Assessment of the Knowledge, Perceptions, and Reactions towards the African Apefly (Spalgis lemolea lemolea) in Tanzania
Abstract
1. Introduction
2. Materials and Methods
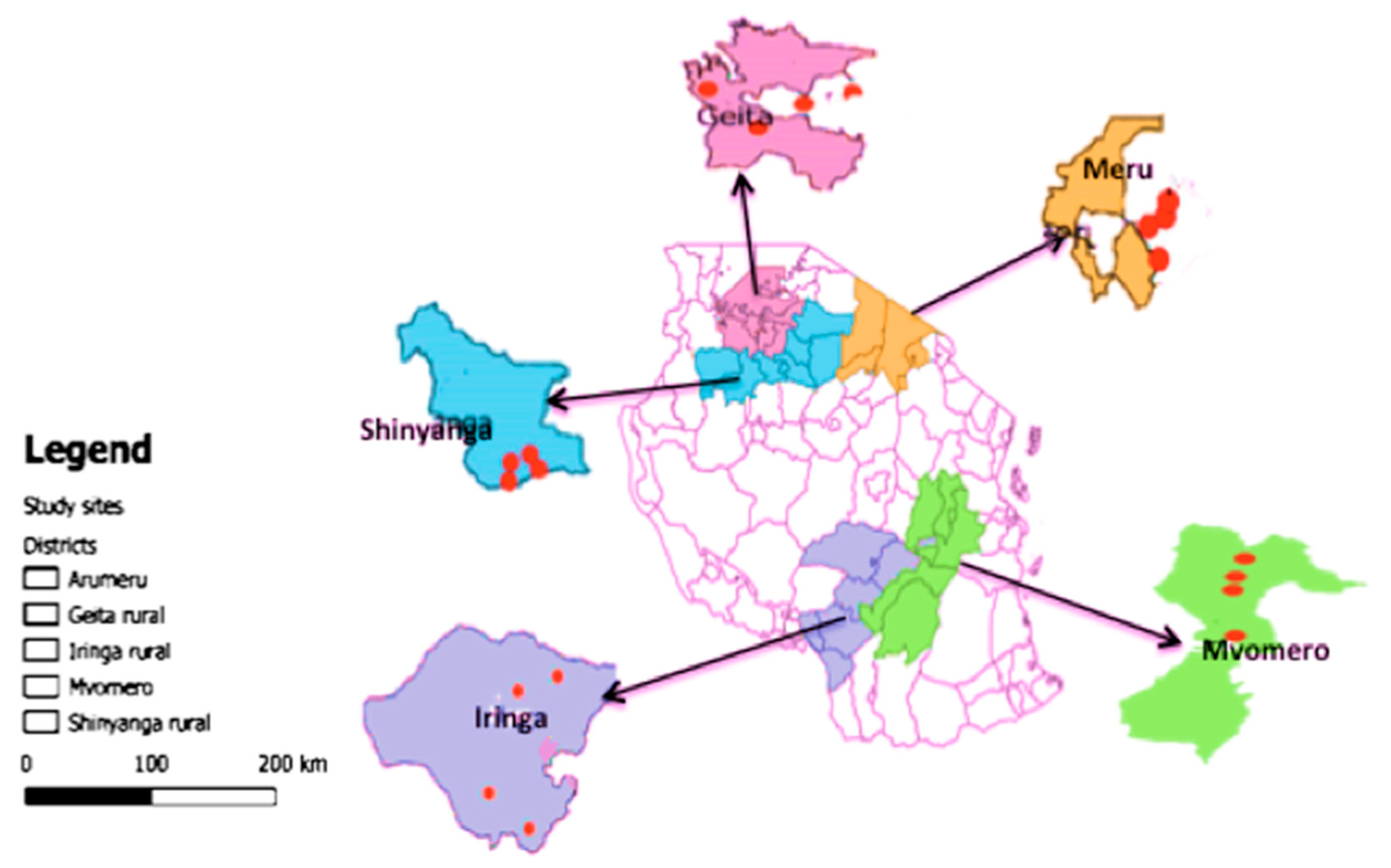
2.1. Study Areas
2.2. Survey
2.3. Toxicity Tests
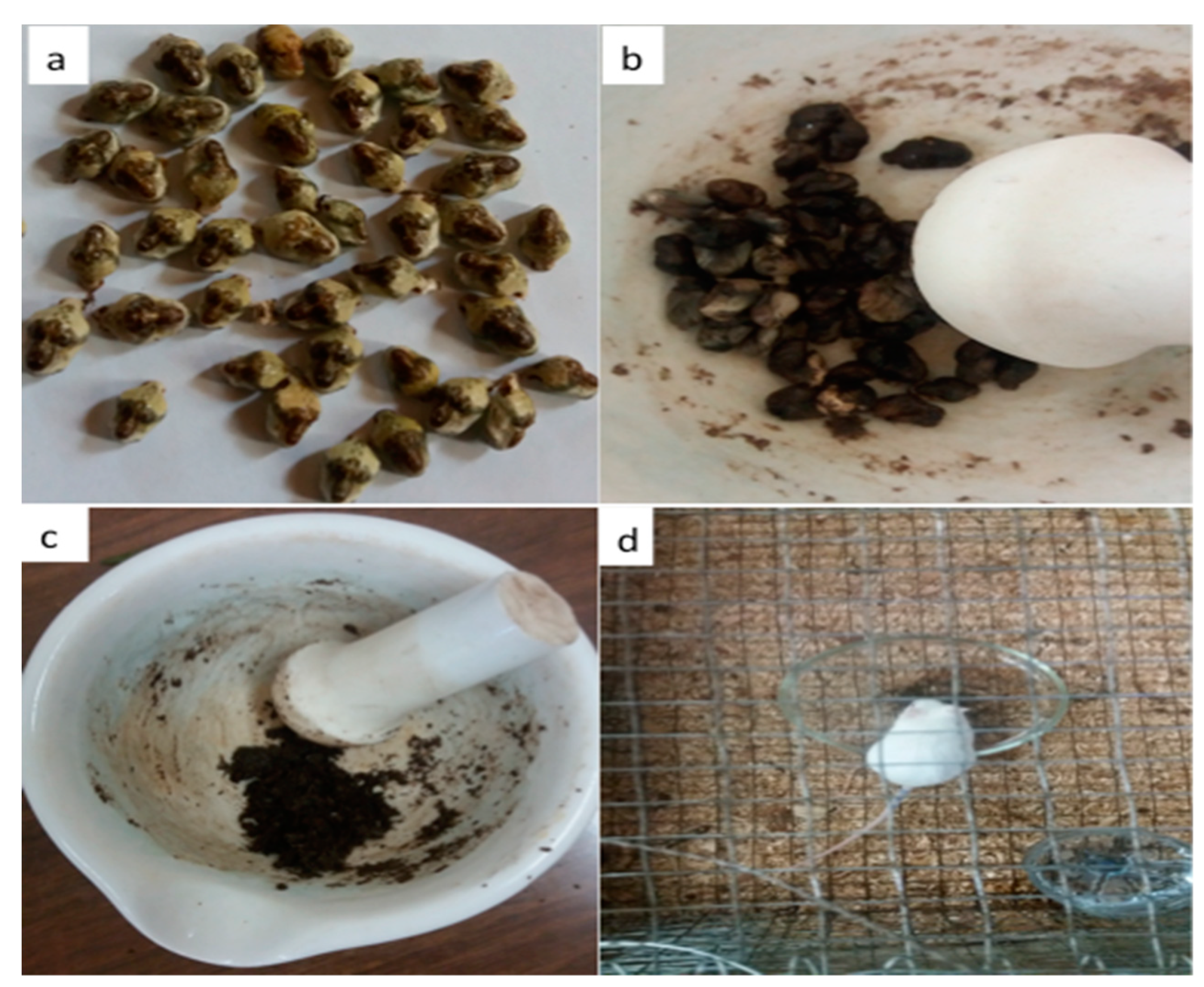
2.3.1. Collection and Preparation of S. lemolea lemolea Pupa
2.3.2. Experimental Animals
2.3.3. Ethical Consideration
2.3.4. Acute Toxicity Tests
2.3.5. Sub-Acute Toxicity Tests
2.3.6. Hematological and Biochemical Examinations
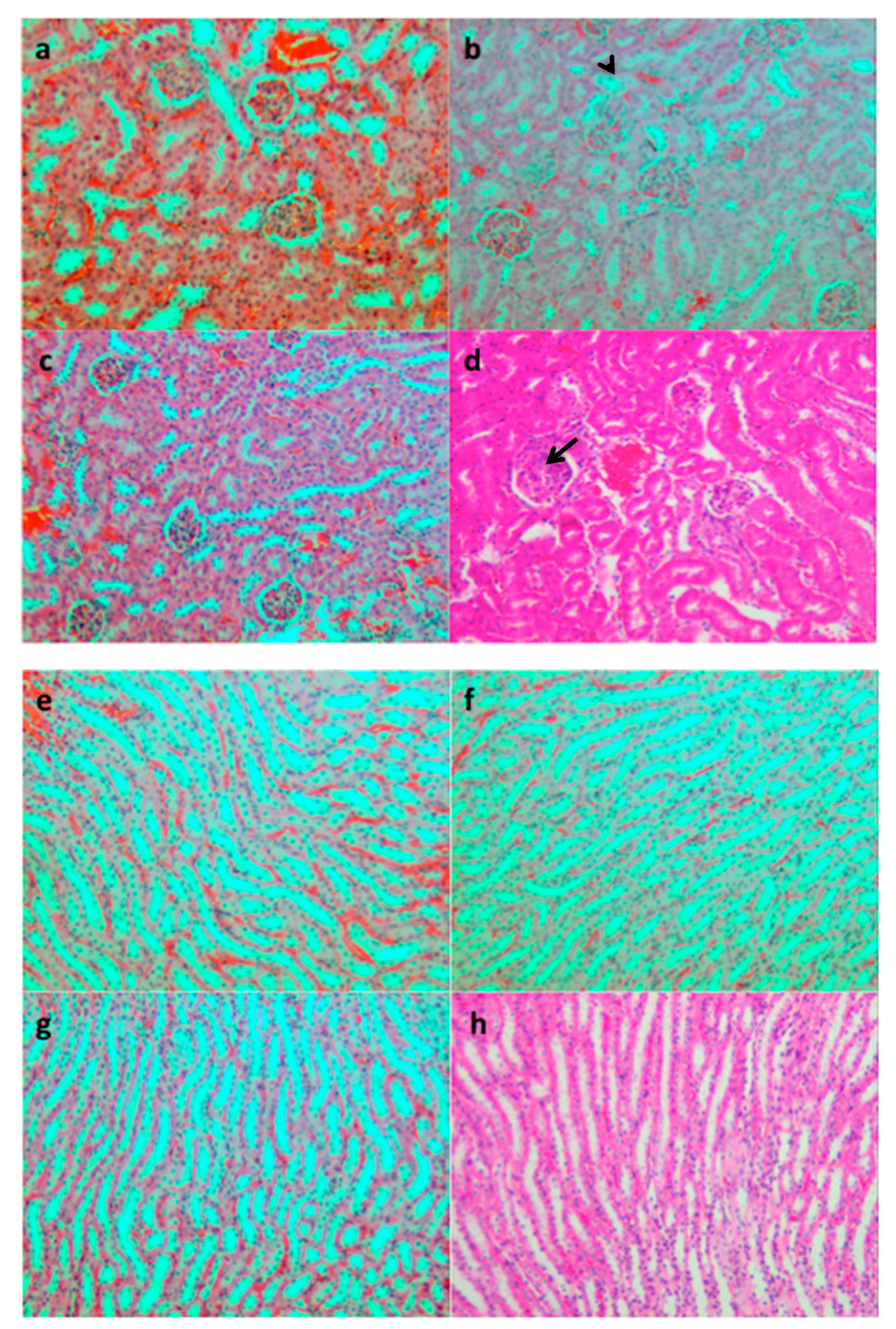
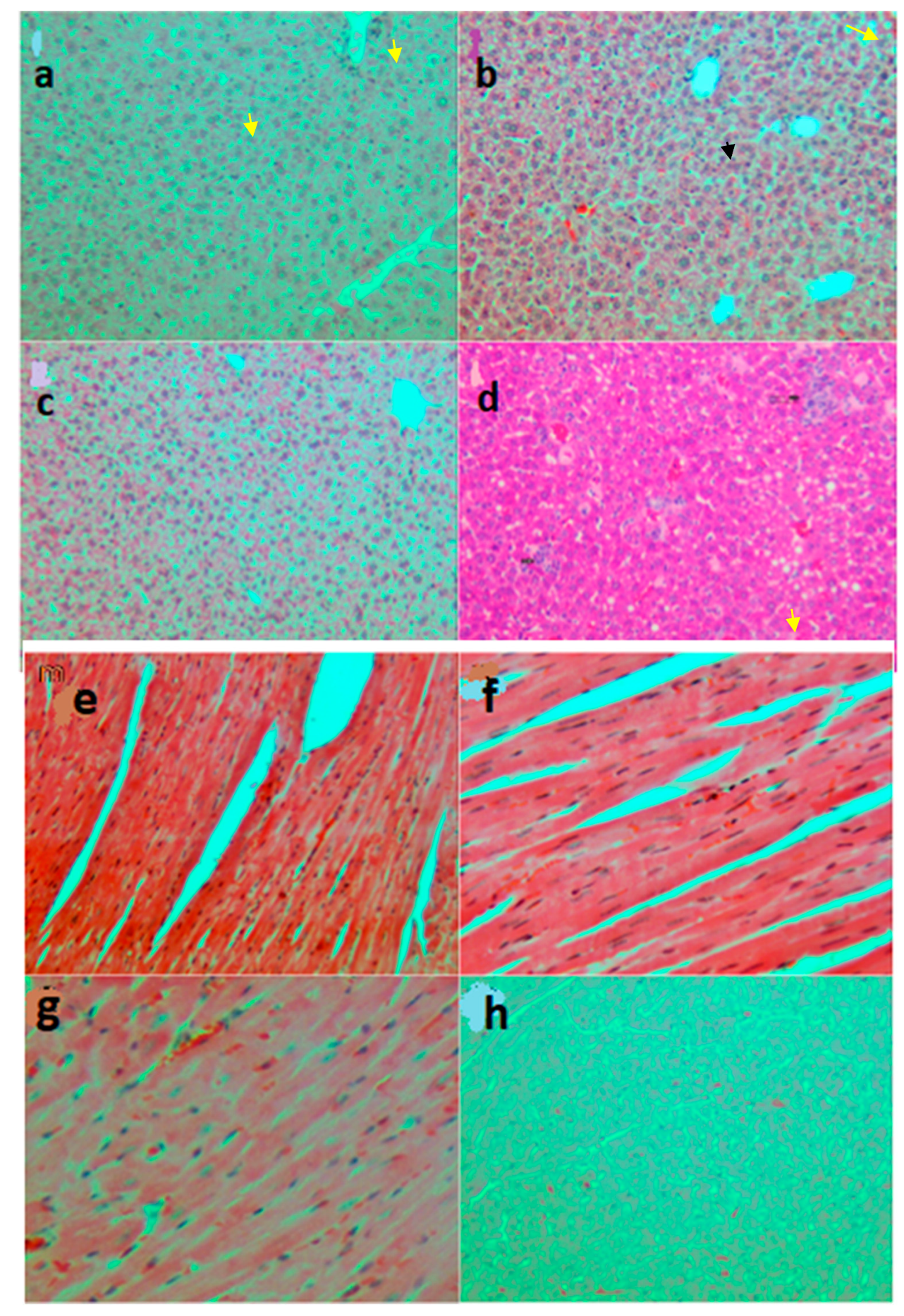
2.3.7. Histopathological Analysis
2.4. Statistical Analyses
3. Results
3.1. Survey Results
3.2. Toxicity Tests
3.2.1. Acute Toxicity
3.2.2. Sub-Acute Toxicity
3.2.3. Biochemical and Microscopic Examinations
4. Discussion
4.1. Knowledge, Perceptions, and Reactions about the Apefly
4.2. Toxicity Tests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagler, R.; Wagler, A. External insect morphology: A negative factor in attitudes toward insects and likelihood of incorporation in future science education settings. Int. J. Environ. Sci. Educ. 2012, 7, 313–325. [Google Scholar]
