Clustering the Adsorbents of Horizontal Series Filtration in Greywater Treatment
Abstract
1. Introduction
2. Materials and Methods
Clustering Methodology
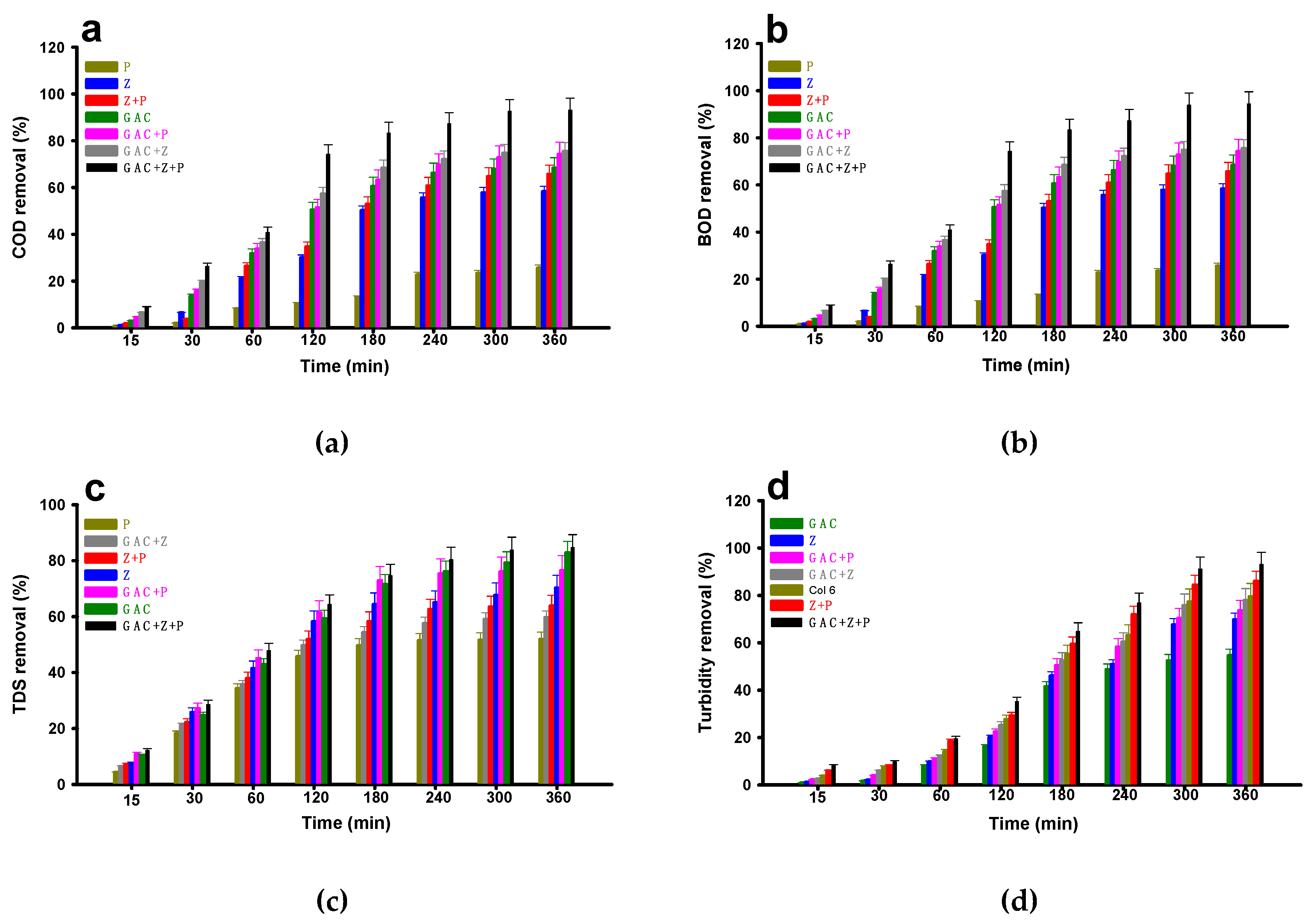
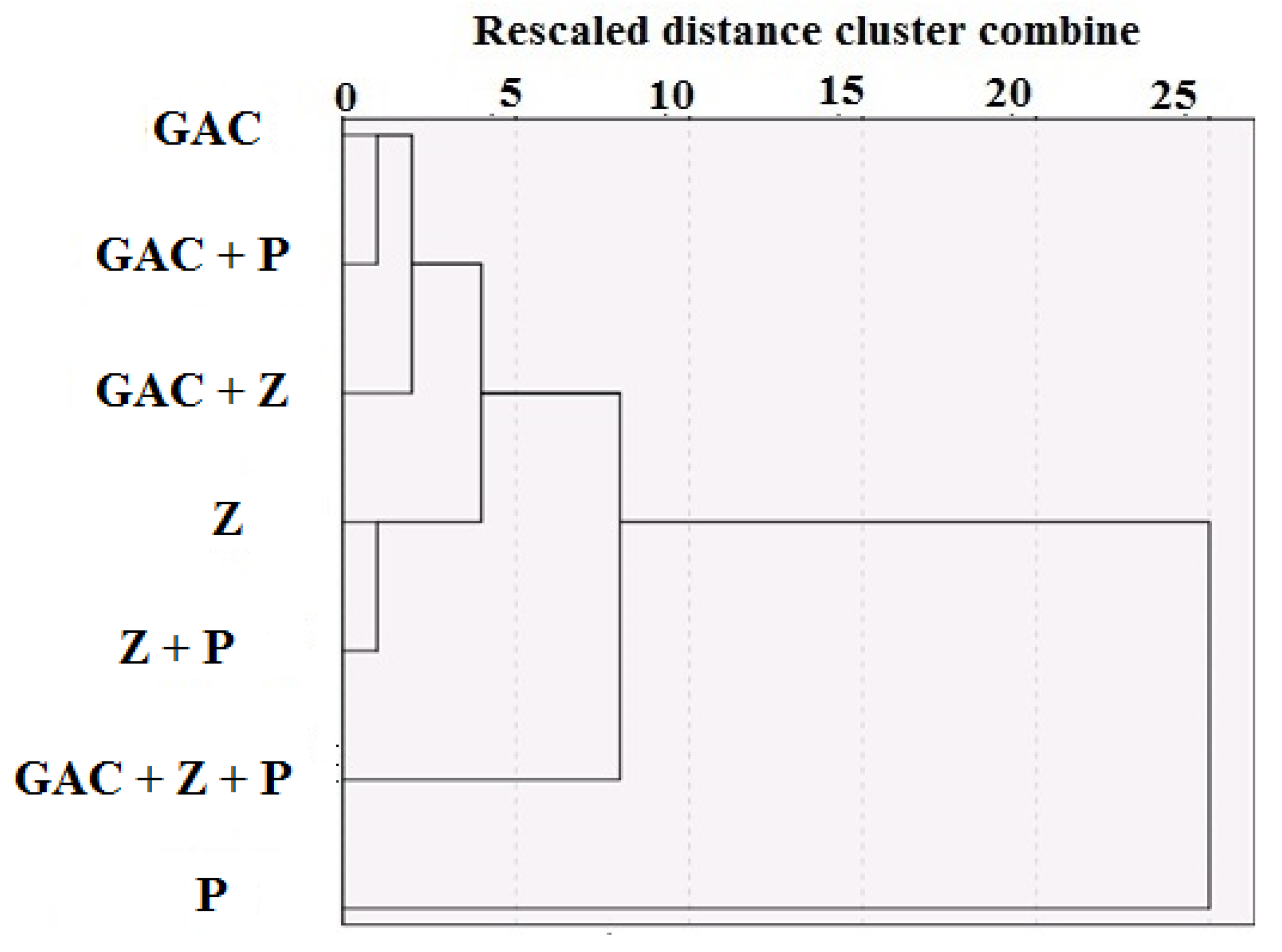
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Clustering Methodology
References
- Friedler, E.; Galil, N.I. On-site greywater reuse in multi-storey buildings: Sustainable solution for water saving. In Proceedings of the 2nd Int. Conf. on Efficient Use and Management of Urban Water Supply, Tenerife, Spain, 2–4 April 2003. [Google Scholar]
- Venot, P.; Molle, F.; Hassan, Y. Irrigated Agriculture, Water Pricing and Water Savings in the Lower Jordan River Basin; International Water Management Institute: Colombo, Sri Lanka, 2007. [Google Scholar]
- Friedler, E.; Hadari, M. Economic feasibility of on-site greywater reuse in multi-storey buildings. Desalin 2006, 190, 221–234. [Google Scholar] [CrossRef]
- Pidou, M.; Avery, L.; Stephenson, T.; Jeffrey, P.; Parsons, S.A.; Liu, S.; Memon, F.; Jefferson, B. Chemical solutions for greywater recycling. Chemosphere 2008, 71, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cornel, P.; Wagner, M. Treatment of greywater for urban water reuse. In Proceedings of the IWA Conference– Advanced Sanitation, Aachen, Germany, 12–13 March 2007. [Google Scholar]
- Halalsheh, M.; Dalahmeh, S.; Sayed, M.; Suleiman, W.; Shareef, M.; Mansour, M.; Safi, M. Grey water characteristics and treatment options for rural areas in Jordan. Bioresour. Technol. 2008, 99, 6635–6641. [Google Scholar] [CrossRef] [PubMed]
