Abstract
This study aimed to assess the effect of mechanical pretreatment on bleached eucalyptus kraft pulp fibers and investigate the influence of reaction time and temperature on the properties and yield of nanocrystalline cellulose (NCC) and microcrystalline cellulose (MCC). Two types of pulps were hydrolyzed, pulp 1 (control, whole fibers) and pulp 2 (mechanically pretreated, disintegrated fibers). NCC and MCC particles were obtained by sulfuric acid hydrolysis (60% w/w) of eucalyptus pulps under different conditions of time (30–120 min) and temperature (45–55 °C). Physical treatment of kraft pulp facilitated acid hydrolysis, resulting in higher NCC yields compared with no pretreatment. The morphologic properties and crystallinity index (CI) of NCC and MCC were little affected by pulp pretreatment. NCC particles obtained from pulps 1 and 2 were needle-shaped, with mean diameters of 6 and 4 nm, mean lengths of 154 and 130 nm, and CI of 74.6 and 76.8%, respectively. MCC particles obtained from pulps 1 and 2 were rod-shaped, with mean diameters of 2.4 and 1.4 µm, mean lengths of 37 and 22 µm, and CI of 73.1 and 74.5%, respectively. Pulps 1 and 2 and their respective NCC and MCC derivatives had a cellulose I crystalline structure.
1. Introduction
Nanocrystalline cellulose (NCC) particles, also known as nanowhiskers, and microcrystalline cellulose (MCC) particles can be obtained by means of acidic hydrolysis of fibers. In this process, the amorphous regions of pulp are solubilized while small fragments with high crystallinity indices remain in the solid fraction [1,2,3]. When isolated, NCC and MCC differ in physicochemical properties from native cellulose. NCC exhibits low density, high degree of crystallinity, high stiffness [1,4], high modulus of elasticity [5], low thermal expansion coefficient [6], and high surface area for polymer and resin bonding [7,8,9,10]. Such properties make NCC promising for a wide range of applications with important commercial potential [11,12,13,14]. MCC can be obtained by specific acid hydrolysis processes or as a by-product of NCC. The properties of MCC are also superior to those of native cellulose [15,16], explaining its wide use in chemical and pharmaceutical industries [17,18,19].
Some reaction conditions for obtaining nanocrystals and microcrystals are well documented, such as acid concentration and type [20,21,22]. However, reaction time and temperature need to be further studied to determine optimal parameters according to the raw material used. Reaction time and temperature are known to influence NCC yield, crystallinity, and morphology [20,21,22,23,24].
Wood fibers are composed of microfibrils of cellulose, lignin, hemicellulose, and extractive contents as well as lignin in the middle lamella between fibers [25,26]. These compounds must be eliminated before subjecting the fibers to acid hydrolysis for NCC production [27,28,29]. Bleached eucalyptus cellulose pulp stands out as an excellent raw material for the production of NCC because of its high cellulose content and high availability in the market, especially in Brazil [30,31,32,33,34].
We highlight that it is not trivial to obtain cellulose nanocrystals in high yields and that optimal reaction conditions may differ according to fiber morphology and chemical composition. Furthermore, some pretreatments have been used to facilitate accessibility of the acid to substrate and, consequently, enhance pulp hydrolysis, including mechanical pretreatment [20,21] and chemical pretreatment for improved fiber swelling [35]. Pretreatments not only positively influence NCC yield but also alter crystallinity degree, surface charge, and dimensions.
This study aimed to evaluate the effect of mechanical pretreatment on bleached eucalypt kraft pulp fibers and investigate the influence of reaction time and temperature on NCC and MCC yield.
2. Materials and Methods
2.1. Materials
A bleached eucalyptus kraft dry lap pulp (Cenibra S.A, Belo Oriente, MG, Brazil) was used as raw material, reagent-grade sulfuric acid (Synth) was used for hydrolysis, and cellulose membrane tubes (43 mm × 27 mm × 40 cm, Sigma–Aldrich, St. Louis, MO, USA) were used for dialysis.
2.2. Preparation and Characterization of Kraft Pulps
The control consisted of disaggregated fibers (pulp 1). Briefly, pulp fibers were separated at 5% consistency using 5000 revolutions at 312 rpm, subsequently centrifuged to 40% absolutely dry, and again disaggregated at 40% absolutely dry. Then, samples were oven-dried at 65 °C for 48 h. For mechanical pretreatment, the pulp was disintegrated into sheets using a Wiley mill (American Lab/Star FT 50) and sieved at 20 mesh, affording pulp 2.