- Getanjaly, V.L.R.; Sharma, P.; Kushwaha, P. Beneficial insects and their value to agriculture. Res. J. Agric. For. Sci. 2015, 3, 25–30. [Google Scholar]
- Gredler, G. Encouraging Beneficial Insects in Your Garden; Oregon State University Extension Service: Covallis, OR, USA, 2001. [Google Scholar]
- Pickett, C.H.; Bugg, R.L. Enhancing Biological Control: Habitat Management to Promote Natural Enemies of Agricultural Pests; University of California Press: Berkeley, CA USA; Los Angeles, CA, USA; London, UK, 1998; p. 422. [Google Scholar]
- Gurung, A.B. Insects—A mistake in God’s creation? Tharu farmers’ perception and knowledge of insects: A case study of Gobardiha Village Development Committee, Dang-Deukhuri, Nepal. Agric. Hum. Values 2003, 20, 337–370. [Google Scholar] [CrossRef]
- Day, J.F.; Edman, J.D.; Kunz, S.E.; Wikel, S.K. Direct Injury: Phobias, Psychoses, Annoyance, Allergies, Toxins, Venoms and Myiasis. In Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods; Eldrige, B.F., Edman, J.D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 99–149. [Google Scholar]
- Gavkare, O.M.; Sharma, P.L. New record of Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) associated with Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) on tomato from Maharashtra, India. J. Biol. Control 2014, 28, 53–54. [Google Scholar]
