. Techno-Economic Assessment
Apart from technological glitches, seaweed is at the center of attention for its potential to substitute fossil-based products and positive environmental impacts, such as nutrients recovery. However, seaweed farming has to be compatible and sustainable to accomplish either of these needs. Presently, seaweed is primarily produced for consumption purposes, whereas its production for high/low value-added products such as fertilizers, bioenergy, and biomaterials is still at a developmental stage. Scarce information is available in the literature for techno-economic assessment of seaweed-based value-added products; however, published literature implies that seaweed cultivation and harvesting is the decisive factor in estimating the cost of bio-based products [66,139]. It was reported that the seaweed harvesting cost would vary from USD 200–900/ton of dry mass, based on the type of seaweed cultivation [65]. Moreover, the use of seaweed as food and chemical seems a worthwhile option. Clarens et al., 2010 suggested the optimization of seaweed farming by expanding the current production line and producing value-added products or selling wet seaweed at a higher price (USD 2/kg) to have profitable farming [140]. Electricity production from seaweed has often been discussed in the literature, and a break-even selling price of USD 154/MWh was estimated. This break-even price of seaweed-derived electricity is comparable with the existing renewable sources like solar (USD 150 ± 10 MWh) and thermal (USD 250 ± 10 MWh). Jorquera O., 2010 [141] assumed that nearly 16 MMT of dry seaweed would be required to sell the electricity to the industrial sector at the rate of USD 70/MWh. However, it was also assumed that if the government subsidizes 20% of the production cost, then the required seaweed production would go down to nearly 11 MMT. The UK government has taken an initiative to subsidize the electricity production from seaweed anaerobic digestion, by which the failure in time price of the electricity has been set to 147 GBP/MWh for small-scaled units [66]. Dave et al., 2013 have reported the break-even selling price of 178 GBP/MWh at an internal rate of return of 8% while considering 86.4 tons/day dry seaweed consumption, 64% conversion rate at anaerobic digestion, 25% capital fees, and 4% operational cost [66]. Nevertheless, seaweed farming must develop business cases for future value chains and optimize strategies. In order to compete with already established products or chemicals, the risk factors, such as seaweed growth variation, nutrient recovery, CO2 fixation, market volatility, CapEx, OpEx, and supply-demand value chains must be analyzed. Government viable funding, subsidies, and seed funding are needed to develop a viable seaweed market; otherwise, the seaweed business will not be sustainable.
11. A Way Forward
It is understood that seaweed has a great potential to go beyond its application as food and support the transition to a bio-based economy. However, specific shortfalls in knowledge have been identified based on the listed scenarios. It is unknown from the available literature how seaweed can mitigate climate change’s ongoing problems and support the concept of a circular economy. The available literature does not discuss viable business models for seaweed apart from its application as food. The social aspects are still missing from the available documents, which play a significant role in product establishment. Producing seaweed-based products at a laboratory scale and comparing them with already established benchmarks will not elucidate the barriers to commercialization. Therefore, a clear path forward is needed to consider the aspects which would lead to the commercialization of financially viable seaweed-based bioproducts. Based on the present understanding, we have summarized the “PBFS” approach, which is essential while considering seaweed-based products’ feasibility. The PBFS based product feasibility approach is presented in Figure 5 below. This approach considers four significant aspects of commercialization, including process development (P), by-product promotion (B), financial assistance (F), and social acceptance (S).
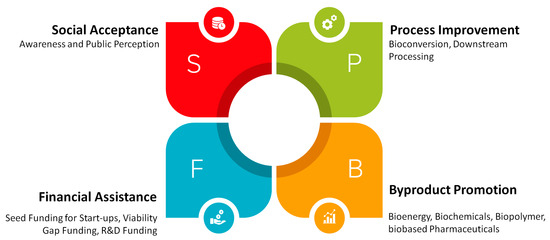
Figure 5.
Seaweed product feasibility approach.
The first and foremost step is to achieve technological enhancement. In the process’ economics, it is understood that nearly 30% of the production cost is accompanied by feedstock processing and handling. Technological improvements will help in increasing product yield, thereby reducing the feedstock processing cost. It may include better storage facilities, separation processes, enzymatic hydrolysis, pre-treatments, fermentation, etc. Emerging technologies and R&D in the field of biotechnology and biochemical processing can improve seaweed-based product economics. Apart from this, skilled labor would play a significant role in R&D, thereby developing novel technologies and improving the overall process.
