Effect of Mechanical Treatment of Eucalyptus Pulp on the Production of Nanocrystalline and Microcrystalline Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Kraft Pulps
2.3. Acid Hydrolysis
2.4. Yield Determination and Particle Characterization
2.5. Statistical Analysis
3. Results and Discussions
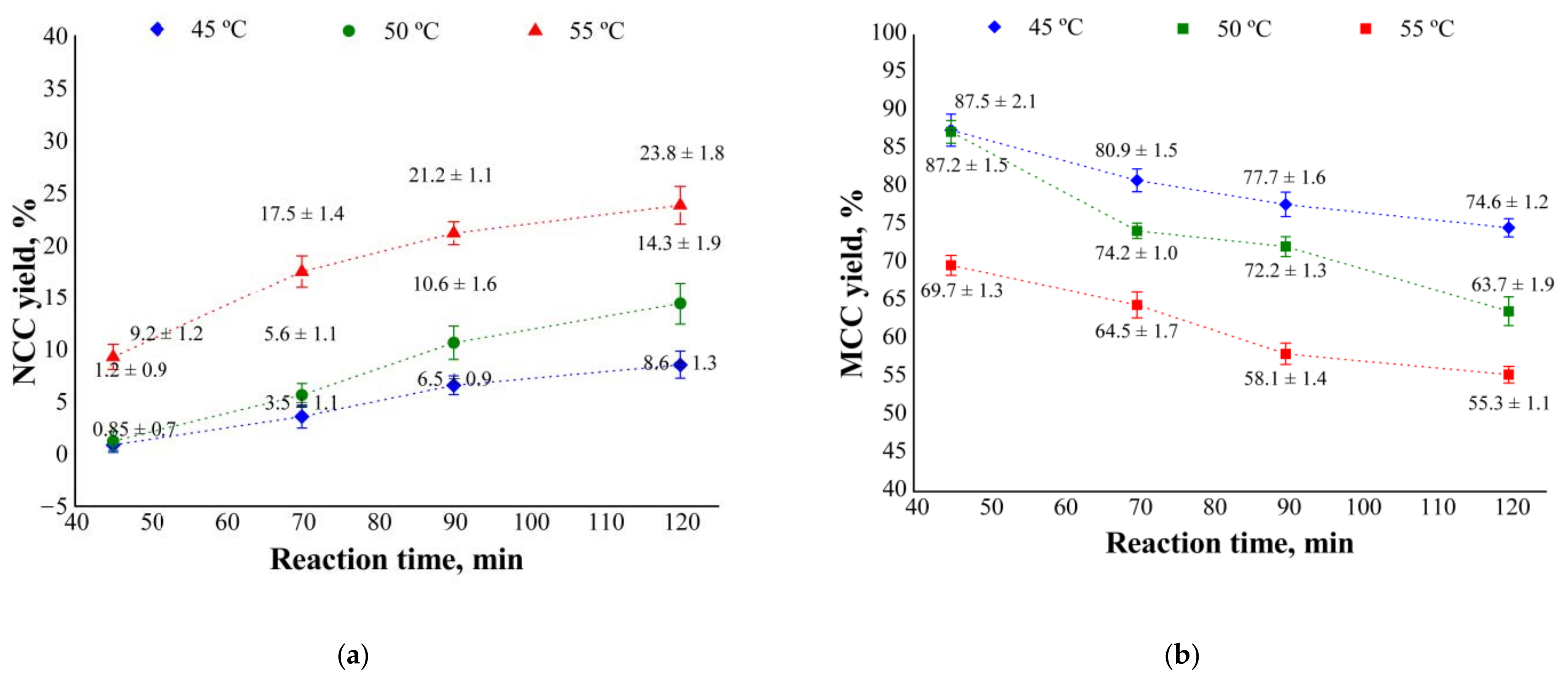
3.1. NCC and MCC Yields
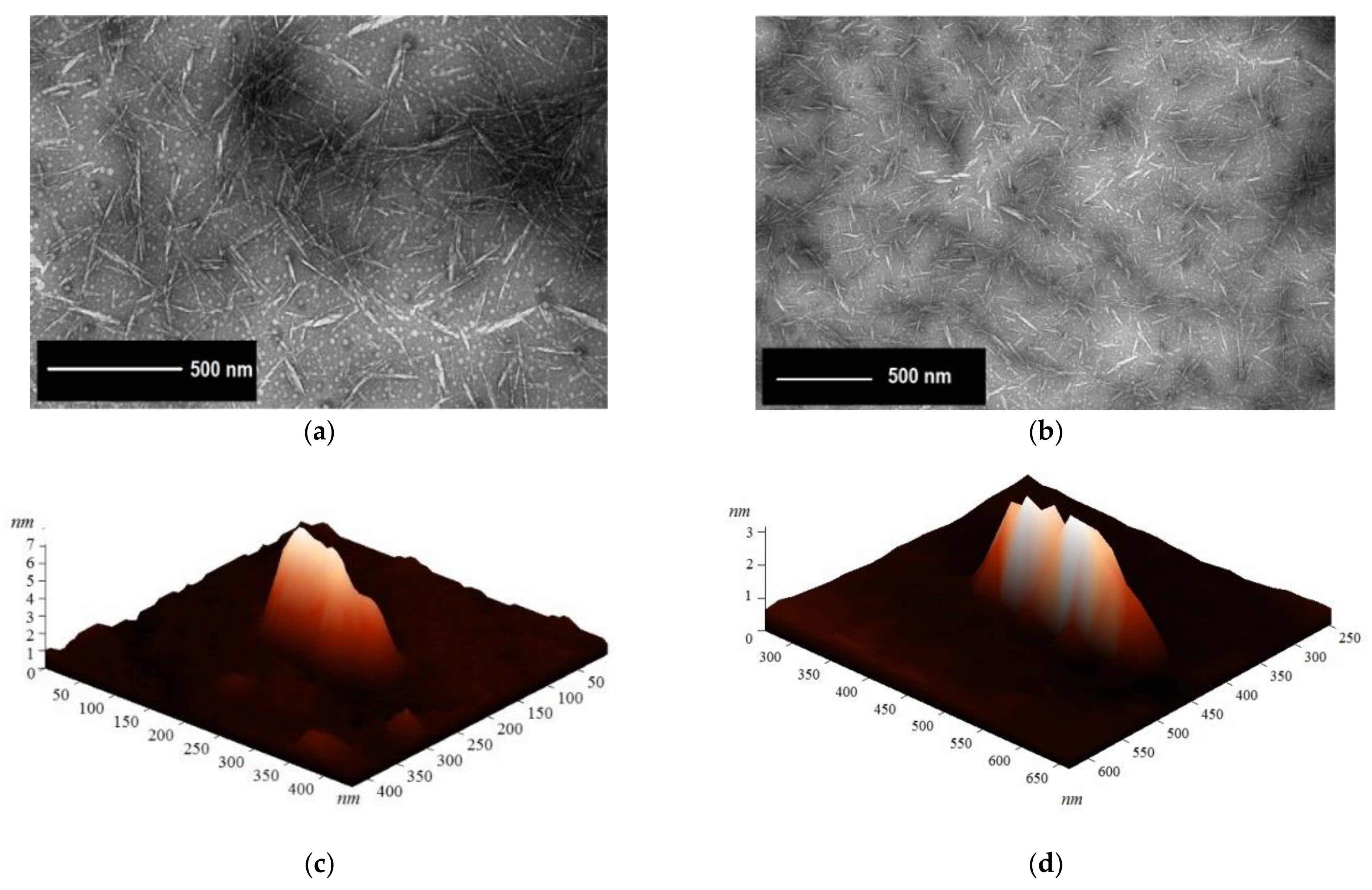
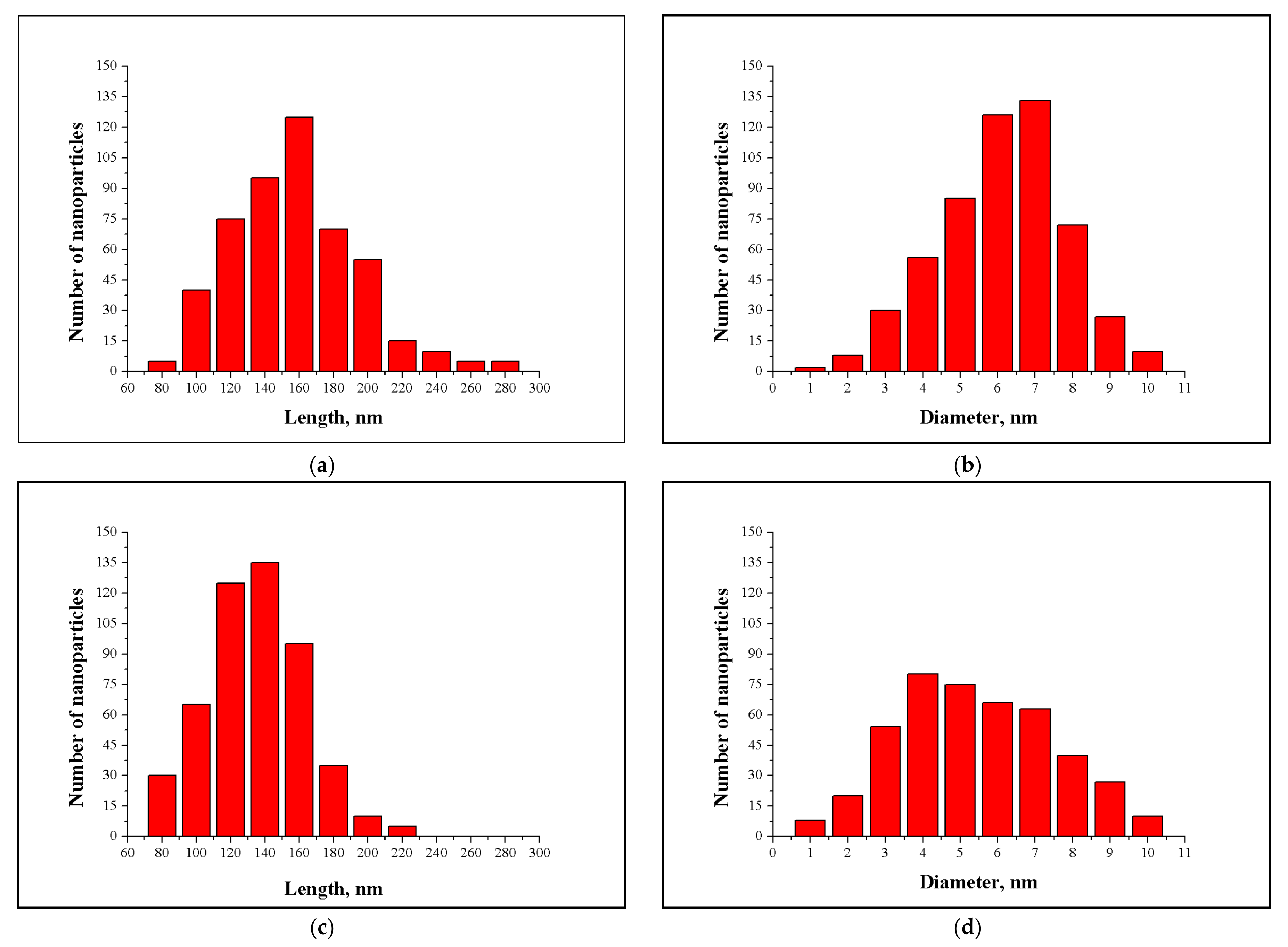
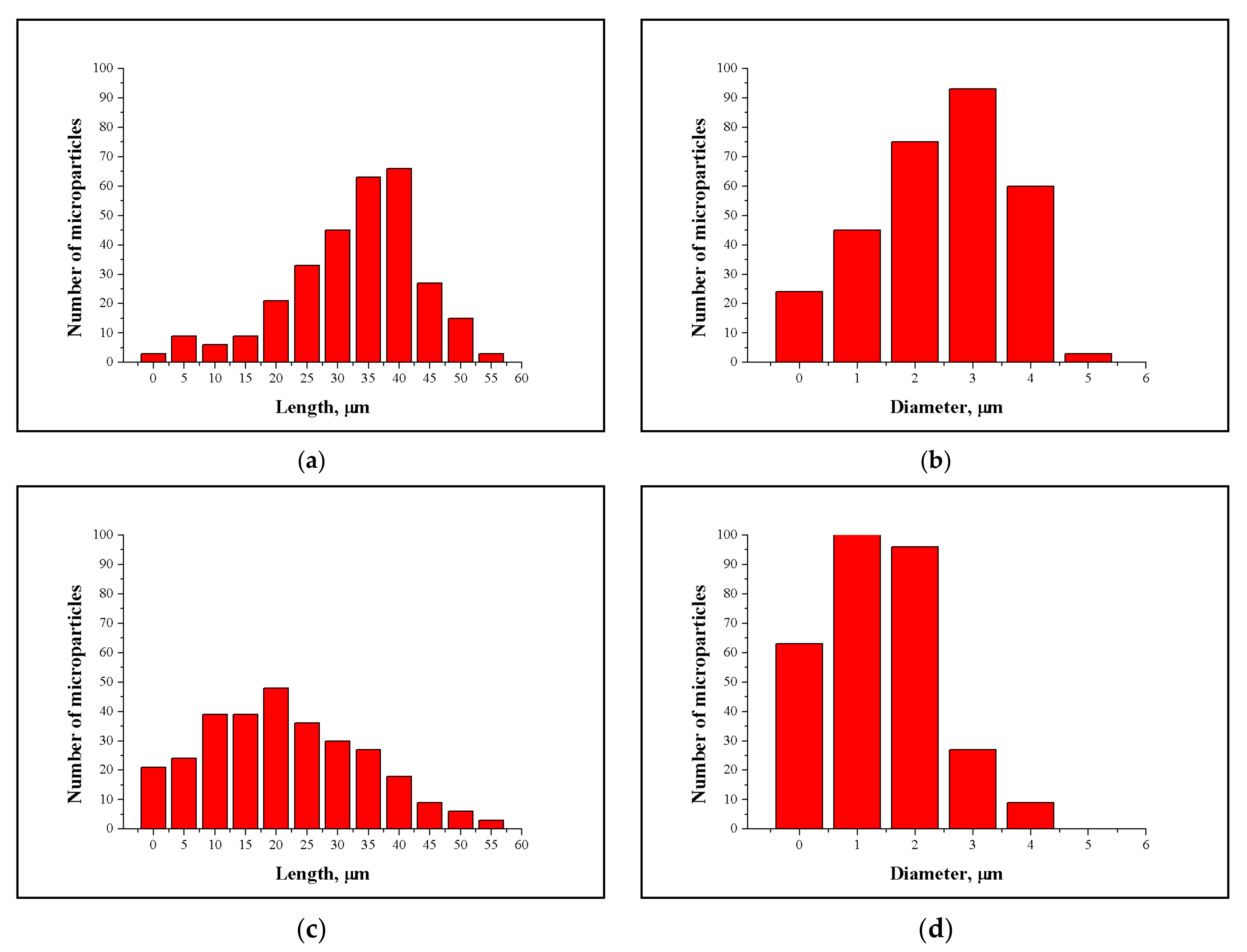
3.2. NCC and MCC Morphology
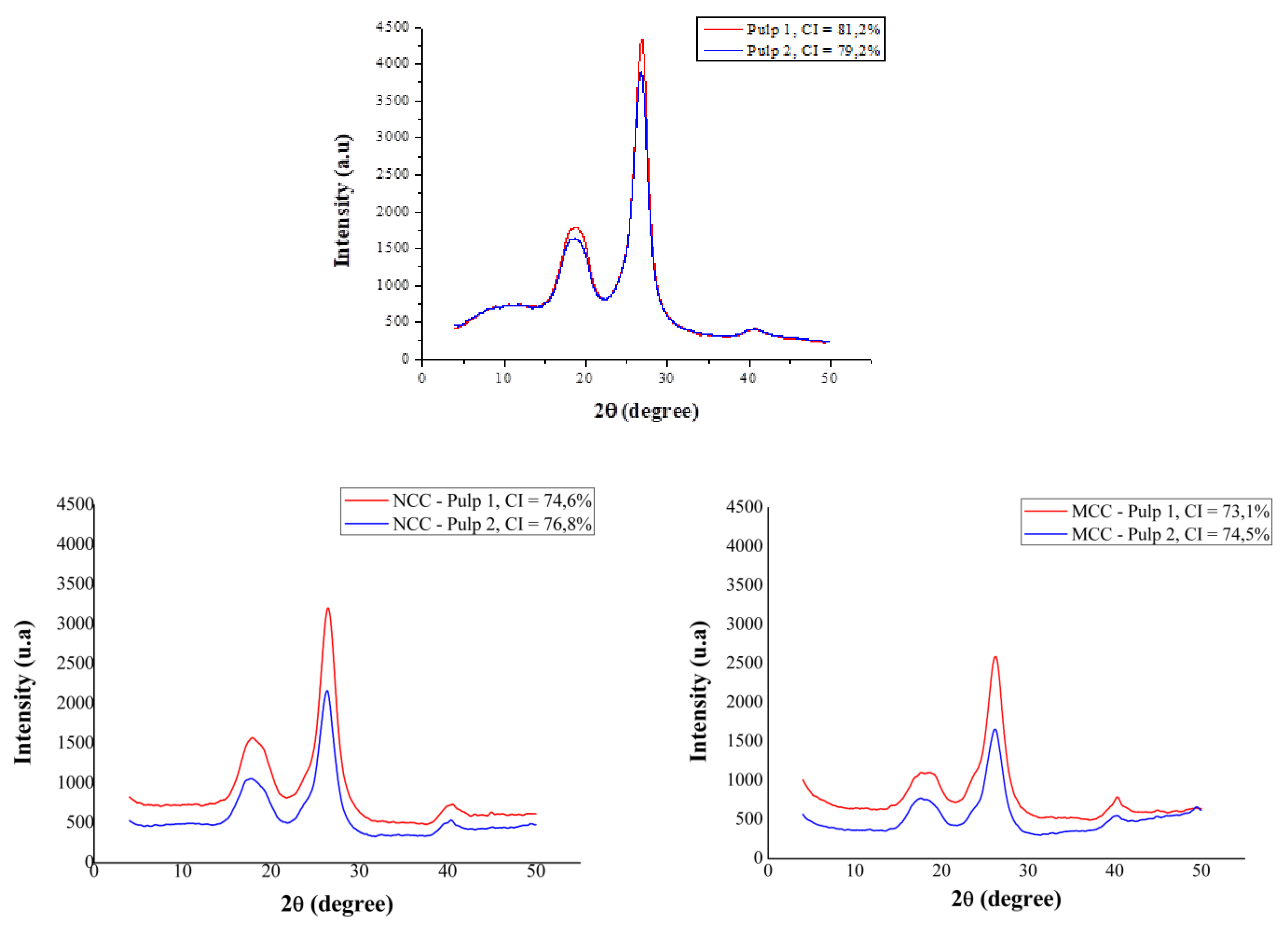
3.3. Crystallinity Index of NCC and MCC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindström, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s Modulus of Bacterial and Microfibrillated Cellulose Fibrils in Fibrous Networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of Surface Energy on Dispersion and Mechanical Properties of Polymer/Nanocrystalline Cellulose Nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef]
- Diddens, I.; Murphy, B.; Krisch, M.; Muưller, M. Anisotropic Elastic Properties of Cellulose Measured Using Inelastic X-ray Scattering. Macromolecules 2008, 41, 9755–9759. [Google Scholar] [CrossRef]