- Dinesh, A.; Venkatesha, M. Prey consumption by the mealybug predator Spalgis epius on pink hibiscus mealybug (Maconellicoccus hirsutus). Phytoparasitica 2011, 39, 11–17. [Google Scholar] [CrossRef][Green Version]
- Saengyot, S.; Burikam, I. Bionomics of the apefly Spalgis epius (Lepidoptera: Lycaenidae) predatory on the papaya mealybug Paracoccus marginatus (Hemiptera: Pseudococcidae) in Thailand. Songklanakarin J. Sci. Technol. 2012, 34, 17. [Google Scholar]
- Ackery, P.R. Bio control potential of African lycaenid butterflies entomophagous on Homopterous. J. Afr. Zool. 1990, 104, 582–591. [Google Scholar]
- Kumar, P.K.V.; Vasudev, V.; Seetharama, H.G.; Irulandi, S.; Sreedharan, K. Biology and biometry of the Lycaenid predator Spalgis epius. J. Coffee Res. 2006, 34, 72–104. [Google Scholar]
- Lohman, D.J.; Samarita, V.U. The biology of carnivorous butterfly larvae (Lepidoptera: Lycaenidae: Miletinae: Miletini) and their ant-tended hemipteran prey in Thailand and the Philippines. J. Nat. Hist. 2009, 43, 569–581. [Google Scholar] [CrossRef]
