From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Catalyst Synthesis
2.2.2. Catalyst Heat Treatment
2.2.3. Catalytic Test
2.2.4. Characterization of the Catalyst
3. Results and Discussion
3.1. Characterization of the ZnO-Ni0.5Zn0.5Fe2O4 Catalyst
3.1.1. X-ray Diffraction
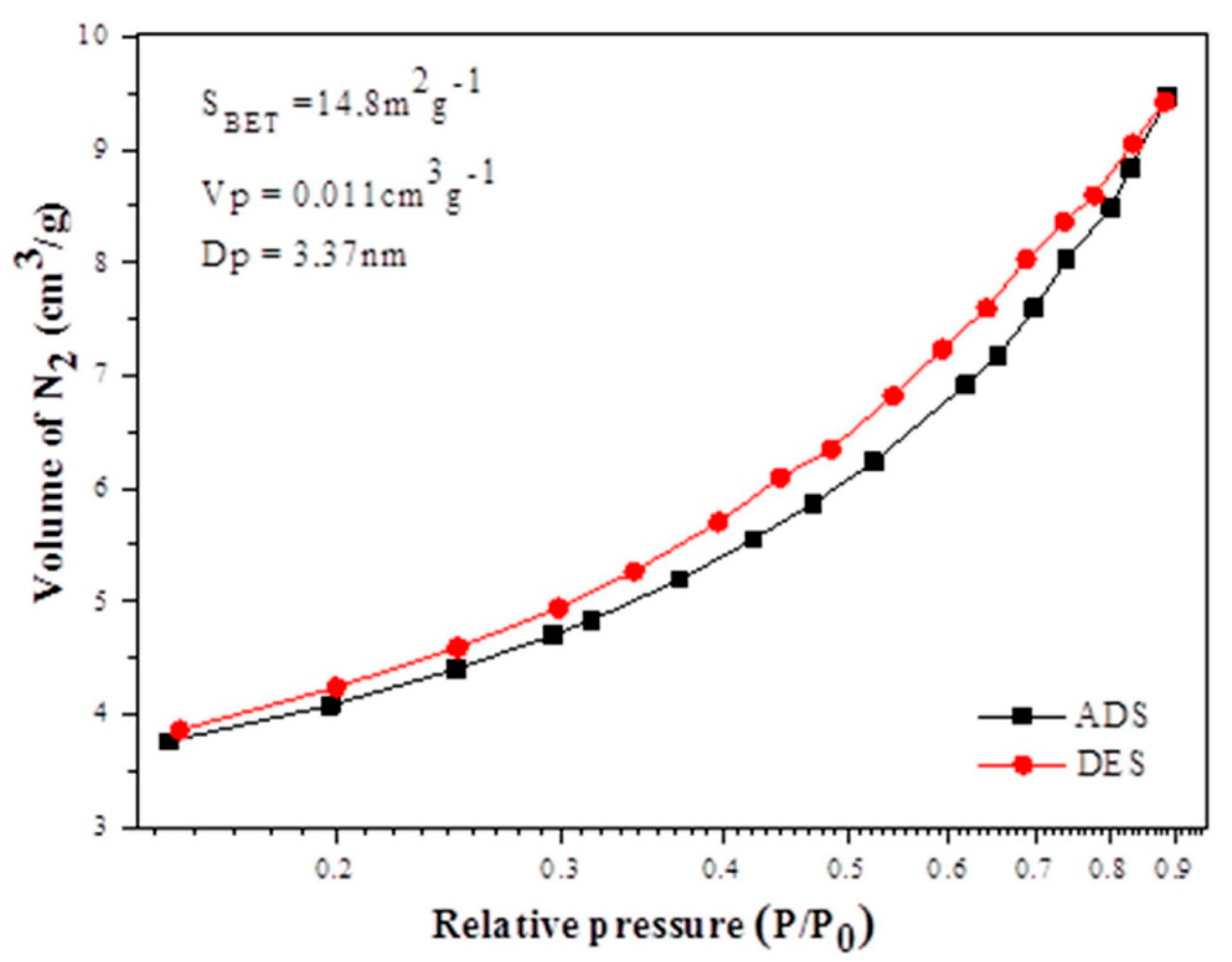
3.1.2. Texture Analysis
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Granulometric Analysis
3.1.5. Fourier Transform Infrared Spectroscopy (FTIR) and Raman
3.1.6. Thermogravimetry (TG)
3.1.7. Magnetic Evaluation
3.1.8. NH3 Temperature Programmed Desorption Analysis (TPD)
3.1.9. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luna, C.B.B.; Siqueira, D.D.; Ferreira, E.d.S.B.; da Silva, W.A.; Nogueira, J.A.d.S.; Araújo, E.M. From disposal to technological potential: Reuse of polypropylene waste from industrial containers as a polystyrene impact modifier. Sustainability 2020, 12, 5272. [Google Scholar] [CrossRef]
- Sales, H.B.; Menezes, R.R.; Neves, G.A.; Souza, J.J.N.d.; Ferreira, J.M.; Chantelle, L.; Oliveira, A.L.M.d.; Lira, H.d.L. Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents. Sustainability 2020, 12, 7393. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, V.; Sharma, Y.C. Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Conv. Manag. 2017, 148, 1438–1452. [Google Scholar] [CrossRef]
- De Campos, T.M.P.; Antunes, M.C. Cadeia reversa do óleo de cozinha residual: O papel do Ponto de Entrega Voluntária (PEV). Dig. Re Vista 2018, 3, 96–111. [Google Scholar]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Mandatory recycling of waste cooking oil from residential and commercial sectors in Taiwan. Resources 2019, 8, 38. [Google Scholar] [CrossRef]
- Said, N.H.; Ani, F.N.; Said, M.F.M. Review of the production of biodiesel from waste cooking oil using solid catalysts. J. Mech. Eng. Sci. 2015, 8, 1302–1311. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Huang, Y.; Li, M.; Lin, X.; Qiu, T. Reusable and efficient heterogeneous catalysts for biodiesel production from free fatty acids and oils: Self-solidifying hybrid ionic liquids. Energy 2020, 211, 118631. [Google Scholar] [CrossRef]
- Gopan, S.N.; Rajan, A.V.; Krishnan, B.R. Review of Bio-diesel production from waste cooking oil and analyze the IC engine performance. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Mandolesi De Araújo, C.D.; De Andrade, C.C.; De Souza, E.; Silva, E.; Dupas, F.A. Biodiesel production from used cooking oil: A review. Renew. Sustain. Energy Rev. 2013, 27, 445–452. [Google Scholar] [CrossRef]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management—A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Mapossa, A.B.; Dantas, J.; Diniz, V.C.S.; Silva, M.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M.; Federal, U.; Grande, D.C.; Grande, C.; Africa, S. Síntese e caracterização do ferroespinélio Ni0.7Zn0.3Fe2O4: Avaliação de desempenho na esterificação metílica e etílica. Cerâmica 2017, 63, 223–232. [Google Scholar] [CrossRef][Green Version]