The characteristics of pulps 1 and 2 are described in Table 1. The following parameters were determined: crystallinity [36], brightness [37], total lignin content [38,39], degree of polymerization (DP), and carbohydrate content [40]. DP was estimated by its correlation with intrinsic viscosity (|ɳ|), as shown by the equation DP0.905 = 0.75 × |ɳ|, described by Inmergut, Shurtz, and Mark [41]. The cellulose and hemicellulose contents of eucalyptus kraft pulp (hardwood) were determined using the following considerations:
Cellulose content = glycan − mannan;
Hemicellulose content = xylan + galactan + mannan + arabinan.

Table 1.
Characteristics of bleached kraft eucalyptus.
2.3. Acid Hydrolysis
NCC production was performed by acid hydrolysis [20]. Two sets of acid hydrolysis experiments were carried out, one with disaggregated fibers (pulp 1) and the other with mechanically pretreated fibers (pulp 2), using a sulfuric acid solution (60% w/w) and an acid-to-pulp (oven-dry weight) ratio of 8:1 (v/w) for pulp 1 and 10:1 (v/w) for pulp 2.
The hydrolysis reaction was carried out in a 1 L Erlenmeyer flask in a thermostat-controlled water bath. Pulps were hydrolyzed at three different temperatures: 45, 50, and 55 °C. Reaction times ranged from 30 to 120 min. The pulp/acid suspension was manually mixed at 5 min intervals during hydrolysis.
Upon completion, the reaction was stopped by diluting the sulfuric acid solution 10 times with deionized water, followed by immersion in an ice bath. The sample was centrifuged at 4000 rpm for 5 min to separate the acid solution from the hydrolyzed pulp. Subsequently, the hydrolyzed pulp was washed with deionized water and subjected to a further centrifugation step; this process was repeated twice. The hydrolyzed washed pulp was dialyzed against deionized water using a cellulose membrane. After pH stabilization, the solution was centrifuged at 12,000 rpm for 10 min to separate the supernatant NCC suspension from the sedimented MCC.
2.4. Yield Determination and Particle Characterization
Yield was determined as the ratio of the final weight (g) to the initial weight (10 g). The final weight was determined by the concentration of final solutions and by weighing the absolutely dry particles in powder form, obtained by lyophilization or concentration of solutions. For lyophilization, NCC and MCC were stored in capped aluminum tubes, frozen at −80 °C for 24 h, and dehydrated in a LIOTOP/LP510 freeze dryer for 72 h. Subsequently, particles were processed in a pulverizing mill (Fritsch/Pulverisette 14).
Characterization of the morphology and crystallinity index of NCC and MCC was performed only for samples obtained using the best hydrolysis conditions, which were defined as those that resulted in the highest yield and the lowest mass loss for each pulp.
Morphological characterization of NCC was carried out with the material in solution (0.5 g L−1). Images were obtained using a transmission electron microscope (TEM) (Libra 120, Zeiss) at an acceleration of 80 kV. NCC particles were sonicated for 5 min at 16 kHz (Soni-Tech, Soni-Top 412), deposited onto 300-mesh copper grids coated with Formvar (0.5% in chloroform), and subsequently stained with 2% uranyl acetate to facilitate observation of crystal structures. The length and diameter of nanocrystals were measured using Image Pro-Plus 4.5 from 100 measurements taken after individual calibration of each image.
MCC samples were examined under a scanning electron microscope (SEM) (LEO, 1430 VP) at an acceleration of 20 kV and a working distance of 16–18 mm. Powdered microparticles were fixed onto metal supports using double-sided carbon tapes and sputter-coated with gold (Balzers, Balzers, Liechtenstein, SCD50). The length and diameter of microcrystals were measured using Image Pro-Plus 4.5 after individual calibration of each image.
Morphological analysis of NCC and MCC was also performed using atomic force microscopy (AFM). NCC/MCC samples were suspended by sonication and applied onto mica slides, which were then oven-dried at 100 °C and attached to an AFM disk. Topography images were recorded using a probe scanning microscope (Ntegra Prima, NT-MDT) and measured automatically using NT-MDT SPM software version 1.0.
The crystallinity index of NCC and MCC were determined in three replicates, with one reading for each hydrolysis condition. X-ray diffraction analysis (X’Pert PRO) was performed at room temperature using Ni filter and Co-kα radiation (λ = 1.78890 Å) at an angular variation of 4–50° (2θ), rate of 3° min−1, 40 kV, and 30 mA. Crystalline and amorphous peak intensities were calculated using Origin 8.0 by applying a Fourier-transform filter to smooth the data and reduce output noise by 15%. Crystallinity index was estimated from the relationship between the crystalline peaks of maximum intensity (I002) and the amorphous halo (Iam) [36].