The second part of the feasibility approach is promoting co-products and by-products developed during seaweed processing. It is understood that the bio-based products are not economically viable compared to their chemical counterparts if produced in a stand-alone facility. It is evident from the overall scheme in Figure 4 that several by-products and co-products are generated while processing seaweed, including proteins, polysaccharides, pigments, extraction residues, etc., have a commercial value in the market. If the production line were strategized so that co-products/by-products are separated efficiently, and their respective processing is considered within the system boundary, then the seaweed-based product would become more economically viable.
The third approach is assisting these projects financially. Stand-alone facilities with the production of seaweed-based products will not survive on their own. For promoting the bio-based economy, the EU and other countries must develop a policy bubble for producing bio-based products. These stand-alone companies should be provided with seed funding for start-up and R&D at the initial development stage. To compensate for the production cost, viability gap funding should be provided until the selling price becomes profitable. Other financial initiatives such as collective tax reduction, incentivizing bio-based products, and defining minimum seaweed purchase price are also encouraged.
The fourth important aspect of achieving the feasibility of seaweed-based products is to improve social acceptance. End users are a significant part of the value chain, since it is they who utilize or consume the product. However, the end-user is always willing to get a premium experience at a low cost, and more often than not, is uninterested in changing the conventional route. It is essential to convey the value-added benefits and competitiveness of a bio-based product to the end-user. Target-specific information would help customers to understand the merits and usefulness of seaweed-based products. The EU may play a crucial role in developing a policy, awareness, and advertising benefits to attract potential customers. Apart from this, evaluating socio-economic aspects and developing benchmarks will also assist in decision-making and social acceptance.
12. Conclusions
The bio-based economy’s prospects seem compelling, given that it is an innovation-based sustainable system, fulfilling the circular economy’s goals. The latest understanding, research, modifications, and competitiveness of bio-based products have propelled unprecedented growth in the last couple of decades. However, some market-driven aspects such as commercial success, scale-up, and economic viability are severe obstacles to progress. Seaweed is a significant feedstock for various bio-based products, which is still in its infancy, and has excellent development potential. Seaweed cultivation has been restricted to food applications so far, and its multi-dimensional usage has not yet been explored commercially. Seaweed cultivation ensures several benefits, including its ability to trap sea nutrients, limit eutrophication, increase biodiversity, and achieve carbon sequestration. Seaweed processing requires extra steps, which could, holistically speaking, counteract environmental sustainability. However, full exploitation of seaweed feedstock with targeted biorefineries approaches and novel value chains is expected to bring environmental and socio-economic benefits.
Author Contributions
Conceptualization, formal analysis, resources and data curation, and original draft preparation, P.N.; funding acquisition, reviewing, supervision, and editing, Y.v.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the European Regional Development Fund, the Dutch provinces of Noord-Brabant, Zeeland and Limburg in the context of OP Zuid, grant number PROJ-02125, and Maastricht University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support from the ZCORE (Zeewier naar COating en Resin) consortium partners.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CC | Climate Change |