- Enweremadu, C.C.; Mbarawa, M.M. Technical aspects of production and analysis of biodiesel from used cooking oil-A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C. The potential of waste cooking oil-based biodiesel using heterogeneous catalyst derived from various calcined eggshells coupled with an emulsification technique: A review on the emission reduction and engine performance. Renew. Sustain. Energy Rev. 2015, 47, 589–603. [Google Scholar] [CrossRef]
- Wang, L.; Dong, X.; Jiang, H.; Li, G.; Zhang, M. Ordered mesoporous carbon supported ferric sulfate: A novel catalyst for the esterification of free fatty acids in waste cooking oil. Fuel Process. Technol. 2014, 128, 10–16. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Gardy, J.; Nourafkan, E.; Osatiashtiani, A.; Lee, A.F.; Wilson, K.; Hassanpour, A.; Lai, X. A core-shell SO4/Mg-Al-Fe3O4 catalyst for biodiesel production. Appl. Catal. B Environ. 2019, 259, 118093. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 103219. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Silva, A.S.; Costa, A.C.F.d.M. Synthesis, characterization and catalytic performance of mixed nanoferrites submitted to transesterification and esterification reaction using methyl and ethyl route for biodiesel production. Rev. Mater. 2016, 21, 1080–1093. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, M.; Liu, Y.; Fan, M.; Jiang, P.; Dong, Y. Sr doping magnetic CaO parcel ferrite improving catalytic activity on the synthesis of biodiesel by transesterification. Fuel 2016, 186, 787–791. [Google Scholar] [CrossRef]
- Ashok, A.; Kennedy, L.J.; Vijaya, J.J. Structural, optical and magnetic properties of Zn1−xMnxFe2O4 (0 ≤ x ≤ 0.5) spinel nano particles for transesterification of used cooking oil. J. Alloys Compd. 2019, 780, 816–828. [Google Scholar] [CrossRef]
- Ashok, A.; Kennedy, L.J. Magnetically Separable Zinc Ferrite Nanocatalyst for an Effective Biodiesel Production from Waste Cooking Oil. Catal. Lett. 2019, 149, 3525–3542. [Google Scholar] [CrossRef]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Sano, N.; Yamada, K.; Tsunauchi, S.; Tamon, H. A novel solid base catalyst for transesterification of triglycerides toward biodiesel production: Carbon nanohorn dispersed with calcium ferrite. Chem. Eng. J. 2017, 307, 135–142. [Google Scholar] [CrossRef]
- Ashok, A.; Ratnaji, T.; John Kennedy, L.; Judith Vijaya, J.; Gnana Pragash, R. Magnetically recoverable Mg substituted zinc ferrite nanocatalyst for biodiesel production: Process optimization, kinetic and thermodynamic analysis. Renew. Energy 2020, 163, 480–494. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel production by aspergillus Niger lipase immobilized on barium ferrite magnetic nanoparticles. Bioengineering 2016, 3, 14. [Google Scholar] [CrossRef]