2.5. Statistical Analysis
The yields of NCC and MCC were analyzed by descriptive statistics (mean and standard deviation) for all treatments, with three replicates. The results of morphological characterization (length and diameter) of NCC and MCC obtained under the best hydrolysis conditions were analyzed by descriptive statistics (mean, standard deviation, and class distribution), with 100 replicates. Crystallinity index data of NCC and MCC obtained under the best hydrolysis conditions were analyzed by descriptive statistics (mean and standard deviation), with three replicates.
3. Results and Discussions
3.1. NCC and MCC Yields
Yield measurements obtained by concentration of the final solutions were confirmed by weight differences between samples before and after lyophilization. Hydrolysis time and temperature influenced NCC and MCC yields from pulp 1 (Figure 1) and pulp 2 (Figure 2).
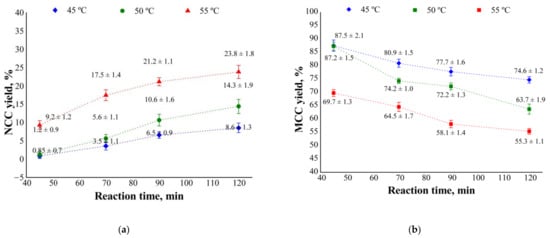
Figure 1.
Effect of hydrolysis time and temperature on the yield of (a) nanocrystalline (NCC) and (b) microcrystalline (MCC) cellulose from eucalyptus kraft pulps.
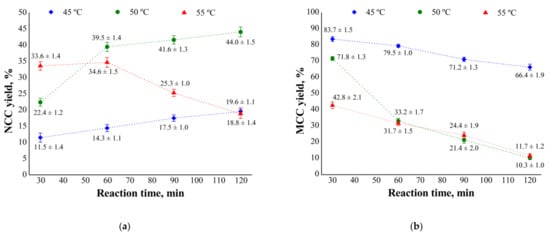
Figure 2.
Effect of hydrolysis time and temperature on the yield of (a) nanocrystalline (NCC) and (b) microcrystalline (MCC) cellulose from mechanically pretreated eucalyptus kraft pulps.
Hydrolysis of pulp 1 with 60% (w/w) sulfuric acid at 45 or 50 °C did not afford significant amounts of NCC, regardless of the reaction time; under these conditions, NCC yields were lower than 15% (Figure 1). Hydrolysis of pulp 1 for 90 and 120 min at 55 °C resulted in NCC yields of 21 and 24%, respectively, values that, although higher, are still unsatisfactory. Reports of NCC yields lower than 30% are common in the literature, under the most diverse hydrolysis conditions [12,19,25,26,42,43].
The low NCC yields obtained from pulp 1 can be explained by its preserved fiber morphology. This characteristic makes it difficult for the acid to penetrate into the fibers, especially from the primary wall to the cellular lumen, which contained large amounts of partially hydrolyzed cellulosic solid residue (MCC).
MCC yields greater than 63% were obtained by hydrolysis of pulp 1 at 45 or 50 °C. Yields increased to more than 75% under shorter reaction periods (45 and 70 min) (Figure 1). In the present study, MCC is a co-product obtained from NCC extraction, and the mass balance between NCC, MCC, and total mass was used to estimate the mass loss during pulp hydrolysis.
When analyzing total mass balance, we observed that mass loss increased as temperature and reaction time increased. Mass losses of about 15, 18, and 20% occurred at 45, 50, and 55 °C, respectively. Reaction times of 45, 70, 90, and 120 min resulted in mass losses of 15, 18, 19, and 20%, respectively. These mass loss values are higher than the total hemicellulose content of pulps (14%) and are greater than the percentage of amorphous regions (18.8%), which indicates that solubilization of crystalline cellulose regions occurred.
Hemicelluloses are low molecular weight polymers with amorphous structures that are easily solubilized [44]. Cellulose can be solubilized from amorphous regions [40], in which reaction rates are about 30 times higher than those in crystalline regions [45,46]. This leads to chain fractionation, especially under severe conditions, such as prolonged reaction times and high temperatures.
Mechanical pretreatment of pulp, that is, disintegration of fibers before hydrolysis, improved the access of acid to fibers, thereby increasing NCC yields compared with no pretreatment. NCC yield from pulp 2 varied with reaction time and temperature (Figure 2).
Pulp 2 had increased fiber exposure, reduced fiber size, and exposed cellular lumen resulting from external fibrillation, likely contributing to the higher NCC yield. Such factors increased the impregnation of acid onto the cell wall and doubled the access path for the acid, toward the primary wall into the cell wall and vice versa. The greater access of acid to the cell wall might have resulted in a more homogeneous hydrolysis of disintegrated fiber.
The highest NCC yields from pulp 2 (39, 42, and 44%) were obtained by hydrolysis at 50 °C for 60, 90, and 120 min, respectively. At 45 °C and 120 min of reaction, the maximum yield was 20%, and yields decreased with shorter reaction times. At 55 °C, the maximum yield (35%) was obtained in 60 min of reaction. In 90 and 120 min of reaction, yields were lower than 35%.