- Jiang, W.; Gu, J. Nanocrystalline cellulose isolated from different renewable sources to fabricate natural rubber composites with outstanding mechanical properties. Cellulose 2020, 27, 5801–5813. [Google Scholar] [CrossRef]
- Brinkmann, A.; Chen, M.; Couillard, M.; Jakubek, Z.J.; Leng, T.; Johnston, L.J. Correlating Cellulose Nanocrystal Particle Size and Surface Area. Langmuir 2016, 32, 6105–6114. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Lim, K.-T. Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 2019, 9, 19143–19162. [Google Scholar] [CrossRef]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Ghosh, T.; Mohanty, A.K.; Misra, M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 2009, 16, 783–793. [Google Scholar] [CrossRef]
- Ahmad, U.; Akhtar, J. MCC used in pharmaceutical industry. In Pharmaceutical Formulation Design-Recent Practices; Juber, A., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Xiu, H.; Ma, F.; Li, J.; Zhao, X.; Liu, L.; Feng, P.; Yang, X.; Zhang, X.; Kozliak, E.; Ji, Y. Using fractal dimension and shape factors to characterize the microcrystalline cellulose (MCC) particle morphology and powder flowability. Powder Technol. 2020, 364, 241–250. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose Nanocrystals for Health Care Applications. In Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–459. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Kusumattaqiin, F.; Chonkaew, W. Preparation and Characterization of Microcrystalline Cellulose (MCC) by Acid Hydrolysis Using Microwave Assisted Method from Cotton Wool. Macromol. Symp. 2015, 354, 35–41. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Melelli, A.; Arnould, O.; Beaugrand, J.; Bourmaud, A. The Middle Lamella of Plant Fibers Used as Composite Reinforcement: Investigation by Atomic Force Microscopy. Molecules 2020, 25, 632. [Google Scholar] [CrossRef]
- Hao, L.C.; Sapuan, S.M.; Hassan, M.R.; Sheltami, R.M. Natural fiber reinforced vinyl polymer composites. In Natural Fibre Reinforced Vinyl Ester and Vinyl Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–70. [Google Scholar]
- Magaton, A.S.; Oliveira, R.; Lopes, O.R.; Milagres, F.R.; Colodette, J.L.; Piló-Veloso, D. Chemical Composition of Wood of Eucalyptus Species. J. Wood Chem. Technol. 2006, 29, 43–58. [Google Scholar]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Colodette, J.L.; Gomes, F.J.B. Branqueamento de Polpa Celulosica: Da Produção da Polpa Marrom ao Produto Acabado; UFV: Abbotsford, BC, Canada, 2016. [Google Scholar]