- Pontes, J.R.M.; Leal, E.; Costa, A.C.F.M. Estudo da atividade catalítica do compósito cerâmico magnético γ-Fe2O3/Ba3Co2Fe24O41 para produção de biodiesel. Rev. Eletr. Mater. Process. 2020, 15, 43–49. [Google Scholar]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Farias, A.F.F.; Pontes, J.R.M.; Rodrigues, A.M.; Costa, A.C.F.d.M. Synthesis of the ZnO-Ni0.5Zn0.5Fe2O4-Fe2O3 magnetic catalyst in pilot-scale by combustion reaction and its application on the biodiesel production process from oil residual. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Costa, A.C.F.M.; Kiminamin, R.H.G.A. Dispositivo para a produção de manocompósitos cerâmicos em larga escala por reação de combustão e processo contínuo de produção de nanocompósitos. Rev. Propr. Ind. RPI 2012, BR 10 2012, 002181-3. [Google Scholar]
- Jain, S.R.; Adiga, K.C.; Pai Verneker, V.R. A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame 1981, 40, 71–79. [Google Scholar] [CrossRef]
- Avila, P.R.T.; da Silva, E.P.; Rodrigues, A.M.; Aristizabal, K.; Pineda, F.; Coelho, R.S.; Garcia, J.L.; Soldera, F.; Walczak, M.; Pinto, H.C. On manufacturing multilayer-like nanostructures using misorientation gradients in PVD films. Sci. Rep. 2019, 9, 15898. [Google Scholar] [CrossRef] [PubMed]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974; ISBN 978-0-471-49369-3. [Google Scholar]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Pereira da Costa, F.; Rodrigues da Silva Morais, C.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Costa, F.P.d.; Morais, C.R.d.S.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Olhero, S.M.; Soma, D.; Amaral, V.S.; Button, T.W.; Alves, F.J.; Ferreira, J.M.F. Co-precipitation of a Ni-Zn ferrite precursor powder: Effects of heat treatment conditions and deagglomeration on the structure and magnetic properties. J. Eur. Ceram. Soc. 2012, 32, 2469–2476. [Google Scholar] [CrossRef]
- Hajalilou, A.; Hashim, M.; Mohamed Kamari, H. Structure and magnetic properties of Ni0.64Zn0.36Fe2O4 nanoparticles synthesized by high-energy milling and subsequent heat treatment. J. Mater. Sci. Mater. Electron. 2015, 26, 1709–1718. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Kiminami, R.H.G.A.; Silva, M.R.; Costa, A.C.F.M. Síntese do ferroespinélio ZnFe2O4 e avaliação do seu desempenho em reações de esterificação e transesterificação via rota metílica. Rev. Eletr. Mater. Process. 2015, 10, 137–143. [Google Scholar]
- Li, J.; Ng, D.H.L.; Song, P.; Song, Y.; Kong, C. Bio-inspired synthesis and characterization of mesoporous ZnFe2O4 hollow fibers with enhancement of adsorption capacity for acid dye. J. Ind. Eng. Chem. 2015, 23, 290–298. [Google Scholar] [CrossRef]