- Chrispim, M.C.; Nolasco, M.A. Greywater treatment using a moving bed biofilm reactor at a university campus in Brazi. J. Clean. Prod. 2017, 142, 290–296. [Google Scholar] [CrossRef]
- Shamabadi, N.; Bakhtiari, H.; Kochakian, N.; Farahani, M. The investigation and designing of an onsite grey water treatment systems at Hazrat-e-Masoumeh University, Qom, IRAN. Energy Procedia 2015, 74, 1337–1346. [Google Scholar] [CrossRef]
- Zipf, M.S.; Pinheiro, I.G.; Conegero, M.G. Simplified greywater treatment systems: Slow filters of sand and slate waste followed by granular activated carbon. J. Environ. Manag. 2016, 176, 119–127. [Google Scholar] [CrossRef]
- Assayed, A.; Chenoweth, J.; Pedley, S. Assessing the efficiency of an innovative method for onsite greywater treatment: Drawer compacted sand filter—A case study in Jordan. Ecol. Eng. 2015, 81, 525–533. [Google Scholar] [CrossRef]
- Abed, S.N.; Almuktar, S.A.; Scholz, M. Treatment of contaminated greywater using pelletised mine watersludge. J. Environ. Manag. 2017, 197, 10–23. [Google Scholar] [CrossRef]
- Song, H.; Alfiya, Y.; Dubowski, Y.; Friedler, E. Sorption and biodegradation of propylparaben in greywater by aerobic attached-growth biomass. Sci. Total Environ. 2017, 598, 925–930. [Google Scholar] [CrossRef]
- Ramprasad, C.; Smith, C.S.; Memon, F.A.; Philip, L. Removal of chemical and microbial contaminants from greywater using a novel constructed wetland: GROW. Ecol. Eng. 2017, 106, 55–65. [Google Scholar] [CrossRef]