- Tanwar, R.K.; Jeyakumar, P.; Vennila, S. Papaya Mealybug and Its Management Strategies; National Centre for Integrated Pest Management: New Delhi, India, 2010; p. 26. Available online: http://www.ncipm.res.in/NCIPMPDFs/Publication/Swarming_caterpillar.pdf (accessed on 22 October 2019).
- Athumani, M. Kidudu Mtu Ndio Hiki 2017. Available online: https://www.youtube.com/watch?v=XzUFO-MHn5A (accessed on 30 June 2019).
- Brown, S. Huyu Ndo Kidudu Mtu Anaesemekana Kusumbua Walaji Wa Matunda Na Mboga Mboga 2017. Available online: https://www.youtube.com/watch?v=DvUPUpcwXP0 (accessed on 18 June 2019).
- Choke, S. www Geita info Mboga. 2017. Available online: https://www.youtube.com/watch?v=OCfDbRAR678 (accessed on 9 June 2019).
- Organization for Economic Cooperation and Development (OECD). Acute oral toxicity: Up-and-Down procedure. In Guidelines for Testing of Chemicals; OECD Publishers: Paris, France, 2001. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf (accessed on 18 July 2019).
- Bunger, M.K.; Moran, S.M.; Glover, E.; Thomae, T.L.; Lahvis, G.P.; Lin, B.C.; Bradfield, C.A. Resistance to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin toxicity and abnormal liver development in mice carrying a mutation in the nuclear localization sequence of the aryl hydrocarbon receptor. J. Biol. Chem. 2003, 278, 17767–17774. [Google Scholar] [CrossRef]