- Abdullah Dar, M.; Shah, J.; Siddiqui, W.A.; Kotnala, R.K. Study of structure and magnetic properties of Ni–Zn ferrite nano-particles synthesized via co-precipitation and reverse micro-emulsion technique. Appl. Nanosci. 2014, 4, 675–682. [Google Scholar] [CrossRef]
- Salvador, V.L.R. Separação de Efeitos de Sobreposição de Espectros Obtidos por Wdxrf Usando o Método de Rietveld Vera Lucia Ribeiro Salvador; Instituto de Pesquisas Energéticas e Nucleares: São Paulo, Brazil, 2005. [Google Scholar]
- Pauluk, S. Estudo da Estrutura Cristalina de Pigmentos de Zirconitas a Partir dos Sistemas ZrSiO4-Co3O4 e ZrSiO4-Cr2O3 Utilizando o Método de Rietveld; Universidade de Ponta Grossa: Ponta Grossa, Brazil, 2008. [Google Scholar]
- ALOthman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Dantas, J.; Silva, A.S.A.; Costa, A.C.F.M.; Freitas, N.L. Síntese, caracterização dos espinélios NiFe2O4 e CoFe2O4 e avaliação do desempenho na transesterificação e esterificação do óleo de algodão. Rev. Eletr. Mater. Process. 2012, 7, 174–179. [Google Scholar]
- Silva, A.L.; Luna, C.B.B.; Chaves, A.C.; Neves, G.A. Avaliação de novos depósitos de argilas provenientes da região sul do Amapá visando aplicação na indústria cerâmica. Ceramica 2018, 64, 69–78. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Luna, C.B.B.; Chaves, A.C.; Neves, G.d.A. Caracterização tecnológica de novos depósitos de argilas da região sul do Amapá visando aplicações na indústria Cerâmica. Rev. Mater. 2017, 22. [Google Scholar] [CrossRef][Green Version]
- Michot, L.J.; Bihannic, I.; Thomas, F.; Lartiges, B.S.; Waldvogel, Y.; Caillet, C.; Thieme, J.; Funari, S.S.; Levitz, P. Coagulation of Na-montmorillonite by inorganic cations at neutral pH. A combined transmission X-ray microscopy, small angle and wide angle X-ray scattering study. Langmuir 2013, 29, 3500–3510. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Samoila, P.M.; Nica, V.; Doroftei, F.; Iordan, A.R.; Palamaru, M.N. Study of the chelating/fuel agents influence on NiFe2O4 samples with potential catalytic properties. Powder Technol. 2013, 243, 9–17. [Google Scholar] [CrossRef]
- Jandl, S.; Deslandes, J. Infrared spectra of HfS3. Phys. Rev. B 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Lazarević, Z.Z.; Jovalekić, C.; Milutinović, A.; Sekulić, D.; Slankamenac, M.; Romčević, M.; Romčević, N.Z. Study of nife2O4 and ZnFe2O4 spinel ferrites prepared by soft mechanochemical synthesis. Ferroelectrics 2013, 448, 1–11. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Preparation and properties of nickel ferrite (NiFe2O4) nanoparticles via sol-gel auto-combustion method. Mater. Res. Bull. 2011, 46, 2204–2207. [Google Scholar] [CrossRef]