- Moges, M.E.; Todt, D.; Eregno, F.E.; Heistad, A. Performance study of biofilter system for on-site greywater treatment at cottages and small households. Ecol. Eng. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Karabelnik, K.; Kõiv, M.; Kasak, K.; Jenssen, P.D.; Mander, Ü. High-strength greywater treatment in compact hybrid filter systems with alternative substrates. Ecol. Eng. 2012, 49, 84–92. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Andreadakis, A.; Kouris, N.; Charchousi, D.; Mendrinou, P.; Galani, A.; Mantziaras, I.; Koumaki, E. Greywater characterization and loadings- Physicochemical treatment to promote onsite reuse. J. Environ. Manag. 2017, 216, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.A.; Calijuri, M.L.; Assemany, P.P.; Santiago, A.F.; Lopes, L.S. Greywater treatment in airports using anaerobic filter followed by UV disinfection: An efficient and low cost alternative. J. Clean. Prod. 2015, 106, 372–379. [Google Scholar] [CrossRef]
- Oh, K.S.; Poh, P.E.; Chong, M.N.; Chan, E.S.; Lau, E.V.; Saint, C.P. Bathroom greywater recycling using polyelectrolyte-complex bilayer membrane: Advanced study of membrane structure and treatment efficiency. Carbohydr. Polym. 2016, 148, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Prodanovic, V.; Hatt, B.; McCarthy, D.; Zhang, K.; Deletic, A. Green walls for greywater reuse: Understanding the role of media on pollutant removal. Ecol. Eng. 2017, 102, 625–635. [Google Scholar] [CrossRef]
- Maimon, A.; Friedler, E.; Gross, A. Parameters affecting greywater quality and its safety for reuse. Sci. Total Environ. 2014, 487, 20–25. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A.; deVries, N.K. Greywater characteristics, treatment systems, reuse strategies and user perception—A review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Opher, T.; Shapira, A.; Friedler, E. A comparative social life cycle assessment of urban domestic water reuse alternatives. Int. J. Life Cycle Assess. 2017. [Google Scholar] [CrossRef]
