1. Introduction
Poultry farming is one of the fastest livestock sub-sector that has rapidly grown in recent decades globally. The fast-growing poultry sector continues to attract formation of small-scale farmer cooperatives across the world, employing millions of people directly in the production and marketing, and indirectly through linkages with input suppliers (day-old-chicks, feeds, and veterinary services) [
1]. Due to the rapid growth in the poultry sector, poultry farming has become a much attractive agribusiness to resource poor communities because of they require low starting capital, space and maintenance costs [
1]. This demand in turn is driven by rapid economic growth as a result of the alarmingly growing population that has created an increasing need to consume more animal proteins [
2]. The demand in developing countries for poultry products is projected to increase by 70% to feed 9.2 billion people worldwide by 2050 [
3], thus putting even more pressure on the need to produce more animal proteins such as poultry eggs and meat.
Isa Brown (IB) chicken is one of the most common commercial layers bred and supplied to enfranchised local companies for multiplication worldwide. In many countries, the current focus is to upscale improved hybrid layer birds to smallholder households [
2] because they are commercially highly productive and profitable. The IB chicken are considered an excellent bioconverters of compounded feeds to high quality eggs, consume a relatively low amount of feed and yet produce more eggs with better shell quality than other common layer breeds [
4]. Thus, the poultry farmers make very quick returns on investment because of the short generation time required before the hen lays eggs. Although, the fast-rising section continued to attract formation of many small-scale farmer co-operatives across different countries, the lack of high-quality feed has hampered most of the birds from being able to express their full genetic egg laying potential.
Good nutrition is key for efficient and profitable poultry production but the high price of poultry feeds, which represents over 70 percent of total cost of production, has hampered the sector from realizing its full potential [
5,
6]. This cost is mainly driven by the growing scarcity of feed protein ingredient resources like fishmeal (FM) and soybean [
7]. The prices of fish and soya bean have doubled globally during the last 5 years [
7], leaving farmers with very limited profit margins. Furthermore, FM in many countries have been reported to be adulterated, leading to lower quality being available in the market. Low crude protein (CP) values ranging from 40.3% to 55.1% DM have commonly been reported against the expected CP content of >65% DM of FM [
8,
9,
10]. Continued dependence on FM and SBM for poultry feed is not a sustainable option, especially for smallholder farmers, thus necessitating the search for sustainable alternatives such as insect-based larval proteins [
5,
11].
In recent years, the utilization of insect meal as high-quality ingredients in chicken, pig and fish diets has grown rapidly [
7,
12,
13]. Insects have high, good quality protein contents and can be mass produced with a low environmental footprint due to low generation of greenhouse gases [
14]. The use of insect meals in animal diets is economically more competitive compared to diets containing conventional protein ingredients in poultry diets [
7]. Morever, consumers have also been reported to accept products from livestocks reared on compounded feeds containing insect meal [
15].
Hermetia illucens L is commonly called black soldier fly (BSF), are potentially low-cost nutrient-rich alternative protein source, that is similar or superior in protein quality to FM and plant sources [
16,
17]. The processed larvae of this insect is rich in nutrients such as crude protein content (38.5–62.7%) with well-balanced amino acids profile, good quality fatty acids (14.0–39.2%) and micronutrients such as iron and zinc [
7,
11,
13,
17]. However, the nutritional status of these insects might vary depending on the species, developmental stage, and rearing substrates [
18,
19]. Several studies on the use of BSF larvae meal (BSFLM) in commercial feeds have largely focused on broiler [
7], pig [
13] and fish [
12]) rather than layers. In literature, few studies on chicken layers have largely focused from the point of egg laying both for non-defatted BSFLM [
20] or defatted BSFLM [
21,
22] based feeds. Also, chick, pullet, and layer birds, each has its own specific nutritional requirements, which must be considered when formulating their feed [
23]. The studies described illustrated differences in body weight only at the onset of egg production because it is a major factor influencing the efficiency of egg production [
20,
21,
22]. Although, laying hen are not raised for meat, the lack of information on the growth of the visceral organs could have a detrimental impact on egg productivity. For example, the weights of some
visceral organs have been shown to be affected by dietary treatment [
24]. Also, during feed restriction or change, the physical development of birds usually gives priority to the development of the internal organs, which are capable of recovering more quickly than other parts of the body [
25]. According to Obeng et al. [
26], changes of internal organs in growing birds could improve or hinder the utilization rate of energy, protein, amino acids, and other nutrients required to enhance their performance and resistance to many diseases. Globally, the effects of diets with varying inclusion levels of non-defatted BSFLM on IB chick and pullet (growers) are less well understood, though these developmental stages of the birds are considered to be highly susceptible to changes in dietary nutrients [
24]. Therefore, the aim of this study was to evaluate differences in performance, feed utilization efficiency, body weight composition (carcass parts and internal organ development) and potential returns of investment of layer IB chick and grower bird fed
ad libitum on diets with strategically inclusion levels of non-defatted BSFLM.