| CED-T | Cumulative Energy Demand, Total |
| ME | Marine Eutrophication |
| FWE | Fresh Water Eutrophication |
| HT-C | Human Toxicity, Cancer |
| HT-NC | Human Toxicity, Non-Cancer |
| OLD | Ozone Layer Depletion |
| HTP | Human Toxicity Potential |
| FAETP | Fresh Aquatic Eco-toxicity Potential |
| MAETP | Marine Aquatic Eco-toxicity Potential |
| TETP | Terrestrial Eco-toxicity Potential |
| AD | Abiotic Depletion |
| EP | Eutrophication Potential |
| GWP | Global Warming Potential |
| AP | Acidification Potential |
| ODP | Ozone Depletion Potential |
| TA | Terrestrial Acidification |
| FD | Fossil Depletion |
| WD | Water Depletion |
| IR | Ionizing Radiation |
| GHG | Green House Gas |
| 5-HMF | 5-Hydroxymethylfurfural |
| FFC | Fossil fuel consumption |
| RD | Resource Depletion |
| NMVOC | Non-Methane Volatile Organic Compound |
| PM | Particulate Matter |
| NREU | Non-Renewable Energy Use |
| PBFS | Process development, By-product promotion, Financial assistance, and Social acceptance |
References
- Dupont-inglis, J.; Borg, A. Destination bioeconomy—The path towards a smarter, more sustainable future. New Biotechnol. 2017, 6784, 30041–30049. [Google Scholar] [CrossRef] [PubMed]
- Mengal, P.; Wubbolts, M.; Zika, E.; Ruiz, A.; Brigitta, D.; Pieniadz, A.; Black, S. Bio-based Industries Joint Undertaking: The catalyst for sustainable bio-based economic growth in Europe. New Biotechnol. 2018, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Schütte, G. What kind of innovation policy does the bioeconomy need? New Biotechnol. 2017, 40, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Paula, L.; Dodd, T.; Németh, S.; Nanou, C.; Mega, V.; Campos, P. EU ambition to build the world’s leading bioeconomy—Uncertain times demand innovative and sustainable solutions. New Biotechnol. 2017, S1871-6784, 30022–30025. [Google Scholar] [CrossRef] [PubMed]
- Dietz, T.; Börner, J.; Förster, J.J.; Von Braun, J. Governance of the Bioeconomy: A Global Comparative Study of National Bioeconomy Strategies. Sustainability 2018, 10, 3190. [Google Scholar] [CrossRef]
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Luxembourg, 2018; pp. 1–107. [Google Scholar]
- Moreno, A.D.; Susmozas, A.; Oliva, J.M.; Negro, M.J. Overview of bio-based industries. In Biobased Products and Industries; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–40. [Google Scholar]
- Krüger, A.; Schäfers, C.; Schröder, C.; Antranikian, G. Towards a sustainable biobased industry—Highlighting the impact of extremophiles. New Biotechnol. 2018, 40, 144–153. [Google Scholar] [CrossRef]
- United States Department of Agriculture. An Economic Impact Analysis of the U.S. Biobased Products Industry; United States Department of Agriculture: Washington, DC, USA, 2018; pp. 1–108.
- Andrea, C.; Nicolas, R.; Klas, J.; Roberto, P.; Sara, G.-C.; Raul, L.-L.; van der Marijn, V.; Tevecia, R.; Patricia, G.; Robert, M.; et al. Biomass production, supply, uses and flows in the European Union. Environ. Impacts Bioenergy 2018, 1–126. [Google Scholar] [CrossRef]
- Resurreccion, E.P.; Colosi, L.M.; White, M.A.; Clarens, A.F. Comparison of algae cultivation methods for bioenergy production using a combined life cycle assessment and life cycle costing approach. Bioresour. Technol. 2012, 126, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.R.; Ilangovan, K.; Khan, A.B. Floristic and macro faunal diversity of Pondicherry mangroves, South India. Trop. Ecol. 2008, 49, 91–94. [Google Scholar]
- Ramani, G.; Tulasi, M.S.; Bhai, V.A. Seaweed: A Novel Biomaterial. Int. J. Pharm. Pharm. Sci. 2013, 5, 40–44. [Google Scholar]
- Delaney, A.; Frangoudes, K.; Li, S. Society and Seaweed: Understanding the Past and Present. Seaweed Health Dis. Prev. 2016, 2, 7–40. [Google Scholar]