- Demers, G.; Griffin, G.; De Vroey, G.; Haywood, J.; Zurlo, J.; Bedard, M. Harmonization of animal care and use guidance. Science 2006, 5774, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Culling, C. Handbook of Histopathological and Histochemical Techniques (Including Museum Techniques), 3rd ed.; Butterworth and Co. Ltd.: London, UK, 1974; p. 419. [Google Scholar]
- Curtis, B.A.; Mannheimer, C.A.; Loutit, B. Tree Atlas of Namibia; National Botanical Research Institute: Windhoek, Namibia, 2005; pp. 406–407. [Google Scholar]
- Haddad, J.V.; Amorim, P.C.H.D.; Junior, H.; Teixera, W.; Cardoso, J.L.C. Venomous and poisonous arthropods: Identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev. Soc. Bras. Med. Trop. 2015, 6, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Longley, M.; Sotherton, N.W. Factors determining the effects of pesticides upon butterflies inhabiting arable farmland. Agric. Ecosyst. Environ. 1997, 61, 1–12. [Google Scholar] [CrossRef]
- Schreiner, I.H.; Donald, M.N. Butterflies of Micronesia. 1997, pp. 1–40. Available online: https://cnas-re.uog.edu/wp-content/uploads/2016/03/ButterfliesOfMicronesia.pdf (accessed on 12 November 2019).