- Siqueira, G.A.; Dias, I.K.R.; Arantes, V. Exploring the action of endoglucanases on bleached eucalyptus kraft pulp as potential catalyst for isolation of cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 133, 1249–1259. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Caixeta, L.A.; Pereira-da-Silva, M.A.; Mattoso, L.H.C. Cellulose micro/nanofibres from Eucalyptus kraft pulp: Preparation and properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef]
- Gullichsen, J.; Paulapuro, H. Forest Products Chemistry; Stenius, P., Ed.; Fapet Oy: Helsinki, Finland, 2000. [Google Scholar]
- Afsahi, G.; Isaza Ferro, E.; Ruuttunen, K.; Vuorinen, T. Optimized Catalytic Bleaching Sequences for Eucalyptus Kraft Pulp. J. Wood Chem. Technol. 2019, 39, 178–186. [Google Scholar] [CrossRef]
- Hashim, M.Y.; Roslan, M.N.; Amin, A.M.; Zaidi, A.M.A.; Ariffin, S. Mercerization treatment parameter effect on natural fiber reinforced polymer matrix composite: A brief review. World Acad. Sci. Eng. Technol. 2012, 68, 1638–1644. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- TAPPI. Diffuse Brightness of Paper, Paperboard and Pulp (d/0)—Ultraviolet Level C; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, November 2000. [Google Scholar]