Similar yields were reported in previous studies that subjected bleached eucalyptus kraft pulp to sulfuric acid hydrolysis (using the same concentration as that applied here) at 45 or 55 °C for 102 min [13,21]. Acid concentration is a critical factor influencing NCC yield and morphology. Some studies have shown that concentrations close to 60% (w/w) afford higher yields [47]. Other studies used different sulfuric acid concentrations for the hydrolysis of disintegrated eucalyptus pulp fibers and obtained NCC yields of 70% [47,48]. The referred studies conducted kinetic analyses to optimize NCC yield [47,48].
A large amount of pulp was solubilized during hydrolysis at 50 or 55 °C for 60 min. A mass loss of more than 37% occurred during hydrolysis at 50 °C for 90 min, reaching 46% in 120 min of reaction. At 55 °C, mass losses were even higher, with a mean of 37% in 60 min of reaction, 50% in 90 min, and 70% in 120 min. These findings indicate that hemicelluloses, amorphous cellulose regions, and most regions of crystalline cellulose were solubilized under severe time–temperature conditions. The best hydrolysis conditions for pulp 1 were 55 °C and 120 min of reaction and those for pulp 2, 50 °C and 120 min of reaction.
Degradation of hemicelluloses and parts of cellulose in hydrolyzed pulps can be observed by changes in color. Pulps subjected to hydrolysis at 50 °C for more than 60 min acquired a greenish-brown color (Figure 3a,b).

Figure 3.
Visual appearance of eucalyptus kraft pulps hydrolyzed at 50 °C for (a) 60 min or (b) 90 min and nanocrystalline cellulose obtained from pulps hydrolyzed at 50 °C for (c) 30, (d) 60, (e) 90, or (f) 120 min.
Color intensity increased with reaction time. When hydrolysis was carried out at 55 °C, pulps had an altered color after 40 min of reaction. The dark color of hydrolyzed pulps resulted in dark NCC and MCC powders (Figure 3e,f) as a consequence of chromophore formation during hydrolysis. Degradation of hemicellulose in concentrated acid medium gives rise to chromophoric compounds through conversion of pentoses to furfural and of hexoses to hydroxymethylfurfural [49]. Thus, it is recommended to bleach NCC and MCC with 4% (w/w) sodium hypochlorite solution after extraction [50].
3.2. NCC and MCC Morphology
TEM and AFM images of NCC samples showed needle-shaped particles of nanoscale dimensions (length and diameter) (Figure 4). Surface topography of NCC obtained from pulps 1 and 2 revealed rod-like shapes [51].
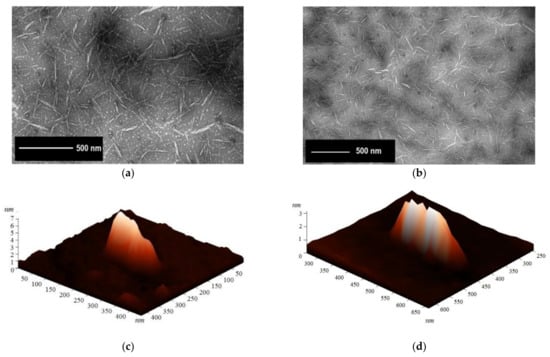
Figure 4.
Transmission electron microscopy images of nanocrystalline cellulose obtained from (a) disaggregated and (b) mechanically disintegrated eucalyptus kraft pulp. 3D atomic force microscopy images of the surface of NCC obtained from (c) disaggregated and (d) mechanically pretreated pulp.
NCC samples obtained from pulp 1 were 6 (±0.5) nm in diameter and 154 (±13) nm in length, as measured by TEM. These values were confirmed by AFM. NCC length ranged from 80–280 nm, and more than 60% of NCC particles were between 140–180 nm in length (Figure 5a). Regarding the diameter, NCC ranged from 1–10 nm, and 80% of NCC particles ranged from 4–8 nm (Figure 5b). Mechanical pretreatment of pulp 2 resulted in smaller NCC particles. Length ranged from 80–220 nm, with a mean of 130 (±12) nm. More than 50% of NCC particles were between 120–140 nm in length (Figure 5c). The mean diameter of NCC obtained from pulp 2 was 4 (±0.5) nm; NCC diameter ranged from 1–10 nm, and 73% of nanocrystals had diameters ranging from 3–7 nm (Figure 5d).