- Raza, M.; Al-Shabanah, O.A.; El-Hadiyah, T.M.; Al Majeed, A.A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. J. Sci. Pharm 2002, 70, 135–145. [Google Scholar] [CrossRef]
- Teo, S.D.; Stirling, S.; Thomas, A.; Kiorpes, A.; Vikram, K. A 90-day oral gavage toxicity study of D-methylphenidate and D, L methylphenidate in Sprague-dawley rats. Toxicology 2002, 179, 183–196. [Google Scholar] [CrossRef]
- Ajagbonna, O.P.; Onifade, K.I.; Suleiman, U. Hematological and biochemical changes in rats given extract of Calotropis procera. Sok. J. Vet. Sci. 1999, 1, 36–42. [Google Scholar]
- Reddy, R.R.; Lokanatha, O.; Ratnam, K.; Reddy, C.S.; Raju, I.N.; Reddy, C.D. Acute and sub-acute toxicity of Moringa oleifera stem bark extract in Swiss albino mice. Int. J. Sci. Biotech. Pharm. Res. 2013, 4, 74–82. [Google Scholar]
- Shah, R.; Parmar, S.; Bhatt, P.; Chandra, S. Evaluation of hepatoprotective activity of ethyl acetate fraction of Tephrosia purpurea. Pharmacology 2011, 3, 188–194. [Google Scholar]
| Characteristics | N | % |
|---|---|---|
| Sex | ||
| Male | 60 | 60.0 |
| Female | 40 | 40.0 |
| Age (Years) | ||
| 21–40 | 35 | 35.0 |
| 41–60 | 54 | 54.0 |
| ≥61 | 18 | 18.0 |
| Education | ||
| No Professional training | 28 | 28.0 |
| College | 54 | 54.0 |
| University | 18 | 18.0 |
| Occupation | ||
| Community leader/elders | 29 | 29.0 |
| Ward/Village staff | 52 | 52.0 |
| District staff | 17 | 17.0 |
| Regional staff | 2 | 2.0 |
| Characteristics | N | % |
|---|---|---|
| How did you know? | ||
| Encountered the insect | 34 | 38.2 |
| Heard about the insect | 55 | 61.8 |
| When? | ||
| 2010–2018 | 71 | 79.8 |
| 2000–2009 | 10 | 11.2 |
| Before 2000 | 8 | 9.0 |
| In your farm? | ||
| Yes | 13 | 14.6 |
| No | 76 | 85.4 |
| In which season? | ||
| Wet season | 5 | 5.6 |
| Dry season | 84 | 94.4 |
| Interaction with other insects? | ||
| Yes | 61 | 68.5 |
| No | 4 | 4.5 |
| I don’t know | 24 | 27.0 |
| Is the apefly useful in agriculture? | ||
| Yes | 3 | 3.4 |
| No | 2 | 2.2 |
| I don’t know | 84 | 94.4 |
| Heard of anyone affected by the apefly? | ||
| Yes | 81 | 91.0 |
| No | 8 | 9.0 |
| Source of information? | ||
| Experts | 3 | 3.4 |
| Media | 74 | 83.1 |
| Farmers | 12 | 13.5 |
| Is the apefly poisonous? | ||
| No | 7 | 7.9 |
| Yes | 82 | 92.1 |
| Any intervention? | ||
| Yes | 12 | 13.5 |
| No | 77 | 86.5 |
| How do you deal with the apefly? | ||
| Chemical spray | 6 | 6.7 |
| Biological | 4 | 4.5 |
| Avoidance | 79 | 88.8 |
| Are farmers affected by the apefly? | ||
| Yes | 54 | 60.7 |
| No | 35 | 39.3 |
| How was your first reaction? | ||
| No reaction | 10 | 11.2 |
| Scared | 79 | 88.8 |
| Districts n (%) | ||||||
|---|---|---|---|---|---|---|
| Meru | Geita | Mvomero | Shinyanga | Iringa | χ2 (p-Value) | |
| How did you know? | 7.401 (0.114) | |||||
| I saw | 7 (35) | 9 (45) | 11 (61.1) | 5 (23.8) | 2 (20) | |
| I heard | 13 (65) | 11 (55) | 7 (38.9) | 16 (76.2) | 8 (80) | |
| When? | 18.550 (0.002) * | |||||
| 2010–2018 | 18 (90) | 16 (80) | 11 (61.1) | 20 (95.2) | 6 (60) | |
| 2000–2009 | 1 (5) | - | 6 (33.3) | - | 3 (30) | |
| Before 2000 | 1 (5) | 4 (20) | 1 (5.6) | 1 (4.8) | 1 (10) | |