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 7–26. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, A.; Hernández-gonzález, M.C.; Pereda, S.V.; Gomez-, J.L.; Golberg, A.; Tadmor-shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Global unbalance in seaweed production, research effort and biotechnology markets. Biotechnol. Adv. 2014, 32, 1028–1036. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Rapid growth of seaweed biotechnology provides opportunities for developing nations. Nat. Publ. Gr. 2013, 31, 591–592. [Google Scholar] [CrossRef]
- Arnaud-haond, S.; Arrieta, J.M.; Duarte, C.M. Marine Biodiversity and Gene Patents. Sci. Glob. Genet. Resour. 2011, 331, 1521–1522. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal Polysaccharides, Novel Application, and Outlook. In Algae Based Polymers, Blends, and Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.; Kang, K.-H.; Kim, S.-K. Seaweed polysaccharides and their potential biomedical applications. Starch 2015, 66, 1–10. [Google Scholar] [CrossRef]
- Werner, A.; Clarke, D.; Kraan, S. Strategic Review of the Feasibility of Seaweed; National Development Plan: Galway, Ireland, 2004; pp. 1–123. [Google Scholar]
- Lunardello, K.A.; Yamashita, F.; Benassi Toledo, M.; Rensis Barros, C.; Maciel, V. The physicochemical characteristics of nonfat set yoghurt containing some hydrocolloids. Int. J. Dairy Technol. 2011, 65, 260–267. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed minor constituents. In Seaweed Sustainability; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 193–242. [Google Scholar]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Colin, B.; Fereidoon, S. Marine Netraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–512. [Google Scholar]
- Laurens, L.M.L.; Lane, M.; Nelson, R.S. Sustainable Seaweed Biotechnology Solutions for Carbon Capture, Composition, and Deconstruction. Trends Biotechnol. 2020, 38, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Masakazu, M.; Nakazoe, J. Production and Use of Marine Algae in Japan. Jpn. Agric. Res. Q. JARQ 2001, 35, 281–290. [Google Scholar]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Comparative Evaluation of Fatty Acid Composition of Different Sargassum (Fucales, Phaeophyta) Species Harvested from Temperate and Tropical Waters. J. Aquat. Food Prod. Technol. 2008, 13, 53–70. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Lipids from the Marine Alga Gracilaria verrucosa. Chem. Nat. Compd. 2005, 41, 230–232. [Google Scholar] [CrossRef]
- Hay, C.H.; Villouta, E. Seasonality of the Adventive Asian Kelp Undaria pinnatifida in New Zealand. Bot. Mar. 1993, 36, 461–476. [Google Scholar] [CrossRef]
- Nelson, M.M.; Nichols, P.D.; Scientific, T.C. Seasonal Lipid Composition in Macroalgae of the Northeastern Pacific Ocean Seasonal Lipid. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- Kim, K.; Lee, O.; Lee, B. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Michel, G.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef]
- Enquist-newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins. In Proteins in Food Processing; Woodhead Publishing Limited.: Cambridge, UK, 1999; pp. 197–213. [Google Scholar]
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 275–318. [Google Scholar]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Fujiwara-arasakjl, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. Hydrobiologia 1984, 116/117, 513–516. [Google Scholar] [CrossRef]
- Baghel, R.S.; Kumari, P.; Reddy, C.R.K.; Bhavanath, J. Growth, pigments, and biochemical composition of marine red alga Gracilaria crassa. J. Appl. Phycol. 2014, 26, 2143–2150. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M.; Munier, M.; Cecile, G.; Fleurence, J. Phycoerythrins: Valuable Proteinic Pigments in Red Seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 321–343. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- South, G.R.; Whittick, A. Introduction to Phycology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–352. [Google Scholar]
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 1, 535–543. [Google Scholar] [CrossRef]