- Vieira, D.A.; Diniz, V.C.S.; Cornejo, D.R.; de Melo Costa, A.C.F.; Kiminami, R.H.G.A. Study of the Reproducibility of Ni-Zn Nanoferrite Obtained by Combustion Reaction. Mater. Sci. Forum 2014, 775–776, 415–420. [Google Scholar] [CrossRef]
- Lima, D.S.; Perez-Lopez, O.W. Conversão catalítica do etanol sobre catalisadores suportados em ZSM-5. Ceramica 2018, 64, 1–9. [Google Scholar] [CrossRef]
- Sawa, M.; Niwa, M.; Murakami, Y. Relationship between acid amount and framework aluminum content in mordenite. Zeolites 1990, 10, 532–538. [Google Scholar] [CrossRef]
- Masiero, S.S.; Marcilio, N.R.; Perez-Lopez, O.W. Aromatization of methane over Mo-Fe/ZSM-5 catalysts. Catal. Lett. 2009, 131, 194–202. [Google Scholar] [CrossRef]
- Holanda, A. Biodiesel e Inclusão Social; Coordenação de Publicações: Brasília, Brazil, 2004; Volume 26. [Google Scholar]
- Dantas, J.; Leal, E.; Mapossa, A.; Cornejo, D.; Costa, A. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]










| Sample | Medium Crystallinity (%) | Average Crystallite Size (nm) |
|---|---|---|
| ZnO-Ni0.5Zn0.5Fe2O4 - Fe2O3 | 43.0 | 25.0 |
| ZnO-Ni0.5Zn0.5Fe2O4-Fe2O3 (650 °C) | 46.5 | 22.8 |
| ZnO-Ni0.5Zn0.5Fe2O4 - Fe2O3 (700 °C) | 44.4 | 26.5 |
| ZnO-Ni0.5Zn0.5Fe2O4 (800 °C) | 54.7 | 32.0 |
| Sample | Lattice Parameters | Crystal System | Percentage of Phases Present (%) | Rwp | Rexp | GOF |
|---|---|---|---|---|---|---|
| ZnO-Ni0.5Zn0.5Fe2O4 (800 °C) | a = b = c = 8.38 | Cubic (Ni-Zn) ferrite | 99.54 | 0.92 | 0.35 | 2.61 |
| Referência1 | ||||||
| a = b = c = 8.38 | ||||||
| a = 3.24 e c = 5.19 | Hexagonal ZnO | 0.46 | ||||
| Referência1 | ||||||
| a = 3.24 e c = 5.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.L.; Luna, C.B.B.; de Farias, A.F.F.; de Medeiros, S.A.S.L.; Meneghetti, S.M.P.; Rodrigues, A.M.; Costa, A.C.F.d.M. From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability 2020, 12, 10159. https://doi.org/10.3390/su122310159
da Silva AL, Luna CBB, de Farias AFF, de Medeiros SASL, Meneghetti SMP, Rodrigues AM, Costa ACFdM. From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability. 2020; 12(23):10159. https://doi.org/10.3390/su122310159
Chicago/Turabian Styleda Silva, Adriano Lima, Carlos Bruno Barreto Luna, Ana Flávia Félix de Farias, Suelen Alves Silva Lucena de Medeiros, Simoni Margareti Plentz Meneghetti, Alisson Mendes Rodrigues, and Ana Cristina Figueiredo de Melo Costa. 2020. "From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst" Sustainability 12, no. 23: 10159. https://doi.org/10.3390/su122310159
APA Styleda Silva, A. L., Luna, C. B. B., de Farias, A. F. F., de Medeiros, S. A. S. L., Meneghetti, S. M. P., Rodrigues, A. M., & Costa, A. C. F. d. M. (2020). From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability, 12(23), 10159. https://doi.org/10.3390/su122310159