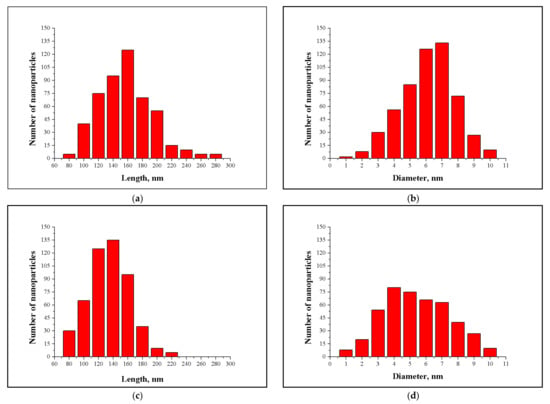
Figure 5.
Length and diameter distribution of nanocrystalline cellulose obtained from (a,b) desegregated and mechanically disintegrated (c,d) eucalyptus kraft pulp.
The mean length and diameter of NCC obtained from pulp 2 were in agreement with literature data. Previous research reported lengths and diameters of 147 (±7) nm and 4.8 (±0.4) nm, respectively [20], and 145 (±25) nm and 6 (±1.5) nm, respectively [52]. In one study, a higher mean diameter was recorded (17.3 nm) [21].
MCC particles were found to have micrometric dimensions. SEM images of microparticles obtained from pulps 1 and 2 show several morphologies, including cylindrical, oval, and irregular shapes. Elongated rod-shaped microparticles were the most frequent (Figure 6a). Many particles formed small aggregates (Figure 6b), probably as a result of lyophilization. Aggregation occurs because of the presence of hydroxyl groups on the particle surface [53]. Cellulose nanofibrils were produced individually and separated by very mild mechanical fibrillation of MCC [21,54].
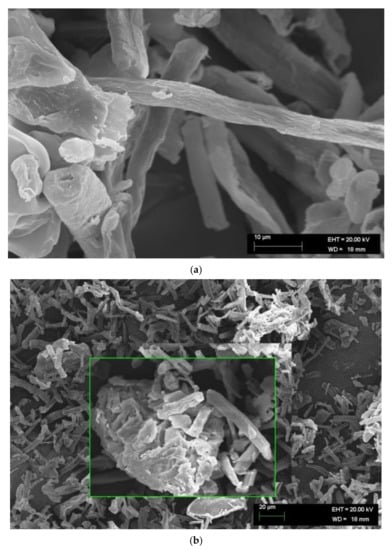
Figure 6.
Scanning electron microscopy images of cellulosic solid residues. (a) Rod-shaped cellulose microcrystals and (b) microparticle agglomerate.
MCC obtained from pulp 1 had a mean length of 37 (±2.2) µm, ranging from 1–55 µm, as measured by SEM. Most MCC particles (43%) were 35–40 µm in length (Figure 7a). The mean diameter was 2.4 (±0.3) µm. Some MCC particles had diameters in the nanoscale range, whereas others were up to 5 µm in diameter. More than 90% of MCC particles were 1–4 µm in diameter (Figure 7b).
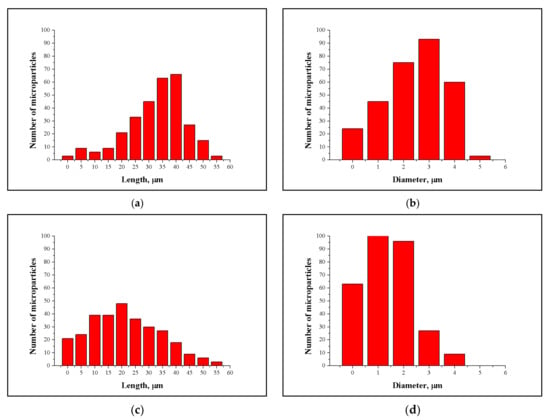
Figure 7.
Length and diameter distribution of cellulosic solid residues obtained from disaggregated (a,b) and mechanically disintegrated (c,d) eucalyptus kraft pulp.
MCC particles obtained from pulp 2 were smaller than those obtained from pulp 1, with a mean length of 22 (±1.2) µm and a mean diameter of 1.4 (±0.2) µm. More than 50% of MCC crystals were 10–30 µm in length, and 90% were 1–3 µm in diameter (Figure 7c,d).
The microscale dimensions of MCC fragments directly reflect the size of eucalyptus fiber cell walls, which are 5 µm thick and 1000 µm long [55]. Fiber length was reduced by 25–30 times and the diameter by half. Previous studies produced MCC with lengths greater than those observed here, as follows: 30–118 µm with a mean of 74 µm [56]; 240 µm [57]; and 50–170 µm [58].