- Xiu-geng, F.; Ying, B.; Shan, L. Seaweed Cultivation: Traditional Way and its Reformation. Chin. J. Oceanol. Limnol. 1999, 17, 193–199. [Google Scholar] [CrossRef]
- Monagail, M.M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- ISO 2006a. Environmental Management: Life Cycle Assessment: Principles and Framework; ISO: Geneva, Switzerland, 2007; Volume 2, pp. 1–20. [Google Scholar]
- ISO 2006b. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO: Geneva, Switzerland, 2007; Volume 2, pp. 1–20. [Google Scholar]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Taelman, S.E.; Champenois, J.; Edwards, M.D.; De Meester, S.; Dewulf, J. Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Seghetta, M.; Goglio, P. Life Cycle Assessment of Seaweed Cultivation Systems. Methods Mol. Biol. 2018, 1980, 103–119. [Google Scholar]
- Seghetta, M.; Romeo, D.; Este, M.D.; Alvarado-morales, M.; Bastianoni, S.; Thomsen, M. Seaweed as innovative feedstock for energy and feed—Evaluating the impacts through a Life Cycle Assessment. J. Clean. Prod. 2017, 150, 1–15. [Google Scholar] [CrossRef]
- Van Oirschot, R.; Thomas, J.E.; Gröndahl, F.; Fortuin, K.P.J.; Brandenburg, W.; Potting, J. Explorative environmental life cycle assessment for system design of seaweed cultivation and drying. Algal Res. 2017, 27, 43–54. [Google Scholar] [CrossRef]
- Aitken, D.; Bulboa, C.; Godoy-faundez, A.; Turrion-gomez, J.L.; Antizar-ladislao, B. Life cycle assessment of macroalgae cultivation and processing for biofuel production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Langlois, J.; Sassi, J.-F.; Jard, G.; Steyer, J.-P.; Delgenes, J.-P.; Helias, A. Life cycle assessment of biomethane from offshore- Cultivated Seaweed. Biofuels Bioprod. Biorefining 2012, 6, 387–404. [Google Scholar] [CrossRef]
- Ghosh, A.; Anand, K.G.V.; Seth, A. Life cycle impact assessment of seaweed based biostimulant production from onshore cultivated Kappaphycus alvarezii (Doty ) Doty ex Silva—Is it environmentally sustainable ? Algal Res. 2015, 12, 513–521. [Google Scholar] [CrossRef]
- Anand, K.G.V.; Eswaran, K.; Ghosh, A. Life cycle impact assessment of a seaweed product obtained from Gracilaria edulis—A potent plant biostimulant. J. Clean. Prod. 2018, 170, 1621–1627. [Google Scholar] [CrossRef]
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, M. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. Part B 2011, 42, 856–873. [Google Scholar] [CrossRef]
- Bouafif, H.; Koubaa, A.; Perré, P.; Cloutier, A. Effects of fiber characteristics on the physical and mechanical properties of wood plastic composites. Compos. Part A 2009, 40, 1975–1981. [Google Scholar] [CrossRef]
- Burg, S.W.; Van Den, K.; Van Duijn, A.P.; Bartelings, H.; Van Krimpen, M.M.; Poelman, M. The economic feasibility of seaweed production in the North Sea. Aquac. Econ. Manag. 2016, 20, 235–252. [Google Scholar] [CrossRef]
- Dave, A.; Huang, Y.; Rezvani, S.; Mcilveen-wright, D.; Novaes, M.; Hewitt, N. Techno-economic assessment of biofuel development by anaerobic digestion of European marine cold-water seaweeds. Bioresour. Technol. 2013, 135, 120–127. [Google Scholar] [CrossRef]
- Zimmermann, A.W.; Wunderlich, J.; Müller, L.; Buchner, G.A.; Marxen, A.; Michailos, S.; Armstrong, K.; Naims, H.; Mccord, S.; Styring, P.; et al. Techno-Economic Assessment Guidelines for CO2 Utilization. Front. Energy Res. 2020, 8, 1–23. [Google Scholar] [CrossRef]
- FAO. Declaration of the world Summit on Food Security. In World Summit on Food Security; FAO: Quebec City, QC, Canada, 2009; pp. 1–7. [Google Scholar]
- Van Hal, J.W.; Huijgen, W.J.J.; Lopez-Contreras, A.M. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014, 32, 231–233. [Google Scholar] [CrossRef]
- Geldermann, J.; Kolbe, L.M.; Krause, A.; Mai, C.; Militz, H.; Osburg, V.-S.; Schöbel, A.; Schumann, M.; Toporowski, W.; Westpha, S. Improved Resource Efficiency and Cascading Utilisation of Renewable Materials. J. Clean. Prod. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S. Present and Future Prospects of Seaweeds in Developing Functional Foods. In Marine Medicinal Foods; Elsevier: Amsterdam, The Netherlands, 2011; Volume 64, pp. 1–15. [Google Scholar]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The main seaweed foods in Japan. Hydrobiologia 1987, 29, 5–29. [Google Scholar] [CrossRef]