- Goldschimid, O. Ultraviolet spectra. In Lignins; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley Interscience: New York, NY, USA, 1971; pp. 241–246. [Google Scholar]
- Gomide, J.L.; Demuner, B.J. Determinação do teor de lignina em material lenhoso: Método Klason modificado. O Pap. 1986, 47, 36–38. [Google Scholar]
- Wallis, A.F.A.; Wearne, R.H.; Wright, P.J. Chemical analysis of polysaccharides in plantation eucalypt woods and pulps. Appita J. 1996, 49, 258–262. [Google Scholar]
- SCAN Scandinavian Pulp, Paper and Board Testing Committee. Proceedings of the SCAN-Test Methods; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 2004. [Google Scholar]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Um, B.-H.; Bae, S.-H. Statistical methodology for optimizing the dilute acid hydrolysis of sugarcane bagasse. Korean J. Chem. Eng. 2011, 28, 1172–1176. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Zhu, J.Y. Kinetics of Strong Acid Hydrolysis of a Bleached Kraft Pulp for Producing Cellulose Nanocrystals (CNCs). Ind. Eng. Chem. Res. 2014, 53, 11007–11014. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Colodette, J.L.; Longue Júnior, D.; Gomes, F.J.B.; Martino, D.C. Preliminary Studies on Furfural Production from Lignocellulosics. J. Wood Chem. Technol. 2014, 34, 178–190. [Google Scholar] [CrossRef]