| In your farm? | 18.422 (<0.001) * | |||||
| Yes | 2 (10) | 2 (10) | 9 (50) | - | - | |
| No | 18 (90) | 18 (90) | 9 (50) | 21 (100) | 10 (100) | |
| Interaction with other insects? | 37.171 (<0.001) * | |||||
| Yes | 15 (75) | 20 (100) | 14 (77.8) | 8 (38.1) | 4 (40) | |
| No | 4 (20) | - | - | - | - | |
| I don’t know | 1 (5) | - | 4 (22.2) | 13 (61.9) | 6 (60) | |
| In which season? | 8.162 (0.012) * | |||||
| Wet season | 4 (20) | - | - | - | 1 (10) | |
| Dry season | 16 (80) | 20 (100) | 18 (100) | 21 (100) | 9 (90) | |
| Is the apefly useful? | 6.020 (0.755) | |||||
| Yes | 1 (5) | 1 (5) | 1 (5.6) | - | - | |
| No | - | - | - | 1 (4.8) | 1 (10) | |
| I don’t know | 19 (95) | 19 (95) | 17 (94.4) | 20 (95.2) | 9 (90) | |
| Heard of anyone affected by the apefly? | 6.989 (0.069) | |||||
| Yes | 20 (100) | 19 (95) | 16 (88.9) | 16 (76.2) | 10 (100) | |
| No | - | 1 (5) | 2 (11.1) | 5 (23.8) | - | |
| Source of information? | 14.162 (0.048) * | |||||
| Experts | - | 3 (15) | - | - | - | |
| Media | 18 (90) | 12 (60) | 14 (77.8) | 20 (95.2) | 10 (100) | |
| Farmers | 2 (10) | 4 (20) | 2 (11.1) | 1 (4.8) | - | |
| No information | - | 1 (5) | 2 (11.1) | - | - | |
| Is the apefly poisonous? | 1.148 (1.000) | |||||
| No | 1 (5) | 2 (10) | 1 (5.6) | 2 (9.5) | 1 (10) | |
| I don’t know | 19 (95) | 18 (90) | 17 (94.4) | 19 (90.5) | 9 (90) | |
| Are farmers affected by the apefly? | 49.554 (<0.001) * | |||||
| Yes | 2 (10) | 20 (100) | 6 (33.3) | 7 (33.3) | - | |
| No | 18 (90) | - | 12 (66.7) | 14 (66.7) | 10 (100) | |
| Interventions? | 16.262 (<0.001) * | |||||
| Yes | 1 (5) | 3 (15) | 8 (44.4) | - | - | |
| No | 19 (95) | 17 (85) | 10 (55.6) | 20 (100) | 10 (100) | |
| How do you deal with the apefly? | 37.378 (<0.001) * | |||||
| Chemical spray | - | 3 (15) | 3 (16.7) | - | - | |
| Biological | - | - | 4 (22.2) | - | - | |
| Avoidance | 20 (100) | 17 (85) | 11 (61) | 20 (100) | 11 (100) | |
| Observation | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (100% Apefly Meal) |
|---|---|---|---|
| Changes in skin and fur | Null | Null | Null |
| Eyes | Normal | Normal | Normal |
| Respiratory activity | Normal | Normal | Normal |
| Tremors | Not observed | Not observed | Not observed |
| Convulsion | Did not occur | Did not occur | Did not occur |
| Salivation | Normal | Normal | Normal |
| Drowsiness | Did not occur | Did not occur | Did not occur |
| Comma | Did not occur | Did not occur | Did not occur |
| Death | Did not occur | Did not occur | Did not occur |
| Weight (g) | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (75% Apefly Meal) | p-Value |
|---|---|---|---|---|
| Day 0 | 26.77 ± 0.25 | 28.20 ± 0.66 | 28.20 ± 0.66 | 0.030 |
| Day14 | 33.23 ± 0.68 | 33.60 ± 1.21 | 31.83 ± 1.01 | 0.149 |
| Parameters | Control (0% Apefly) | Treatment 1 (50% Apefly) | Treatment 2 (75% Apefly) | Treatment 3 (100% Apefly) | p-Value |
|---|---|---|---|---|---|
| WBC M/mm3 | 4.81 ± 0.2 | 4.54 ± 0.68 | 4.62 ± 0.18 | 4.97 ± 0.57 | 0.474 |
| LYM% | 80.8 ± 1.3 | 82.2 ± 3.77 | 85.8 ± 7.09 | 80.4 ± 3.21 | 0.233 |
| RBC M/mm3 | 5.3 ± 2.03 | 5.72 ± 2.64 | 4.16 ± 0.57 | 4.37 ± 0.51 | 0.440 |