- Glicksman, M. Utilization of seaweed hydrocolloids in the food industry. Hydrobiologia 1987, 151/152, 31–47. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweeds as Food. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 149–167. [Google Scholar]
- Li, H.; Ren, J.; Zhong, L.; Sun, R.; Liang, L. Production of furfural from xylose, water-insoluble hemicelluloses and water-soluble fraction of corncob via a tin-loaded montmorillonite solid acid catalyst. Bioresour. Technol. 2015, 176, 242–248. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Effects of careful designing of SAPO-44 catalysts on the efficient synthesis of furfural. Catal. Today 2014, 251, 66–72. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Cponversion of biomass platform mpolecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.; Kim, Y.; Hwang, I.T.; Park, N.; Hwang, Y.K.; Chang, J.; Hwang, J.-S. Furfural: Hemicellulose/xylose- derived biochemical. Biofuels Bioprod. Biorefining 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Park, G.; Jeon, W.; Ban, C.; WOO, H.C.; Kim, D.H. Direct catalytic conversion of brown seaweed-derived alginic acid to furfural using 12-tungstophosphoric acid catalyst in tetrahydrofuran/water co-solvent. Energy Convers. Manag. 2016, 118, 135–141. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Ding, L.; Lin, R.; Song, W.; Su, H.; Zhou, J.; Cen, K. Substrate consumption and hydrogen production via co-fermentation of monomers derived from carbohydrates and proteins in biomass wastes. Appl. Energy 2015, 139, 9–16. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Pierra, M.; Trably, E.; Godon, J.; Bernet, N. Fermentative hydrogen production under moderate halophilic conditions. Hydrog. Energy 2013, 39, 1–10. [Google Scholar] [CrossRef]
- De Vrije, T.; Mars, A.E.; Budde, M.A.W.; Lai, M.H.; Dijkema, C.; De, W.P.; Claassen, P.A.M. Glycolytic pathway and hydrogen yield studies of the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl. Microbiol. Biotechnol. 2007, 74, 1358–1367. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Matsumura, Y.; Sato, K.; Al-saari, N.; Nakagawa, S.; Sawabe, T. Enhanced hydrogen production by a newly described heterotrophic marine bacterium, Vibrio tritonius strain AM2, using seaweed as the feedstock. Int. J. Hydrog. Energy 2014, 39, 7270–7277. [Google Scholar] [CrossRef]
- Xia, A.; Jacob, A.; Herrmann, C.; Tabassum, M.R.; Murphy, J.D. Production of hydrogen, ethanol and volatile fatty acids from the seaweed carbohydrate mannitol. Bioresour. Technol. 2015, 193, 488–497. [Google Scholar] [CrossRef]
- Wang, J.J.; Bensmail, H.; Yao, N.; Gao, X. Discriminative sparse coding on multi-manifolds. Knowl. Based Syst. 2013, 54, 199–206. [Google Scholar] [CrossRef][Green Version]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; ISO: Geneva, Switzerland, 2003; pp. 1–118. [Google Scholar]
- Yeon, J.-H.; Lee, S.-E.; Choi, W.Y.; Kang, D.H.; Lee, H.-Y.; Jung, K.-H. Repeated-batch operation of surface-aerated fermentor for bioethanol production from the hydrolysate of seaweed Sargassum sagamianum. J. Microb. Biotechnol. 2016, 21, 323–331. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Nakamura, K.; Ariga, O.; Nakasaki, K. Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochem. 2011, 46, 2111–2116. [Google Scholar] [CrossRef]
- Kim, H.M.; Wi, S.G.; Jung, S.; Song, Y.; Bae, H. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Council for Agricultural Science and Technology (CAST). Animal Feed vs. Human Food: Challenges and Opportunities in Sustaining Animal Agriculture Toward 2050; Iowa State University, Department of Economics: Ames, IA, USA, 2013; pp. 1–16. [Google Scholar]