3.3. Crystallinity Index of NCC and MCC
X-ray diffractograms of pulps 1 and 2 show typical patterns of semicrystalline materials (Figure 8), with amorphous regions and crystalline peaks. Mechanical pretreatment of pulp 2 reduced the intensity of the crystalline peak by 12% in relation to that of pulp 1 and was responsible for the 2.5% lower crystallinity index (79.2 vs. 81.2%, respectively). Partial degradation of cellulose as a result of fiber disintegration was evidenced by a 5% reduction in the DP of pulp 2 compared with that of pulp 1; cellulose and hemicellulose contents, however, were similar (Table 1).
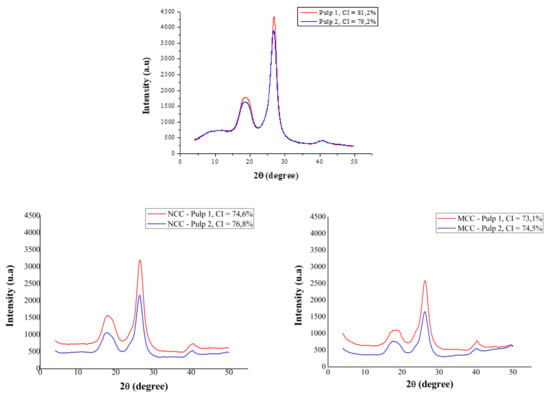
Figure 8.
X-ray diffractograms and crystallinity indices (CI) of disaggregated (pulp 1) and mechanically disintegrated (pulp 2) eucalyptus kraft pulp and nanocrystalline (NCC) and microcrystalline (MCC) cellulose obtained from pulps.
Figure 8 shows that the diffraction angle of amorphous halos (2θ = 22.3°) and crystalline peaks (2θ = 26.8°) were the same in pulps 1 and 2. X-ray diffractogram patterns are characteristic of cellulose I [59,60]. This result shows that fiber disintegration, despite causing a reduction in crystallinity, did not modify the cellulose I crystalline structure of pulps. Furthermore, the alkaline treatments used for bleaching, such as oxygen delignification and alkaline extraction, did not alter the crystalline structure of pulps.
Similar crystallinity indices and polymorphisms were reported by other studies evaluating bleached eucalyptus kraft pulp subjected to the same bleaching sequence used for reaction wood, DHT-EP-D-P [61]. On the other hand, some studies did not observe transformation of cellulose I into cellulose II in eucalyptus pulp [62].
Hydrolysis of pulps 1 and 2 affected the crystallinity index of NCC and MCC. NCC obtained from pulps 1 and 2 had X-ray patterns characteristic of cellulose I [59]. Cellulose I is less reactive and thermodynamically stable than cellulose II [60,63]. The mean crystallinity indices of NCC obtained from pulps 1 and 2 were 74.6 and 76.8%, respectively (Figure 8). These values represent a reduction of 8 and 3% in relation to the crystallinity indices of pulps 1 and 2, respectively.
MCC microparticles obtained from pulps 1 and 2 had X-ray patterns characteristic of cellulose I [59] and mean crystallinity indices of 73.1 and 74.5%, respectively (Figure 8). These values represent a reduction of 10 and 6% in relation to the crystallinity indices of the respective pulps. NCC particles obtained in the current study had similar cellulose I crystallinity indices to particles obtained in other studies (65–83%) [64].
The lower crystallinity indices of NCC and MCC compared with those of the pulps can be explained by the partial hydrolysis of amorphous regions and partial degradation of crystalline structures of NCC and MCC during hydrolysis [43]. The diffractograms of MCC obtained from pulp 1 showed a 26 and 30% lower crystalline peak compared with that of pulp 1, whereas the intensity of the amorphous halo was the same.
For MCC obtained from pulp 2, the intensity of the crystalline peak decreased by 45 and 57% and that of the amorphous halo by 38 and 47%. Reduction of MCC particles was also influenced by partial solubilization of amorphous and crystalline regions, evidenced by a mass loss of 46% after hydrolysis.
4. Conclusions
Physical pretreatment of kraft pulp facilitated acid hydrolysis, enhancing NCC yield compared with no pretreatment. Pretreatment, however, did not markedly influence NCC and MCC properties. NCC particles obtained from pulps 1 and 2 were needle-shaped, with mean diameters of 6 and 4 nm, mean lengths of 154 and 130 nm, and crystallinity indices of 74.6 and 74.5%, respectively. MCC samples obtained from pulps 1 and 2 were rod-shaped, with mean diameters of 2.4 and 1.4 µm, mean lengths of 37 and 22 µm, and crystallinity indices of 73.1 and 74.5%, respectively. Pulps 1 and 2 and their respective NCC and MCC derivatives exhibited a cellulose I crystalline structure.