- Reiner, R.S.; Rudie, A.W. Preparation and Characterization: Process Scale-Up of Cellulose Nanocrystal Production to 25 kg per Batch at the Forest Prod-ucts Laboratory. In Production and Applications of Cellulose Nanomaterials; Michael, T., Postek, M.T., Moon, R.J., Rudie, A.W., Bilodeau, M.A.T., Eds.; Tappi Press: Peachtree Corners, GA, USA, 2013; p. 321. [Google Scholar]
- De Souza Lima, M.M.; Borsali, R. Rodlike Cellulose Microcrystals: Structure, Properties, and Applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased Nanocomposites from Layer-by-Layer Assembly of Cellulose Nanowhiskers with Chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Santos, F.A.; Tavares, M.I.B. Preraring Films from Poly(Lactid Acid) and Microcrystalline Cellulose and Characterization. Polímeros Ciência Tecnol. 2013, 229–235. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.Y.; Considine, J.M. Strong and Optically Transparent Films Prepared Using Cellulosic Solid Residue Recovered from Cellulose Nanocrystals Production Waste Stream. ACS Appl. Mater. Interfaces 2013, 5, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, W.T.N.; Paes, J.B.; Oliveira, J.T.D.S.; Dudecki, L. Características anatômicas para produção de celulose do lenho de reação de árvores inclinadas de eucalipto. Pesqui. Agropecuária Bras. 2015, 50, 459–467. [Google Scholar] [CrossRef][Green Version]
- Pasqualoto, K.F.; Funck, J.A.B.; Silva, F.E.B.; Kratz, C.D.P. Application of the probit spreadsheet as a simple statistical tool to evaluate the two microcrystalline cellulose types particle size distribution. Rev. Bras. Farmácia 2005, 86, 31–34. [Google Scholar]
- Rojas, J.; Lopez, A.; Guisao, S.; Ortiz, C. Evaluation of several microcrystalline celluloses obtained from agricultural by-products. J. Adv. Pharm. Technol. Res. 2011, 2, 144. [Google Scholar] [CrossRef]
- Tomar, M.; Singh, A.K.; Sinha, A.R. Physicochemical Parameter of Microcrystalline Cellulose and the Most Acceptability in Pharmaceutical Industries. J. Innov. Pharm. Biol. Sci. 2015, 2, 570–578. Available online: https://www.pharmaexcipients.com/wp-content/uploads/attachments/Physicochemical+parameter+of+Microcrystalline+Cellulose+and+most+acceptablility+in+pharmaceutical+Industries%5B1%5D.pdf?t=1483721846 (accessed on 1 March 2021).