4. Discussion
Here, we present the first report globally on the effects of non-defatted BSF meal as a promising and sustainable alternative nutrient-rich protein additive in commercial IB layer chick and grower diets. Protein is an essential component of poultry diet, which accounts for over 70% of total production cost needed for nutrition and growth [
7]. However, this protein source is critical because it strongly impacts accessibility and ease of use of the essential amino acids [
54]. The composition of protein of BSFLM (47% DM) was higher compared to that documented by De Marco et al. [
40], Makkar et al. [
11] and Onsongo et al. [
7]. These variations in CP composition observed among studies can be largely attributed to the substrate (
brewers’
spent grain) used compared to that reported in previous studies [
55]. The methionine content of BSFLM was lower compared to that stated by Onsongo et al. [
7] (0.8%), though higher than that presented by Spranghers et al. [
56]. The content of lysine of BSFLM was comparable to that published by Onsongo et al. [
7]. Similarly, Newton et al. [
57] has demonstrated comparable levels of lysine and methionine in poultry diets. However, lysine and methionine content reported in BSFLM is within the recommended quantity (1.00 and 0.38 g/g, respectively), acceptable for chicken diets [
23,
58]. The considerable variation in the growth and carcass responses to the various diet types might be attributed to deficiency or imbalance of amino acids and other nutrients, which needs further future research exploration. This might be a particular shortcoming due to the method of diet type preparation. Therefore, future research activities should use more advanced methods to effectively formulate poultry diets based on standardized ileal tract digestible (SID) amino acids (AA) for poultry [
31]. Moreover, BSFLM has been shown to exhibit low and variable sulphur amino acids (Met and Cys) concentration and these amino acids are the least digestible [
32,
33].
Growth rate of chicks provided feeds with higher inclusion levels of BSFLM (50, 75 and 100%) was significantly affected. This can be attributed to reduced feed intake observed with the incremental levels of BSFLM as observed in the current study. Besides protein, BSFLM are also known to contain higher levels of chitin than FM (lack chitin), which has been shown to increase the fibre content of the diets [
11,
19]. Diets rich in fiber (chitin) are beneficial for feed application due the influence imposed on the mucosa lining of the intestine, in respective of the amount used [
59], but insoluble fractions such as fibre have also been reported to favor the resulting effects on the intestinal mucus barrier of chickens [
60]. It is common knowledge that the addition of fiber in ration in low levels poses a good effect but levels exceeding 30 g/kg have been shown to negatively impact voluntary uptake of feed and nutrient digestibility [
61,
62,
63], thus poor performance of birds. This explains why formulated poultry diets should not contain more than 3% crude fiber, especially for the younger birds (chicks) to allow for improved feed conversion [
64]. However, mixing fiber fractions in different feeds would be more effective if further research is undertaken particularly for the emerging commercial insect-based feeds.
The NDF content of the chick diets increased with incremental levels of BSFLM, which was associated with reduced feed intake and could potentially affect the growth of the birds due to higher substitution level of chitin, which has been well documented to lessen digestibility and micronutrient utilization [
65,
66]. This effect is especially pronounced for proteins due to poor digestibility of ADF bound nitrogen [
66]. The chicks require more protein than the later stages of growth [
23] and therefore are more affected when protein intake and digestibility are affected. Other studies have reported similar results when insect meals are included in poultry diets. For instance, Awoniyi et al. [
67] reported negative but insignificant body weight gain at 25% to 100% inclusion levels of insects (
Musca domestica and
Tenebrio molitor) in broiler chicken diet. The reduction of feed intake by birds has also been reported by Mohammed et al. [
68], following the incorporation of various levels of BSFLM in their diets.