- Amiri, M.J.; Bahrami, M.; Badkouby, M.; Kalavrouziotis, I.K. Greywater treatment using single and combined adsorbents for landscape irrigation. Environ. Process. 2019, 6, 43–63. [Google Scholar] [CrossRef]
- Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S.S.; Mousavi, S.F.; Arshadi, M. Modelling Pb(II) adsorption based on synthetic and industrial wastewaters by ostrich bone char using artificial neural network and multivariate non-linear regression. Int. J. Hydrol. Sci. Technol. 2013, 3, 221–240. [Google Scholar] [CrossRef]
- Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S.S.; Mousavi, S.F.; Hasheminejad, H. Modeling Pb(II) adsorption from aqueous solution by ostrich bone ash using adaptive neural-based fuzzy inference system. J. Environ. Sci. Health Part A 2013, 48, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.J.; Abedi-Koupai, J.; Jalali, S.M.J.; Mousavi, S.F. Modeling of fixed-bed column system of Hg (II) ions on ostrich bone ash/nZVI composite by artificial neural network. J. Environ. Eng. ASCE 2017, 143, 04017061. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Mahmoudi, M.R.; Koochaki, S. Modeling caffeine adsorption by multi-walled carbon nanotubes using multiple polynomial regression with interaction effects. J Water Health. 2017, 15, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Dacewicz, E.; Chmielowski, K. Application of multidimensional clustering for an assessment of pollutants removal from domestic wastewater using a filter with a plastic waste filling. J. Water Process Eng. 2019, 29, 100794. [Google Scholar] [CrossRef]