| MCV (pg) | 32.16 ± 9.49 | 36.82 ± 11.26 | 32.66 ± 7.83 | 41.1 ± 18.59 | 0.650 |
| MCH (pg) | 31 ± 1.67 | 31.46 ± 2 | 31.26 ± 0.67 | 30.08 ± 2.38 | 0.639 |
| MCHC (g/dL) | 32.5 ± 0.38 | 32.62 ± 0.68 | 33.12 ± 2.35 | 31.96 ± 1.43 | 0.652 |
| Hb (g/dL) | 14.1 ± 1.58 | 12.94 ± 1.1 | 13.7 ± 2.37 | 13.18 ± 1.38 | 0.699 |
| Weight (g) | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (75% Apefly Meal) | Treatment 3 (100% Apefly Meal) | p-Value |
|---|---|---|---|---|---|
| Day 0 | 27.12 ± 0.54 | 27.92 ± 0.62 | 27.76 ± 0.64 | 27.92 ± 0.62 | 0.158 |
| Day 14 | 33.66 ± 0.87 | 33.32 ± 1.22 | 33.72 ± 1.80 | 32.40 ± 1.07 | 0.369 |
| Day 28 | 42.20 ± 1.38 | 40.72 ± 2.22 | 41.68 ± 3.36 | 38.88 ± 2.36 | 0.187 |
| Organ | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (75% Apefly Meal) | Treatment 3 (100% Apefly Meal) | p-Value |
|---|---|---|---|---|---|
| Spleen | 0.2 ± 0 | 0.18 ± 0.02 | 0.18 ± 0.1 | 0.16 ± 0.03 | 0.807 |
| Liver | 1.96 ± 0.01 | 1.97 ± 0.01 | 1.99 ± 0.03 | 1.97 ± 0.02 | 0.001 |
| Kidney | 0.57 ± 0.01 | 0.56 ± 0.01 | 0.57 ± 0.02 | 0.55 ± 0.01 | 0.281 |
| Heart | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.34 ± 0.38 | 0.17 ± 0.01 | 0.425 |
| Organ | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (75% Apefly Meal) | Treatment 3 (100% Apefly Meal) | p-Value |
|---|---|---|---|---|---|
| Spleen | 0.46 ± 0.01 | 0.44 ± 0.04 | 0.41 ± 0.2 | 0.42 ± 0.05 | 0.859 |
| Liver | 3.94 ± 0.11 | 4.86 ± 0.24 | 4.79 ± 0.32 | 5.08 ± 0.27 | <0.001 |
| Kidney | 1.35 ± 0.03 | 1.38 ± 0.05 | 1.36 ± 0.07 | 1.43 ± 0.07 | 0.174 |
| Heart | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.77 ± 0.78 | 0.43 ± 0.01 | 0.422 |
| Parameters | Control (0% Apefly Meal) | Treatment 1 (50% Apefly Meal) | Treatment 2 (75% Apefly Meal) | Treatment 3 (100% Apefly Meal) | p-Value |
|---|---|---|---|---|---|
| Ap | 64.8 ± 1.30 | 65.4 ± 2.70 | 64.8 ± 0.84 | 64.2 ± 1.10 | 0.727 |
| Cr | 0.88 ± 0.12 | 0.98 ± 0.24 | 0.8 ± 0.05 | 0.86 ± 0.17 | 0.373 |
| AST | 22.84 ± 3.00 | 18.26 ± 3.04 | 23.18 ± 11.04 | 23 ± 4.48 | 0.562 |
| ALT | 21.24 ± 2.57 | 18.68 ± 1.17 | 19.52 ± 1.53 | 18.86 ± 1.31 | 0.120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasari, S.P.; Ndakidemi, P.A.; Mbega, E.R. Assessment of the Knowledge, Perceptions, and Reactions towards the African Apefly (Spalgis lemolea lemolea) in Tanzania. Sustainability 2020, 12, 942. https://doi.org/10.3390/su12030942
Nasari SP, Ndakidemi PA, Mbega ER. Assessment of the Knowledge, Perceptions, and Reactions towards the African Apefly (Spalgis lemolea lemolea) in Tanzania. Sustainability. 2020; 12(3):942. https://doi.org/10.3390/su12030942
Chicago/Turabian StyleNasari, Sayuni P., Patrick A. Ndakidemi, and Ernest R. Mbega. 2020. "Assessment of the Knowledge, Perceptions, and Reactions towards the African Apefly (Spalgis lemolea lemolea) in Tanzania" Sustainability 12, no. 3: 942. https://doi.org/10.3390/su12030942
APA StyleNasari, S. P., Ndakidemi, P. A., & Mbega, E. R. (2020). Assessment of the Knowledge, Perceptions, and Reactions towards the African Apefly (Spalgis lemolea lemolea) in Tanzania. Sustainability, 12(3), 942. https://doi.org/10.3390/su12030942