Author Contributions
W.T.N.B. contributed to the experimental design, sample characterization, data analysis, and manuscript drafting. A.M.M.L.C. contributed to the experimental design, supervised the project, and revised the manuscript. D.N.S. contributed to the experimental design, sample characterization, and data analysis and revised the draft. A.d.C.O.C.; G.B.V. and F.J.B.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian agencies: CNPq grant numbers: 307979/2017-2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for the financial support, the Center for Microscopy and Microanalysis (NMM), and the Department of Forestry Engineering of the Federal University of Viçosa (UFV), Brazil, for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindström, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s Modulus of Bacterial and Microfibrillated Cellulose Fibrils in Fibrous Networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of Surface Energy on Dispersion and Mechanical Properties of Polymer/Nanocrystalline Cellulose Nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef]
- Diddens, I.; Murphy, B.; Krisch, M.; Muưller, M. Anisotropic Elastic Properties of Cellulose Measured Using Inelastic X-ray Scattering. Macromolecules 2008, 41, 9755–9759. [Google Scholar] [CrossRef]
- Jiang, W.; Gu, J. Nanocrystalline cellulose isolated from different renewable sources to fabricate natural rubber composites with outstanding mechanical properties. Cellulose 2020, 27, 5801–5813. [Google Scholar] [CrossRef]
- Brinkmann, A.; Chen, M.; Couillard, M.; Jakubek, Z.J.; Leng, T.; Johnston, L.J. Correlating Cellulose Nanocrystal Particle Size and Surface Area. Langmuir 2016, 32, 6105–6114. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162. [Google Scholar] [CrossRef]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Ghosh, T.; Mohanty, A.K.; Misra, M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 2009, 16, 783–793. [Google Scholar] [CrossRef]
- Ahmad, U.; Akhtar, J. MCC used in pharmaceutical industry. In Pharmaceutical Formulation Design-Recent Practices; Juber, A., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Xiu, H.; Ma, F.; Li, J.; Zhao, X.; Liu, L.; Feng, P.; Yang, X.; Zhang, X.; Kozliak, E.; Ji, Y. Using fractal dimension and shape factors to characterize the microcrystalline cellulose (MCC) particle morphology and powder flowability. Powder Technol. 2020, 364, 241–250. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose Nanocrystals for Health Care Applications. In Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–459. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Kusumattaqiin, F.; Chonkaew, W. Preparation and Characterization of Microcrystalline Cellulose (MCC) by Acid Hydrolysis Using Microwave Assisted Method from Cotton Wool. Macromol. Symp. 2015, 354, 35–41. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Melelli, A.; Arnould, O.; Beaugrand, J.; Bourmaud, A. The Middle Lamella of Plant Fibers Used as Composite Reinforcement: Investigation by Atomic Force Microscopy. Molecules 2020, 25, 632. [Google Scholar] [CrossRef]
- Hao, L.C.; Sapuan, S.M.; Hassan, M.R.; Sheltami, R.M. Natural fiber reinforced vinyl polymer composites. In Natural Fibre Reinforced Vinyl Ester and Vinyl Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–70. [Google Scholar]
- Magaton, A.S.; Oliveira, R.; Lopes, O.R.; Milagres, F.R.; Colodette, J.L.; Piló-Veloso, D. Chemical Composition of Wood of Eucalyptus Species. J. Wood Chem. Technol. 2006, 29, 43–58. [Google Scholar]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Colodette, J.L.; Gomes, F.J.B. Branqueamento de Polpa Celulosica: Da Produção da Polpa Marrom ao Produto Acabado; UFV: Abbotsford, BC, Canada, 2016. [Google Scholar]
- Siqueira, G.A.; Dias, I.K.R.; Arantes, V. Exploring the action of endoglucanases on bleached eucalyptus kraft pulp as potential catalyst for isolation of cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 133, 1249–1259. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Caixeta, L.A.; Pereira-da-Silva, M.A.; Mattoso, L.H.C. Cellulose micro/nanofibres from Eucalyptus kraft pulp: Preparation and properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef]
- Gullichsen, J.; Paulapuro, H. Forest Products Chemistry; Stenius, P., Ed.; Fapet Oy: Helsinki, Finland, 2000. [Google Scholar]
- Afsahi, G.; Isaza Ferro, E.; Ruuttunen, K.; Vuorinen, T. Optimized Catalytic Bleaching Sequences for Eucalyptus Kraft Pulp. J. Wood Chem. Technol. 2019, 39, 178–186. [Google Scholar] [CrossRef]