- Washington State Department of Health. Recommended Standards and Guidance for Performance, Application, Design, and Operation & Maintenance; Intermittent Sand Filter Systems; Washington State Department of Health: Washington, DC, USA, 2016.
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: American Water Works Association: Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Ares, G. Cluster Analysis: Application in Food Science and Technology. In Mathematical and Statistical Approaches in Food Science and Technology; Granato, D., Ares, G., Eds.; Wiley Blackwell: Oxford, UK, 2014. [Google Scholar]
- Johnson, R.A.; Wichern, D. Multivariate Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Mahmoudi, M.R.; Avazzadeh, Z. Diagnosis and clustering of power transformer winding fault types by cross-correlation and clustering analysis of FRA results. IET Gener. Transm. Distrib. 2018, 12, 4301–4309. [Google Scholar] [CrossRef]
- Aghakhani, A.; Mousavi, S.F.; Mostafazadeh-Fard, B.; Rostamian, R.; Seraji, M. Application of some combined adsorbents to remove salinity parameters from drainage water. Desalin 2011, 275, 217–223. [Google Scholar] [CrossRef]
- Laaffat, J.; Aziz, F.; Ouazzani, N.; Mandi, L. Biotechnological approach of greywater treatment and reuse for landscape irrigation in small communities. Saudi J. Biol. Sci. 2019, 26, 83–90. [Google Scholar] [CrossRef]