The FCR values decreased with increase inclusion levels of BSFLM, though there was no significant difference for chicks and growers fed on the various diet types. Contrarily, Amao et al. [
68] reported that replacing fishmeal with BSF larvae meal turn to significant increase the FCR values of experimental hens. Chicks fed diets with lower inclusion levels of BSFLM (25%BSFLM +75%FM and 50%BSFLM + 50%FM) were observed to perform better compared to those fed diet with 100% BSFLM. Previous studies have fed broilers on diets supplemented with
Musca domestica [
69] and
Tenebrio molitor [
67,
70,
71] and demonstrated that the FCR values did not vary [
66,
70], which is consistent with the current findings. However, additional studies are warranted to establish the impact of BSFLM on long-term growth rate following the incorporation of higher levels of insect protein in animal feeds.
The incorporation of BSFLM in grower diets did not show any significant effect on the overall weight, ADG, ADFI and FCR of the birds subjected to the various feed regimes. Similarly, no negative dietary effects were observed when soybean meal in poultry feed is replaced by defatted BSFLM [
72]. Mwaniki et al. [
21] also observed an improved feed intake when birds were offered diets with increased insect meals compared to those provided 0 and 50% inclusion levels of the defatted BSFLM. Marono et al. [
20] also reported significant effect of inclusion of BSFLM in laying hens on laying percentage, feed intake, weight gain and egg characteristics. In livestock production including poultry, ADG plays a critical role in growth rate of birds [
73]. The effects of diet types did not significantly impact on the ADG of the growing birds, which implies there was adequate supply of nutrients to the birds provided by the various diet types. In this study, the growth rate of growing birds subjected to diets with BSFLM inclusion up to 2.5 and 5.0% (25 and 50% replacement of FM) was comparable [
74]. However, the discrepancy observed might be attributed to the different strain of birds used in the two experiments.
The FCR of the growers was similar when exposed to the various diet types. Our findings are consistent to that presented by Maurer et al. [
72], whereby the feed consumption and FCR of laying hen (Lohmann Leghorn) were unaffected by the experimental diets with BSFLM replacing soybean cake up to 100%. Contrarily, Mwaniki et al. [
21], revealed increased feed consumption by the Shaver White Leghorns provided diets with defatted BSFLM. According to Mwaniki et al. [
21] and Liu et al. [
75], high feed consumption by birds provided insect-based diet might be due to higher fiber content following increase inclusion levels of BSFLM. This can be attributed to the fact that fibres help to facilitate increased ceca fermentation in birds [
76], thus increased nutrients absorption [
77] and better growth. Bovera et al. [
78] reported, however, that increased intake of chitin through increased inclusion level of BSFLM played a significant probiotic role in enhancing the weight gain in birds.
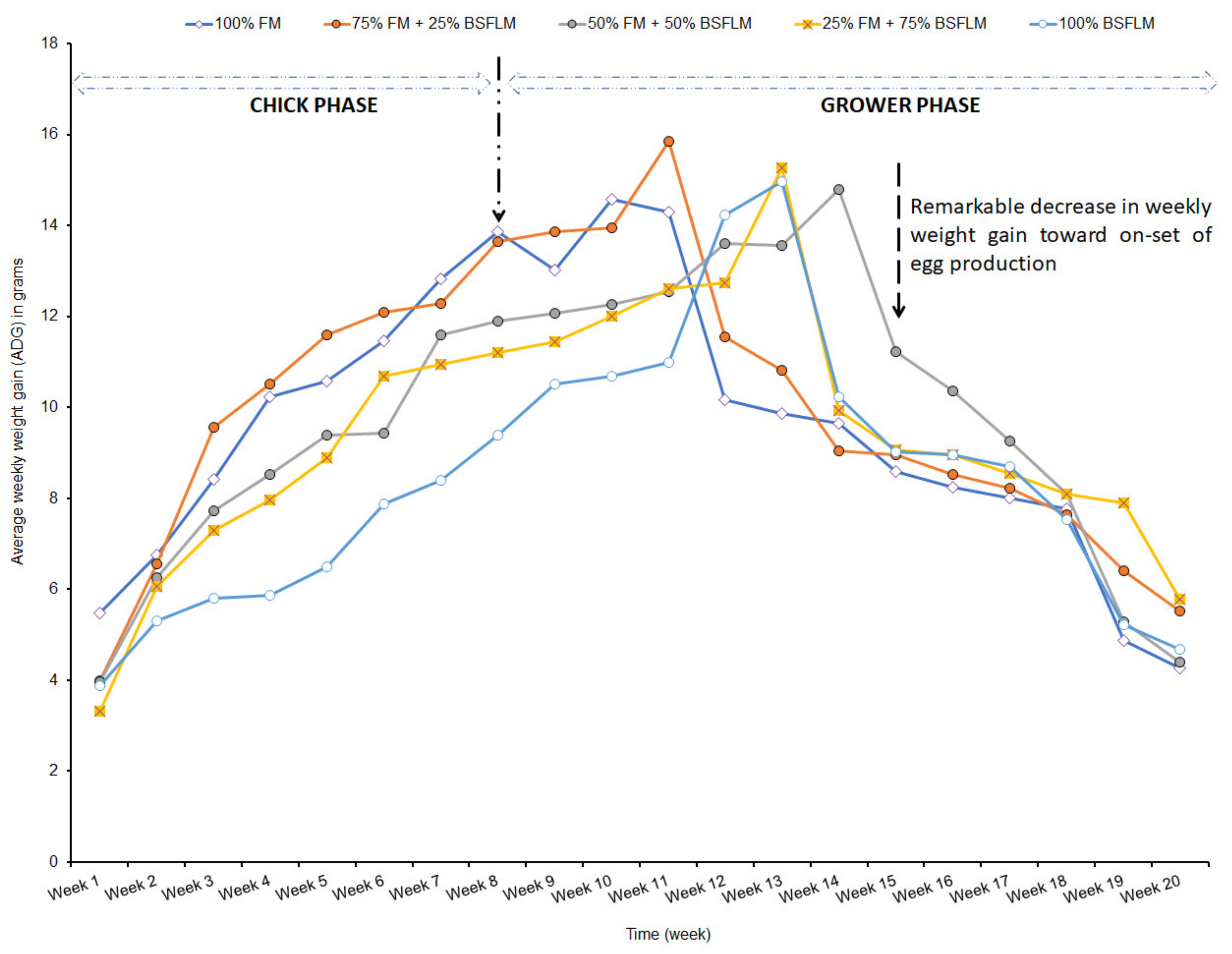