- Van Den Burg, S.; Stuiver, M.; Veenstra, F.; Bikker, P.; Contreras, A.L.; Palstra, A.; Broeze, J.; Jansen, H.; Jak, R.; Gerritsen, A.; et al. A Triple P Review of the Feasibility of Sustainable Offshore Seaweed Production in the North Sea; Wageningen UR: Wageningen, The Netherlands, 2013; pp. 1–108. [Google Scholar]
- Reilly, P.; Doherty, J.V.O.; Pierce, K.M.; Callan, J.J.; Sullivan, J.T.O.; Sweeney, T. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Anim. Int. J. Anim. Biosci. 2008, 2, 1465–1473. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Campbell, A.J.; Doherty, J.V.O.; Pierce, E.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Lawlor, P.G. Effect of Ascophyllum nodosum extract on growth performance, digestibility, carcass characteristics and selected intestinal microflora populations of grower—Finisher pigs. Anim. Feed Sci. Technol. 2008, 141, 259–273. [Google Scholar] [CrossRef]
- Kolb, N.; Vallorani, L.; Milanovi, N.; Stocchi, V. Evaluation of Marine Algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as Food Supplements. Food Technol. Biotehnol. 2004, 42, 57–61. [Google Scholar]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. Microalgae in Novel Food Products. In Food Chemistry Research Development; Nova Science Publishers: New York, NY, USA, 2008; pp. 1–37. [Google Scholar]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame Seaweed Suppresses the Proliferation of 7, 12-Dimethylbenz (a)- anthracene-induced Mammary Tumors in Rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Kim, E. Fucoidan from Seaweed Fucus vesiculosus Inhibits Migration and Invasion of Human Lung Cancer Cell via PI3K-Akt-mTOR Pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Nelson, T.E.; Lewis, B.A. Separation and characterization of the soluble insoluble components of insoluble Laminaran. Carbohydr. Res. 1974, 33, 63–74. [Google Scholar] [CrossRef]
- Park, E.; Pezzuto, J.M. Antioxidant Marine Products in Cancer Chemoprevention. Antioxid. Redox Signal. 2013, 19, 115–140. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J. Seaweeds in Human Health. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 319–367. [Google Scholar]
- Paradis, M.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 423–441. [Google Scholar]
- Wang, H.D.; Chen, C.; Huynh, P.; Chang, J. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Recontstruction Asthetic Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Pallela, R.; Na-young, Y.; Kim, S. Anti-photoaging and Photoprotective Compounds Derived from Marine Organism. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Fishery and Aquaculture Statistics; FAO: Quebec City, QC, Canada, 2016; pp. 1–108. [Google Scholar]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency (IEA) Bioenergy. Biorefineries: Adding Value to the Sustainable Utilization of Biomass; International Energy Agency (IEA) Bioenergy: Paris, France, 2009; pp. 1–16. [Google Scholar]
- Laurens, L.M.; McMillan, J.D.; Baxter, D.; Cowie, A.L.; Saddler, J.; Barbosa, M.; Murphy, J.; Drosg, B.; Elliot, D.C.; Sandquist, J.; et al. State of Technology Review—Algae Bioenergy: An IEA Bioenergy Inter-Task Strategic Project; International Energy Agency (IEA) Bioenergy: Paris, France, 2017; pp. 1–158. [Google Scholar]
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysi; Pacific Northwest National Lab.: Richland, WA, USA, 2010; pp. 1–50. [Google Scholar]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection Against Pathogens in Plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Bikker, P.; Van Krimpen, M.M.; Van Wikselaar, P.; Houweling-tan, B.; Scaccia, N.; Van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; Lopez-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Collet, P.; Lardon, L.; Hélias, A.; Steyer, J.; Bernard, O. Life-cycle assessment of microalgal-based biofuel. In Biomass, Biofuels and Biochemicals; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 507–550. [Google Scholar]
- Urban, R.A.; Bakshi, B.R. 1, 3-Propanediol from Fossils versus Biomass: A Life Cycle Evaluation of Emissions and Ecological Resources. Ind. Eng. Chem. Resour. 2009, 48, 8068–8082. [Google Scholar] [CrossRef]