- Ghawi, A.H. Development of the greywater domestic treatment unit for irrigation of the garden in rural areas. Ecol. Eng. 2019, 20, 46–56. [Google Scholar] [CrossRef]
- Lavanya, V.; Kannan, D.P. Grey water treatment using effective micro-organisms and its impact on water qualities. J. Appl. Sci. 2019, 19, 188–198. [Google Scholar]
- Samayamanthula, D.R.; Sabarathinam, C.; Bhandary, H. Treatment and efective utilization of greywater. Appl. Water Sci. 2019, 9, 90. [Google Scholar] [CrossRef]
- Amiri, M.J.; Bahrami, M.; Beigzadeh, B.; Gil, A. A response surface methodology for optimization of 2, 4-dichlorophenoxyacetic acid removal from synthetic and drainage water: A comparative study. Environ. Sci. Pollut. Res. 2018, 25, 34277–34293. [Google Scholar] [CrossRef] [PubMed]
- Remer, D.; Nieto, A. A compendium and comparison of 25 project evaluation techniques, part 1: Net present value and rate of return methods. Int. J. Prod. Econ. 1995, 42, 72–96. [Google Scholar] [CrossRef]
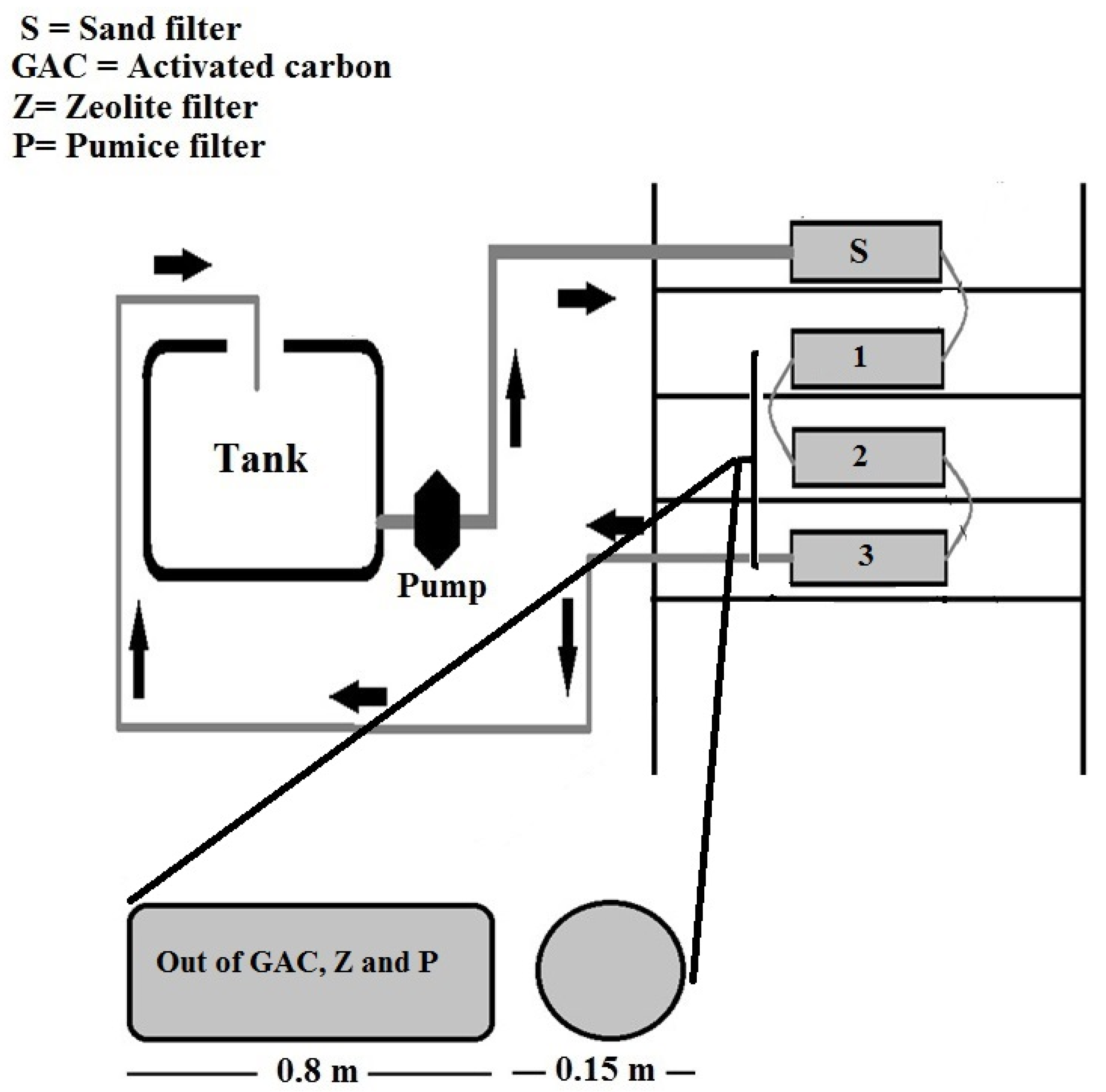
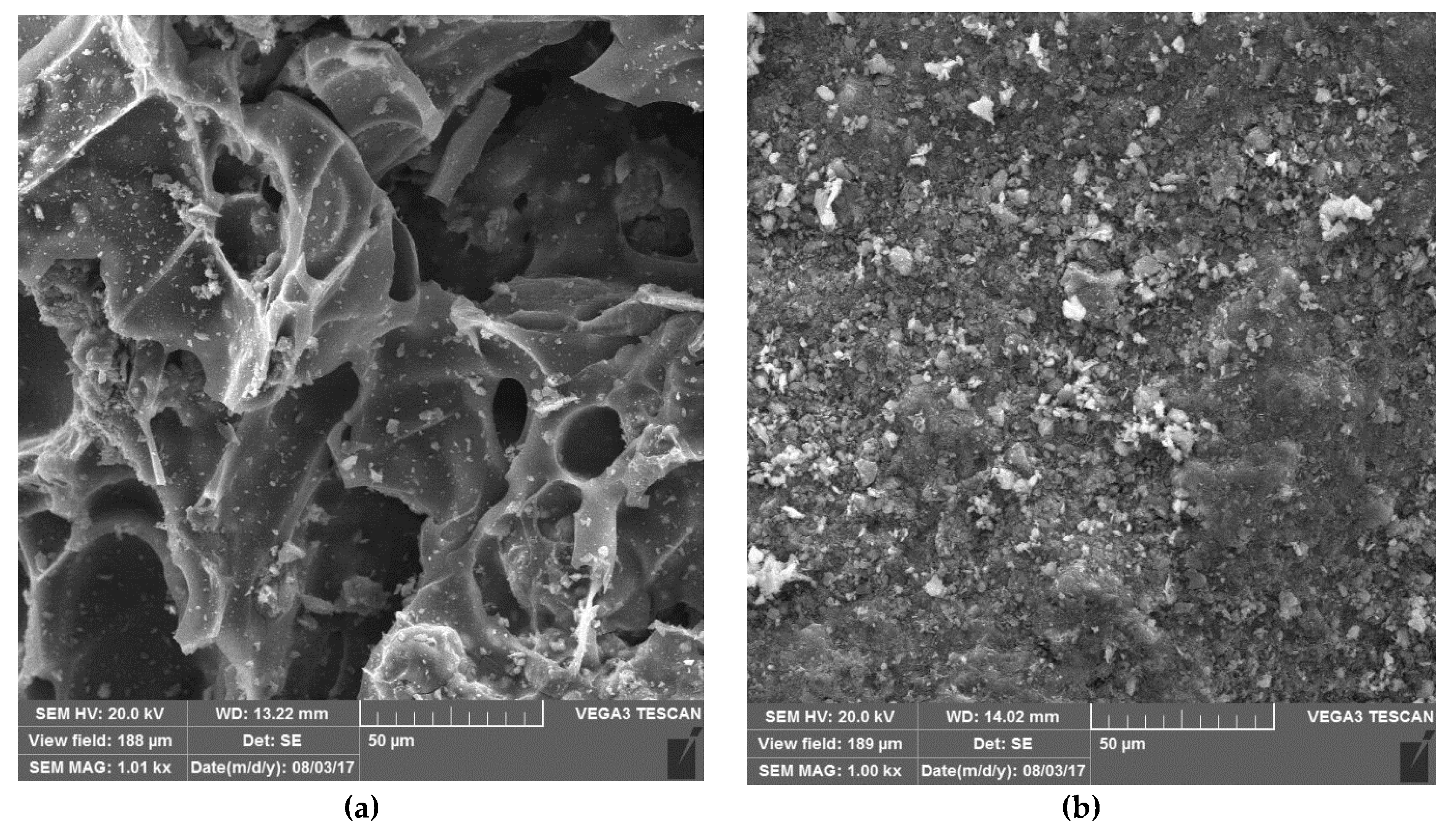
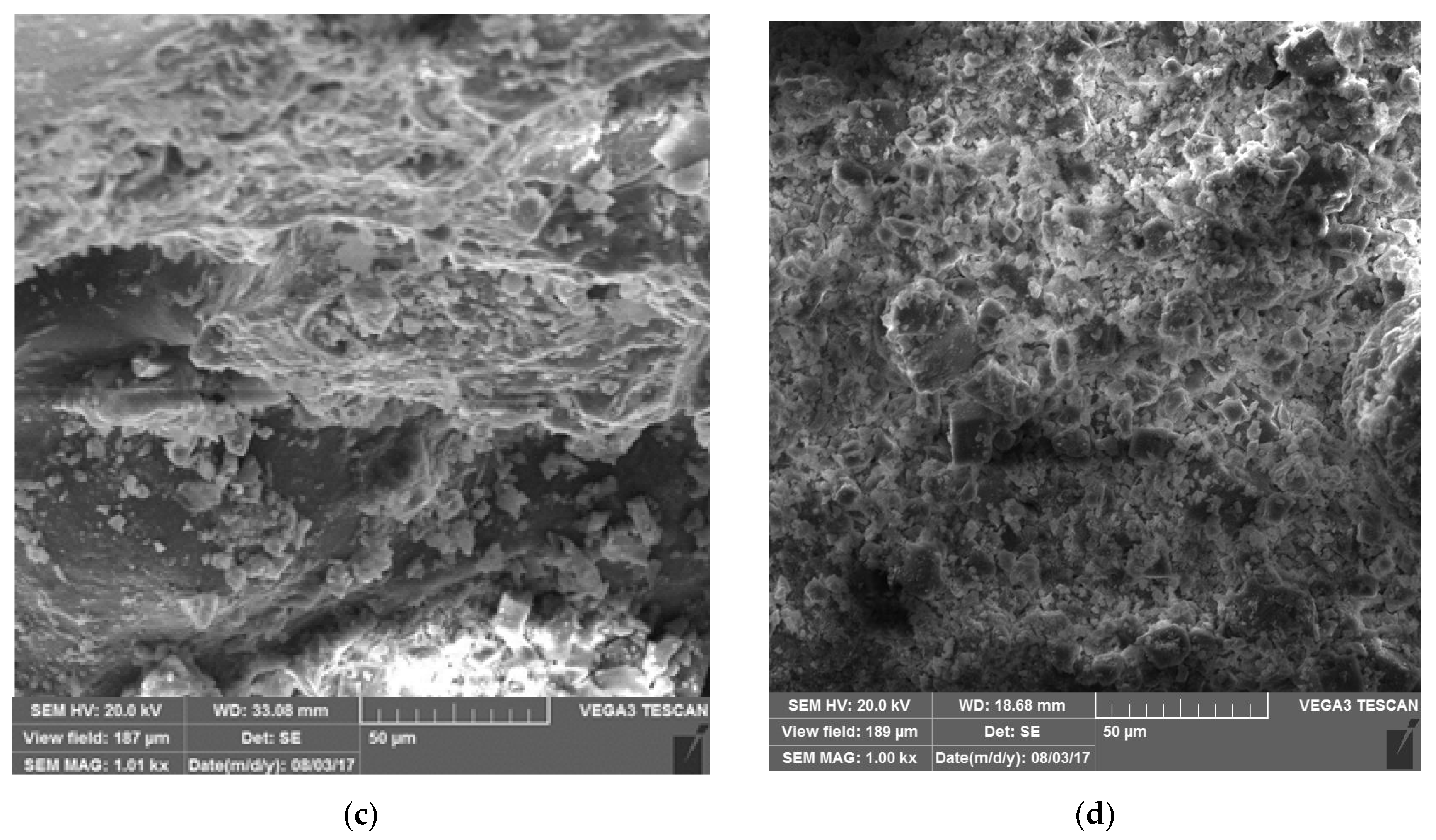
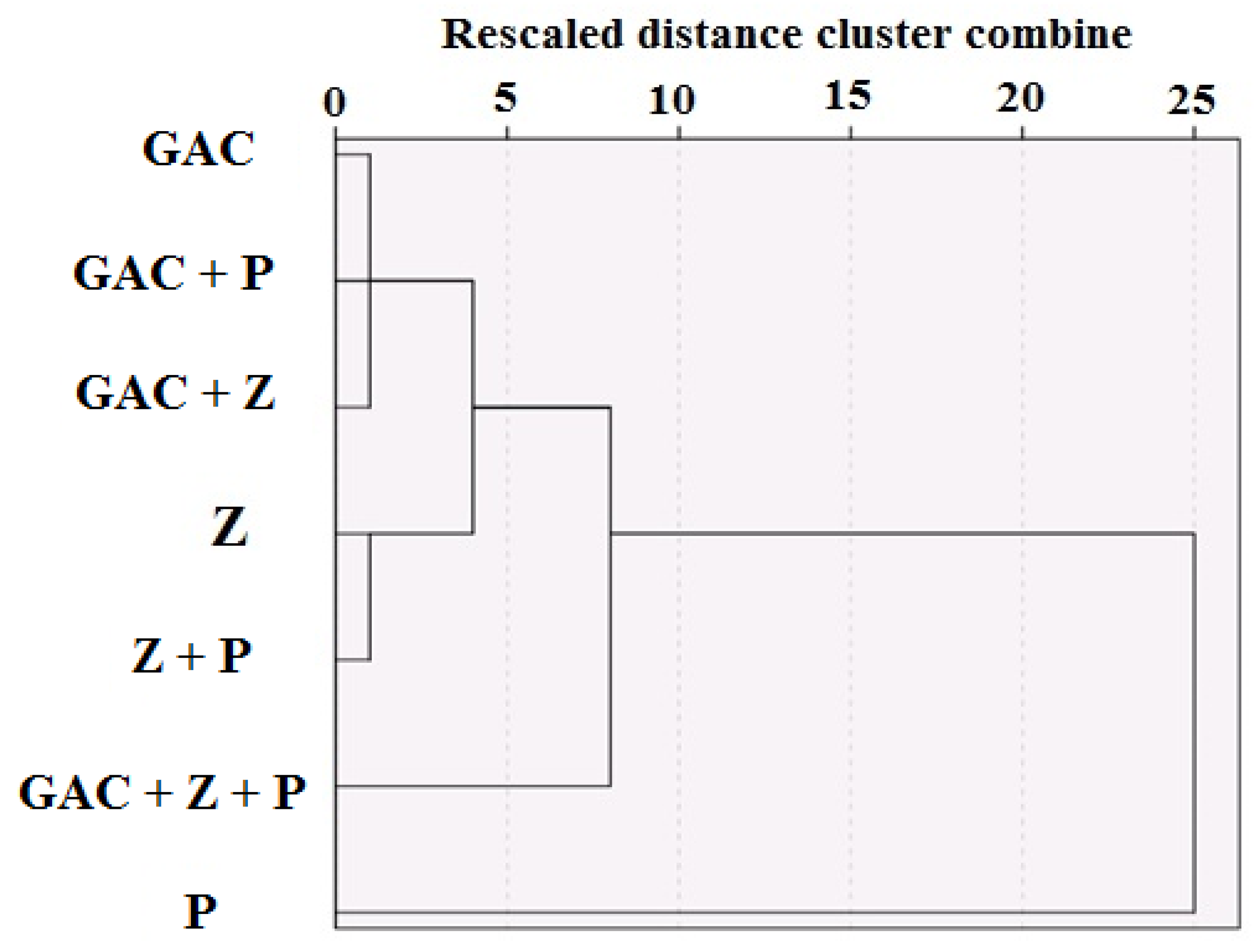
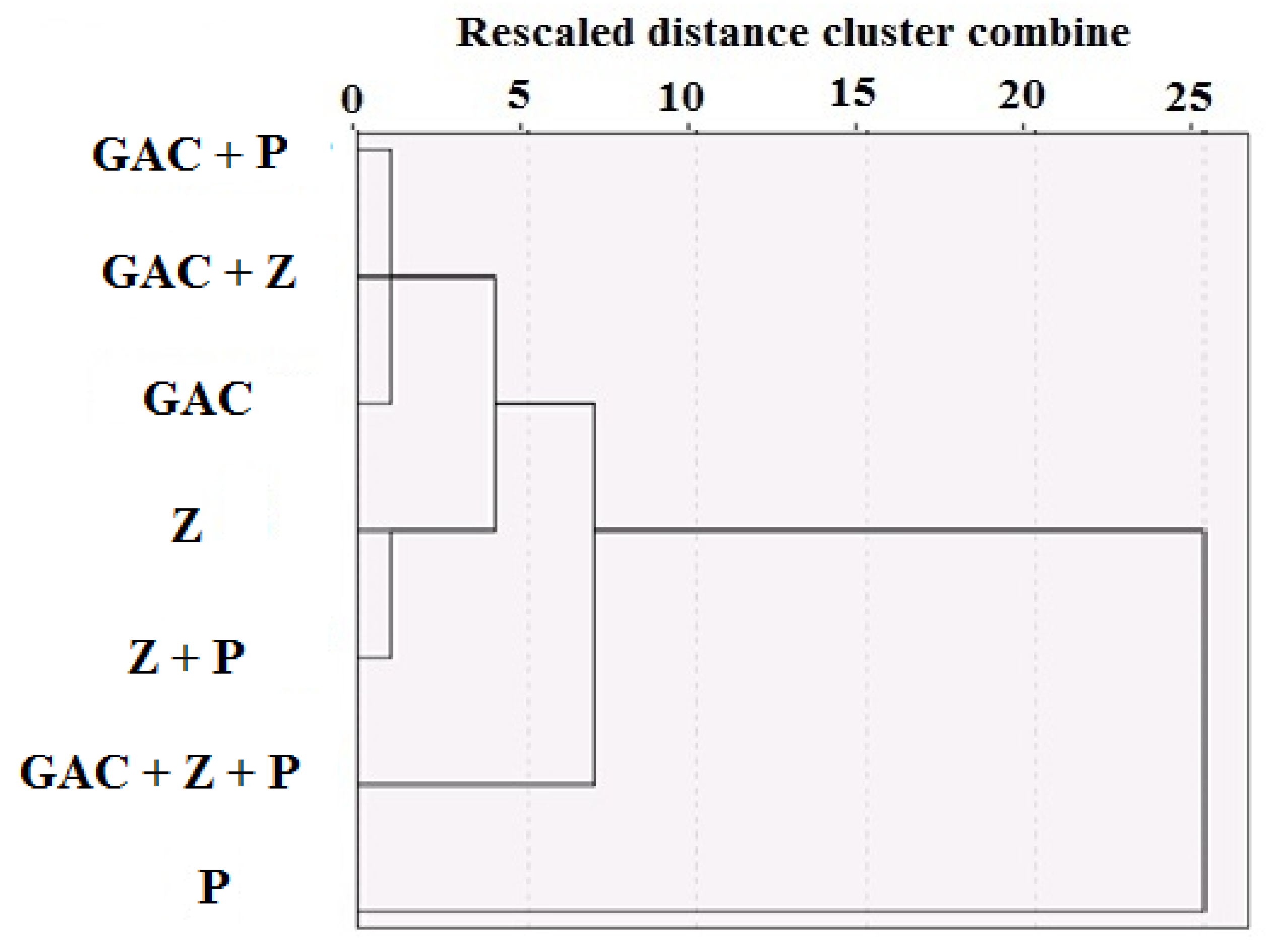
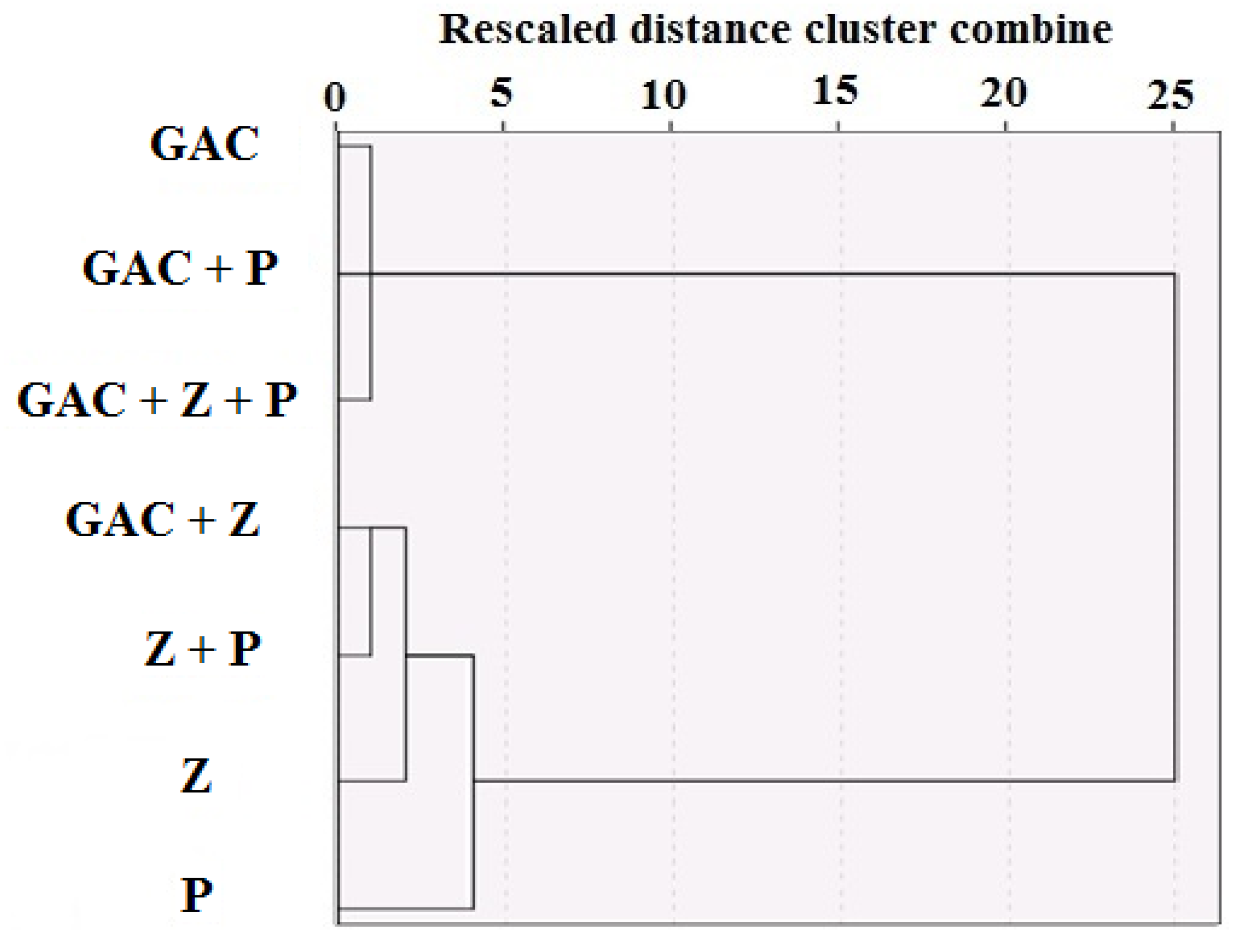
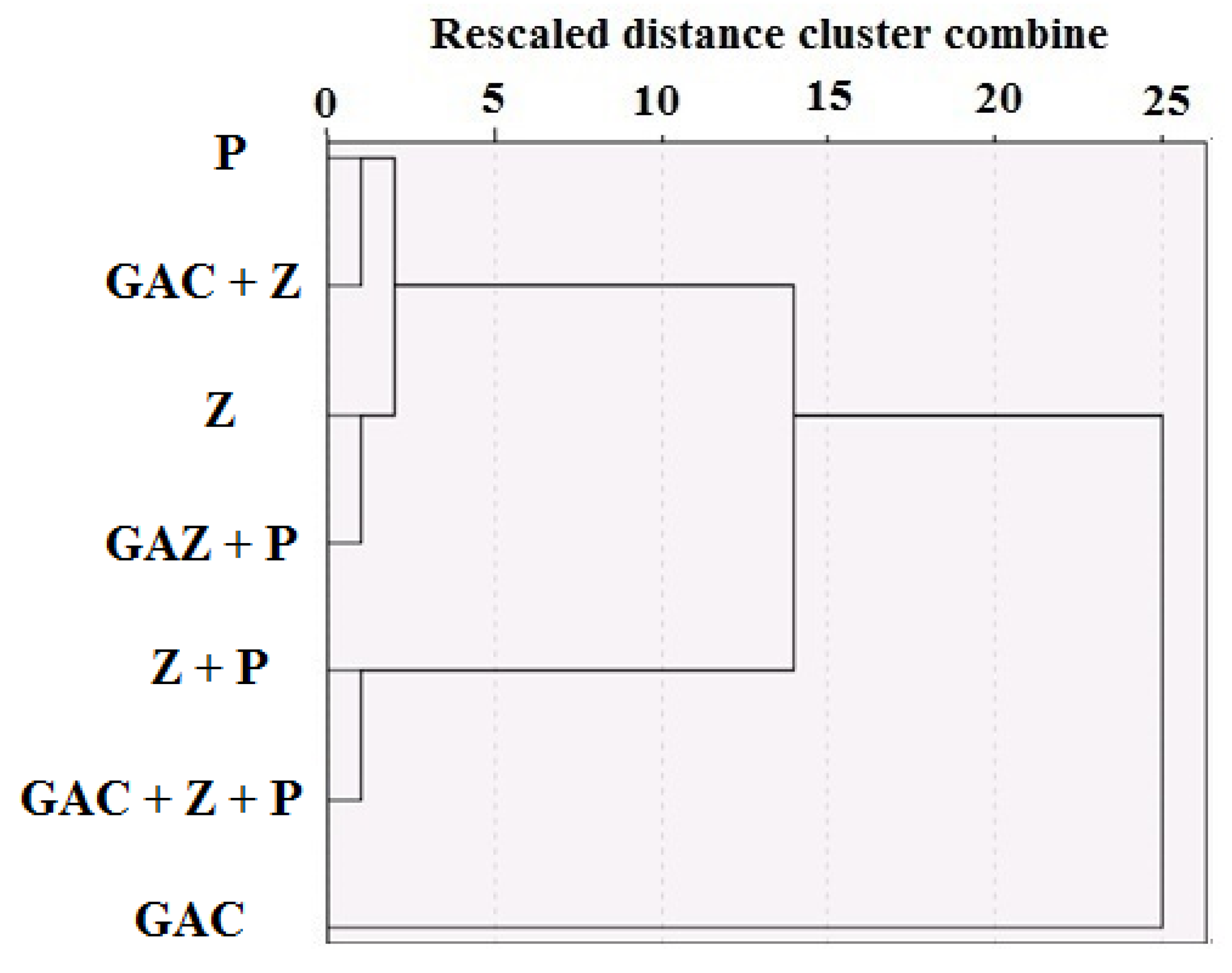
| Material | Particle Size (mm) | Density (Kg/m3) | Specific Surface Area (SSA) (m2/g) | Weight (Kg) | Volume (m3) | Uniformity Coefficient | Permeability (m2) |
|---|---|---|---|---|---|---|---|
| S | - | 1920 | 0.0444 | 26.88 | 0.0141 | 2 | 2.710−10 |
| GAC | 2–2.8 | 305 | 1000 | 4.30 | 0.0141 | 2.1 | 1.710−9 |
| Z | 0.8–1 | 2470 | 805.9 | 34.58 | 0.0141 | 1.6 | 3.110−12 |