- Zugenmaier, P. Crystalline Cellulose and Derivatives; Heidelberg, S.-V.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Muniz, G.I.M.; Nisgoski, S.; Magalhães, W.L.E. Cellulose acquirement evaluation methods with different degrees of crystallinity. Sci. For. 2013, 41, 185–194. [Google Scholar]
- Azevedo, M.A.B.; Pasa, V.m.d.; Colodette, J.L.; Fontes, M.P. The effects of various bleaching conditions on the crystalline structure of eucalyptus kraft pulp. In Proceedings of the XXII Encontro Regional da SBQ, Belo Horizonte, Brasil, 2008. [Google Scholar]
- Kroon-Batenburg, L.M.J.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison be-tween measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]



| Parameter | Pulp 1 | Pulp 2 |
|---|---|---|
| Cellulose, (%) | 82.2 | 82.1 |
| Hemicellulose, (%) | 14.0 | 14.1 |
| Total lignin, (%) | 0.1 | 0.1 |
| Viscosity, cm3 g−1 | 630 | 599 |
| Degree of polymerization | 901 | 853 |
| Brightness, (%) ISO | 86.9 | 87.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetti, W.T.N.; Carvalho, A.M.M.L.; Carneiro, A.d.C.O.; Vidaurre, G.B.; Gomes, F.J.B.; Soratto, D.N. Effect of Mechanical Treatment of Eucalyptus Pulp on the Production of Nanocrystalline and Microcrystalline Cellulose. Sustainability 2021, 13, 5888. https://doi.org/10.3390/su13115888
Boschetti WTN, Carvalho AMML, Carneiro AdCO, Vidaurre GB, Gomes FJB, Soratto DN. Effect of Mechanical Treatment of Eucalyptus Pulp on the Production of Nanocrystalline and Microcrystalline Cellulose. Sustainability. 2021; 13(11):5888. https://doi.org/10.3390/su13115888
Chicago/Turabian StyleBoschetti, Walter Torezani Neto, Ana Márcia Macedo Ladeira Carvalho, Angélica de Cássia Oliveira Carneiro, Graziela Baptista Vidaurre, Fernando José Borges Gomes, and Déborah Nava Soratto. 2021. "Effect of Mechanical Treatment of Eucalyptus Pulp on the Production of Nanocrystalline and Microcrystalline Cellulose" Sustainability 13, no. 11: 5888. https://doi.org/10.3390/su13115888
APA StyleBoschetti, W. T. N., Carvalho, A. M. M. L., Carneiro, A. d. C. O., Vidaurre, G. B., Gomes, F. J. B., & Soratto, D. N. (2021). Effect of Mechanical Treatment of Eucalyptus Pulp on the Production of Nanocrystalline and Microcrystalline Cellulose. Sustainability, 13(11), 5888. https://doi.org/10.3390/su13115888