- Gnansounou, E.; Raman, J.K. Life cycle assessment of algae biodiesel and its co-products. Appl. Energy. 2016, 161, 300–308. [Google Scholar] [CrossRef]
- Corona, A.; Biddy, M.J.; Vardon, D.R.; Birkved, M.; Houschild, M.; Beckkam, T. Life Cycle Assessment of Adipic Acid Production from Lignin. Green Chem. 2018, 20, 3857–3866. [Google Scholar] [CrossRef]
- Montazeri, M.; Eckelman, M.J. Life cycle assessment of UV-Curable bio-based wood flooring coatings. J. Clean. Prod. 2018, 192, 932–939. [Google Scholar] [CrossRef]
- Smidt, M.; Hollander, J.D.; Bosch, H.; Xiang, Y.; Van der Graaf, M.; Lambin, A.; Duda, J.-P. Life Cycle Assessment of Biobased and Fossil Based Succinic Acid. In Sustainability Assessment of Renewables-Based Products: Methods and Case Studies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 307–321. [Google Scholar]
- Brentner, L.B.; Eckelman, M.J.; Zimmerman, J.B. Combinatorial Life Cycle Assessment to Inform Process Design of Industrial Production of Algal Biodiesel. Environ. Sci. Technol. 2011, 45, 7060–7067. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.K.; Gnansounou, E. Life Cycle Assessment of Vetiver- Based Biorefinery With Production of Bioethanol and Furfural. In Life-Cycle Assessment of Biorefineries; Elsevier B.V.: Amsterdam, The Netherlands, 2017; pp. 147–165. [Google Scholar]
- Pilicka, I.; Blumberga, D.; Romagnoli, F. Life Cycle Assessment of Biogas Production from Marine Macroalgae: A Latvian Scenario. Sci. J. Riga Tech. Univ. 2011, 6, 69–78. [Google Scholar] [CrossRef][Green Version]
- Alvarado-morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef]
- Ertem, F.C.; Neubauer, P.; Junne, S. Environmental life cycle assessment of biogas production from marine macroalgal feedstock for the substitution of energy crops. J. Clean. Prod. 2017, 140, 977–985. [Google Scholar] [CrossRef]
- Seghetta, M.; Hou, X.; Bastianoni, S.; Bjerre, A.; Thomsen, M. Life cycle assessment of macroalgal biore fi nery for the production of ethanol, proteins and fertilizers e A step towards a regenerative bioeconomy. J. Clean. Prod. 2016, 137, 1158–1169. [Google Scholar] [CrossRef]
- Cappelli, A.; Gigli, E.; Romagnoli, F.; Simoni, S.; Palerno, M.; Guerriero, E. Co-digestion of macroalgae for biogas production: An LCA-based environmental evaluation. Energy Procedia 2015, 72, 3–10. [Google Scholar] [CrossRef]
- Czyrnek-delêtre, M.M.; Rocca, S.; Agostini, A.; Giuntoli, J.; Murphy, J.D. Life cycle assessment of seaweed biomethane, generated from seaweed sourced from integrated multi-trophic aquaculture in temperate oceanic climates. Appl. Energy 2017, 196, 34–50. [Google Scholar] [CrossRef]
- Giwa, A. Comparative cradle-to-grave life cycle assessment of biogas production from marine algae and cattle manure biorefineries. Bioresour. Technol. 2017, 244, 1470–1479. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Abeln, F.; Mcmanus, M.; Chuck, C.J. Sustainability and life cycle assessment (LCA) of macroalgae-derived single cell oils. J. Clean. Prod. 2019, 232, 1272–1281. [Google Scholar] [CrossRef]
- Pérez-lópez, P.; Balboa, E.M.; González-garcía, S.; Domínguez, H.; Feijoo, G.; Moreira, M.T. Comparative environmental assessment of valorization strategies of the invasive macroalga Sargassum muticum. Bioresour. Technol. 2014, 161, 137–148. [Google Scholar] [CrossRef]
- Plouviez, M.; Shilton, A.; Packer, M.A.; Guieysse, B. N2O emissions during microalgae outdoor cultivation in 50 L column photobioreactors. Algal Res. 2017, 26, 348–353. [Google Scholar] [CrossRef]
- Fagerstone, K.D.; Quinn, J.C.; Bradley, T.H.; De Long, S.K.; Marchese, A.J. Quantitative Measurement of Direct Nitrous Oxide Emissions from Microalgae Cultivation. Environ. Sci. Technol. 2011, 45, 9449–9456. [Google Scholar] [CrossRef]
- Soleymani, M.; Rosentrater, K.A. Techno-Economic Analysis of Biofuel Production from Macroalgae (Seaweed). Bioengineering 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).