| P | 10–40 | 440 | 144.3 | 6.20 | 0.0141 | 5.86 | 9.310−6 |
| Parameter | Initial Concentration |
|---|---|
| COD (mg L−1) | 350 ± 20.35 |
| BOD5 (mg L−1) | 280 ± 15.73 |
| TDS (mg L−1) | 2800 ± 200.12 |
| Turbidity (NTU) | 185 ± 5 |
| DO (mg L−1) Fecal coliforms (NMP/100 mL) | 1.3 ± 0.6 None detected |
| EC (dS/m) | 1.55 ± 0.03 |
| pH | 8.26 ± 0.3 |
| Adsorbent | |||||||
|---|---|---|---|---|---|---|---|
| Feature | GAC | Z | P | GAC+P | GAC+Z | Z+P | GAC+Z+P |
| COD | 69.21 | 60.20 | 30.53 | 76.10 | 77.34 | 68.16 | 93.06 |
| BOD | 69.76 | 60.52 | 29.51 | 76.08 | 77.50 | 67.88 | 94.34 |
| TDS | 84.37 | 72.46 | 53.99 | 79.44 | 61.63 | 65.18 | 84.65 |
| Turbidity | 56.61 | 71.37 | 81.74 | 75.55 | 79.39 | 88.03 | 93.01 |
| Feature | Material | GAC | Z | P | GAC+P | GAC+Z | Z+P | GAC+Z+P |
|---|---|---|---|---|---|---|---|---|
| COD | GAC | 100 | 96 | 75 | 99 | 99 | 96 | 92 |
| Z | 100 | 75 | 96 | 96 | 99 | 92 | ||
| P | 100 | 75 | 75 | 75 | 75 | |||
| GAC+P | 100 | 99 | 96 | 92 | ||||
| GAC+Z | 100 | 96 | 92 | |||||
| Z+P | 100 | 92 | ||||||
| GAC+Z+P | 100 | |||||||
| BOD | GAC | 100 | 96 | 75 | 99 | 99 | 96 | 93 |
| Z | 100 | 75 | 96 | 96 | 99 | 93 | ||
| P | 100 | 75 | 75 | 75 | 75 | |||
| GAC+P | 100 | 99 | 96 | 93 | ||||
| GAC+Z | 100 | 96 | 93 | |||||
| Z+P | 100 | 93 | ||||||
| GAC+Z+P | 100 | |||||||
| TDS | GAC | 100 | 75 | 75 | 99 | 75 | 75 | 99 |
| Z | 100 | 96 | 75 | 98 | 98 | 75 | ||
| P | 100 | 75 | 96 | 96 | 75 | |||
| GAC+P | 100 | 75 | 75 | 99 | ||||
| GAC+Z | 100 | 99 | 75 | |||||
| Z+P | 100 | 75 | ||||||
| GAC+Z+P | 100 | |||||||
| Turbidity | GAC | 100 | 75 | 75 | 75 | 75 | 75 | 75 |
| Z | 100 | 98 | 99 | 98 | 86 | 86 | ||