During the transition phase of the birds from the chicks to the growers (i.e., between week 10 and 12), there was a period of physical adjustment to the new feeds between week 10 and 12. Leeson and Summers [
79] and Mwaniki et al. [
21] revealed that birds are usually physically challenged before the onset of egg laying with great potential for negative nutrient balances to occur. This explains why young birds must be provided with adequate energy and nutrient-rich feeds for proper body build up before the onset of egg laying. This may be due to the growth of the digestive system of the grower birds, which is capable of handling more fibrous materials that might have originated from the increased inclusion of BSFLM. Mwaniki et al. [
21] emphasized that despite the weight gain at the end of the growing phase of the birds, it is imperative that the feed intake of the birds should be increased as the peak egg laying period approaches.
Generally, the weekly average weight gain of the birds subjected to the various feeding regimes declined consistently from week 15 as they approach the egg laying phase. This is a common trend observed in layer birds and have been shown to have associated benefits during the egg laying phase [
80]. Gordon et al. [
80] demonstrated the influence of body weight on egg size, revealing that lighter weight hens might turn to produce smaller eggs than heavier hens. However, we are also undertaken further research studies with the same clusters of grower hens to establish if weight gain observed at the grower phase would affect the dynamics of total egg mass production and egg size when the hens are subjected to feeding regimes with varying inclusion levels of BSFLM as well as associated profitability [
80,
81,
82,
83].
No significant dietary effect was observed on carcass traits (all internal organs, breast meat and drumsticks) of grower pullets except for the dressed carcass weight, thighs, and wings. The weight of body parts of the birds was generally higher for birds that were subjected to diets containing 25, 50 and 75% BSFLM inclusion ratio compared to the other diets. Several studies have reported an increase in dressed carcass weight of birds when fed on diets with BSFLM at moderate inclusion levels [
7,
66], while others have shown that dietary regime of birds with BSFLM does not significantly affect the size of the internal organs [
74,
84] and broiler chickens fed mealworm [
85].
Our findings have proven that the price of feeds for the chicks and grower pullets gradually reduces following increment levels of BSFLM substituting FM. Chick and grower diets with 100% BSFLM inclusion was much cheaper compared to diets with 100% FM. The results also showed that 100% FM diet had the least gross profit margin, CBR and RoI compared to the other diet types thus making BSFLM feed more affordable than the conventional FM feed, that heavily depends on fish importation and overfishing in major waters [
86]. The low cost of BSFLM is reflected in the reduced cost of feed, increased profits, CBR and RoI compared to FM, which is coherent with the information provided by Khan et al. [
86] and Onsongo et al. [
7].