- Hashim, M.Y.; Roslan, M.N.; Amin, A.M.; Zaidi, A.M.A.; Ariffin, S. Mercerization treatment parameter effect on natural fiber reinforced polymer matrix composite: A brief review. World Acad. Sci. Eng. Technol. 2012, 68, 1638–1644. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- TAPPI. Diffuse Brightness of Paper, Paperboard and Pulp (d/0)—Ultraviolet Level C; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, November 2000. [Google Scholar]
- Goldschimid, O. Ultraviolet spectra. In Lignins; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley Interscience: New York, NY, USA, 1971; pp. 241–246. [Google Scholar]
- Gomide, J.L.; Demuner, B.J. Determinação do teor de lignina em material lenhoso: Método Klason modificado. O Pap. 1986, 47, 36–38. [Google Scholar]
- Wallis, A.F.A.; Wearne, R.H.; Wright, P.J. Chemical analysis of polysaccharides in plantation eucalypt woods and pulps. Appita J. 1996, 49, 258–262. [Google Scholar]
- SCAN Scandinavian Pulp, Paper and Board Testing Committee. Proceedings of the SCAN-Test Methods; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 2004. [Google Scholar]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Um, B.-H.; Bae, S.-H. Statistical methodology for optimizing the dilute acid hydrolysis of sugarcane bagasse. Korean J. Chem. Eng. 2011, 28, 1172–1176. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Zhu, J.Y. Kinetics of Strong Acid Hydrolysis of a Bleached Kraft Pulp for Producing Cellulose Nanocrystals (CNCs). Ind. Eng. Chem. Res. 2014, 53, 11007–11014. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Colodette, J.L.; Longue Júnior, D.; Gomes, F.J.B.; Martino, D.C. Preliminary Studies on Furfural Production from Lignocellulosics. J. Wood Chem. Technol. 2014, 34, 178–190. [Google Scholar] [CrossRef]
- Reiner, R.S.; Rudie, A.W. Preparation and Characterization: Process Scale-Up of Cellulose Nanocrystal Production to 25 kg per Batch at the Forest Prod-ucts Laboratory. In Production and Applications of Cellulose Nanomaterials; Michael, T., Postek, M.T., Moon, R.J., Rudie, A.W., Bilodeau, M.A.T., Eds.; Tappi Press: Peachtree Corners, GA, USA, 2013; p. 321. [Google Scholar]
- De Souza Lima, M.M.; Borsali, R. Rodlike Cellulose Microcrystals: Structure, Properties, and Applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased Nanocomposites from Layer-by-Layer Assembly of Cellulose Nanowhiskers with Chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Santos, F.A.; Tavares, M.I.B. Preraring Films from Poly(Lactid Acid) and Microcrystalline Cellulose and Characterization. Polímeros Ciência Tecnol. 2013, 229–235. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.Y.; Considine, J.M. Strong and Optically Transparent Films Prepared Using Cellulosic Solid Residue Recovered from Cellulose Nanocrystals Production Waste Stream. ACS Appl. Mater. Interfaces 2013, 5, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, W.T.N.; Paes, J.B.; Oliveira, J.T.D.S.; Dudecki, L. Características anatômicas para produção de celulose do lenho de reação de árvores inclinadas de eucalipto. Pesqui. Agropecuária Bras. 2015, 50, 459–467. [Google Scholar] [CrossRef][Green Version]
- Pasqualoto, K.F.; Funck, J.A.B.; Silva, F.E.B.; Kratz, C.D.P. Application of the probit spreadsheet as a simple statistical tool to evaluate the two microcrystalline cellulose types particle size distribution. Rev. Bras. Farmácia 2005, 86, 31–34. [Google Scholar]
- Rojas, J.; Lopez, A.; Guisao, S.; Ortiz, C. Evaluation of several microcrystalline celluloses obtained from agricultural by-products. J. Adv. Pharm. Technol. Res. 2011, 2, 144. [Google Scholar] [CrossRef]
- Tomar, M.; Singh, A.K.; Sinha, A.R. Physicochemical Parameter of Microcrystalline Cellulose and the Most Acceptability in Pharmaceutical Industries. J. Innov. Pharm. Biol. Sci. 2015, 2, 570–578. Available online: https://www.pharmaexcipients.com/wp-content/uploads/attachments/Physicochemical+parameter+of+Microcrystalline+Cellulose+and+most+acceptablility+in+pharmaceutical+Industries%5B1%5D.pdf?t=1483721846 (accessed on 1 March 2021).
- Zugenmaier, P. Crystalline Cellulose and Derivatives; Heidelberg, S.-V.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Muniz, G.I.M.; Nisgoski, S.; Magalhães, W.L.E. Cellulose acquirement evaluation methods with different degrees of crystallinity. Sci. For. 2013, 41, 185–194. [Google Scholar]
- Azevedo, M.A.B.; Pasa, V.m.d.; Colodette, J.L.; Fontes, M.P. The effects of various bleaching conditions on the crystalline structure of eucalyptus kraft pulp. In Proceedings of the XXII Encontro Regional da SBQ, Belo Horizonte, Brasil, 2008. [Google Scholar]
- Kroon-Batenburg, L.M.J.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison be-tween measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).