| P | 100 | 98 | 99 | 86 | 86 | |||
| GAC+P | 100 | 98 | 86 | 86 | ||||
| GAC+Z | 100 | 86 | 86 | |||||
| Z+P | 100 | 99 | ||||||
| GAC+Z+P | 100 | |||||||
| Total Features | GAC | 100 | 96 | 75 | 99 | 98 | 96 | 92 |
| Z | 100 | 75 | 96 | 96 | 99 | 92 | ||
| P | 100 | 75 | 75 | 75 | 75 | |||
| GAC+P | 100 | 98 | 96 | 92 | ||||
| GAC+Z | 100 | 96 | 92 | |||||
| Z+P | 100 | 92 | ||||||
| GAC+Z+P | 100 |
| Incomes and Costs | GAC | P | Z | GAC+Z+P |
|---|---|---|---|---|
| Initial investment ($) | 4800 | 3420 | 3800 | 4100 |
| Annual Cost ($) | 2800 | 2100 | 2500 | 2600 |
| Annual Incomes ($) | 4000 | 2800 | 3300 | 4400 |
| Salvage Value ($) | 750 | 500 | 500 | 570 |
| Useful Life (year) | 7 | 7 | 7 | 7 |
| MARR | 15 | 15 | 15 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrami, M.; Amiri, M.J.; Mahmoudi, M.R. Clustering the Adsorbents of Horizontal Series Filtration in Greywater Treatment. Sustainability 2020, 12, 3194. https://doi.org/10.3390/su12083194
Bahrami M, Amiri MJ, Mahmoudi MR. Clustering the Adsorbents of Horizontal Series Filtration in Greywater Treatment. Sustainability. 2020; 12(8):3194. https://doi.org/10.3390/su12083194
Chicago/Turabian StyleBahrami, Mehdi, Mohammad Javad Amiri, and Mohammad Reza Mahmoudi. 2020. "Clustering the Adsorbents of Horizontal Series Filtration in Greywater Treatment" Sustainability 12, no. 8: 3194. https://doi.org/10.3390/su12083194
APA StyleBahrami, M., Amiri, M. J., & Mahmoudi, M. R. (2020). Clustering the Adsorbents of Horizontal Series Filtration in Greywater Treatment. Sustainability, 12(8), 3194. https://doi.org/10.3390/su12083194







