Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Analytical Methods
2.2.1. Phsicochemical Properties of Soils
2.2.2. Ecological Assessment
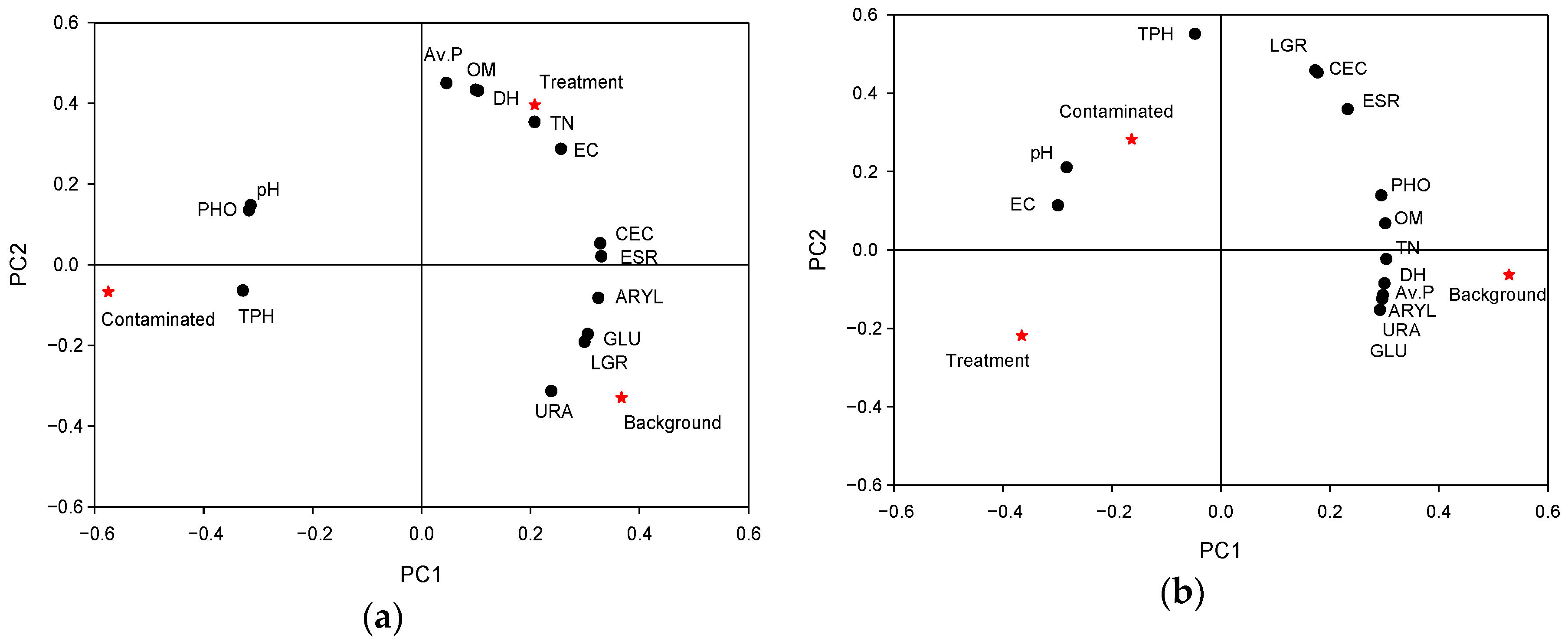
2.3. Data Analysis
3. Results
3.1. Changes in Physico-Chemical Properties of Soils
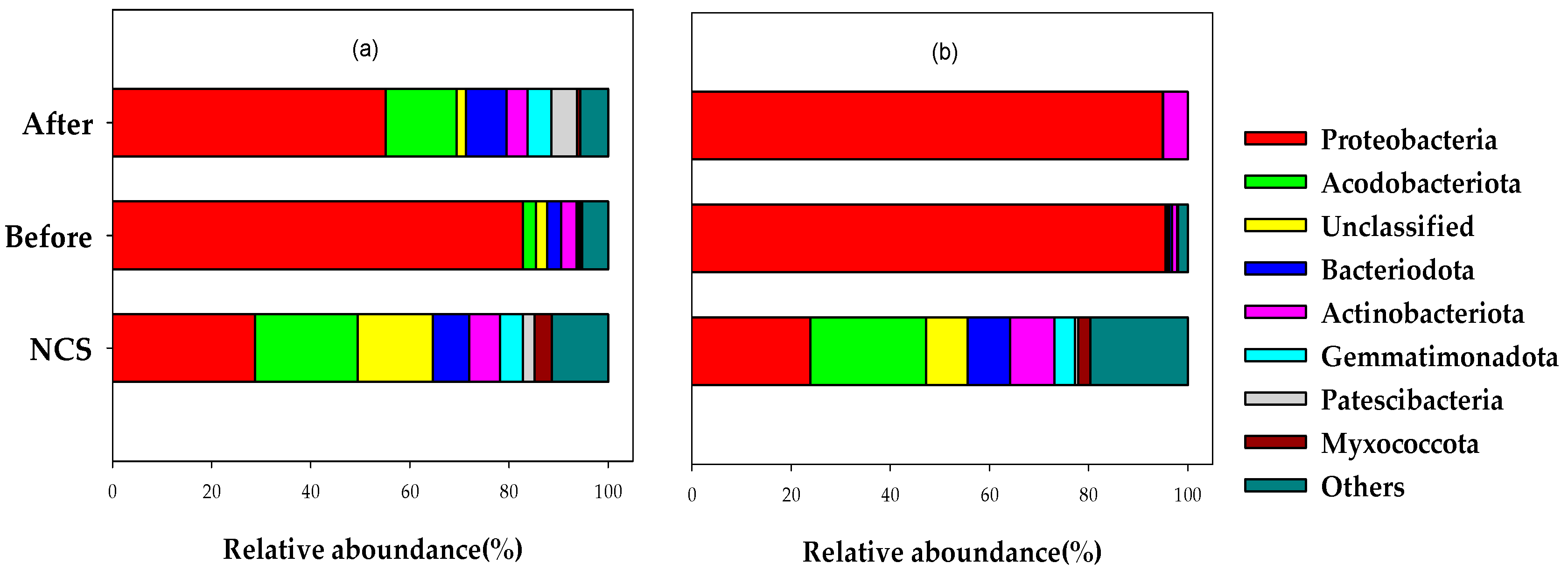
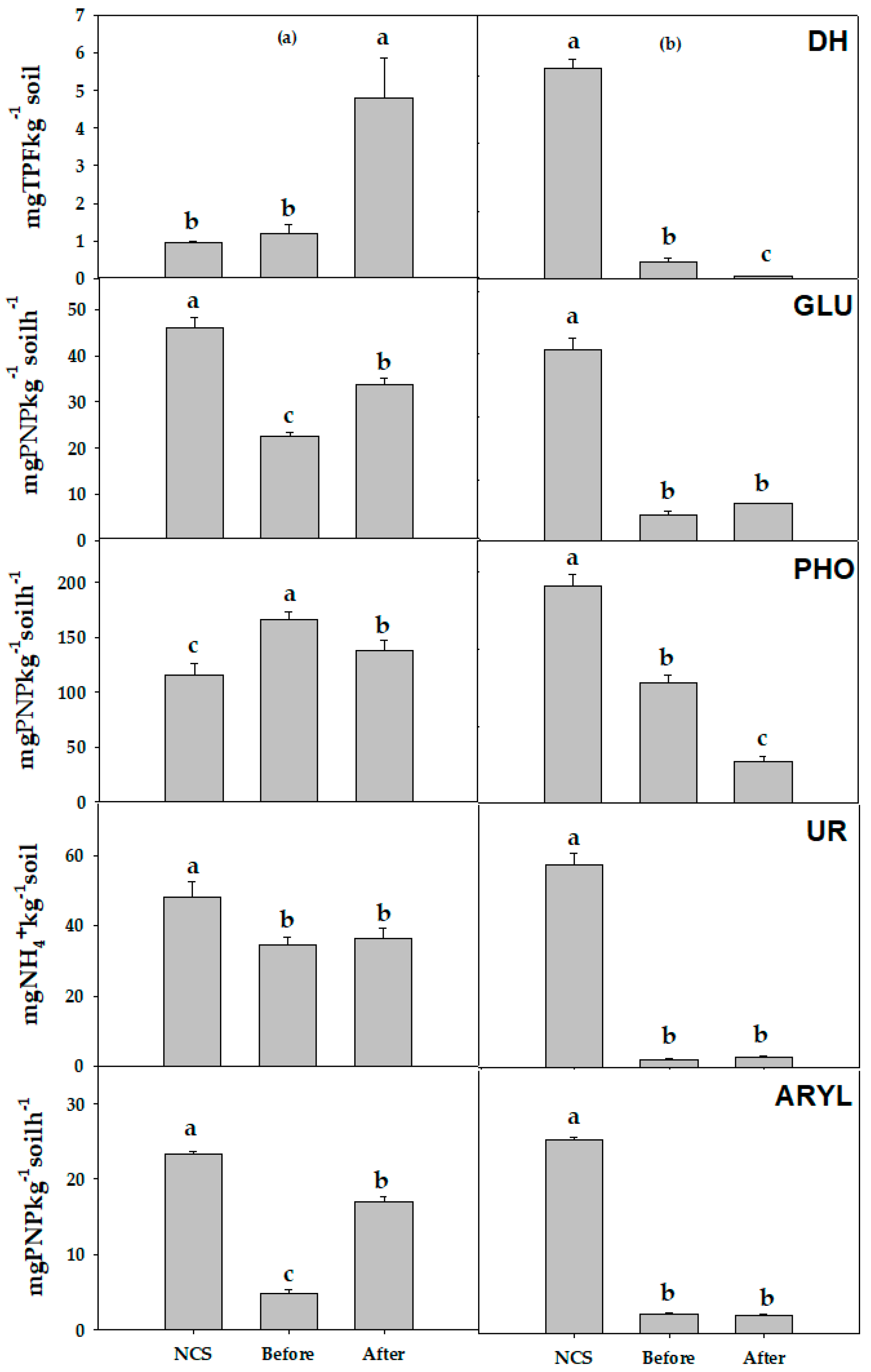
3.2. Changes in Ecological Properties of Soils
3.2.1. Microbial Community
3.2.2. Soil Enzyme Activities
3.2.3. Plant Toxicity Assay
3.2.4. Earthworm Toxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gidarakos, E.; Aivalioti, M. Large scale and longterm application of bioslurping: The case of Greek petroleum refinery site. J. Hazard. Mater. 2007, 149, 574–581. [Google Scholar] [CrossRef]
- Iturbe, R.; Flores, C.; Castro, A.; Torres, L.G. Sub-soil contamination due to oil spills in six oil-pipeline pumping station in northern Mexico. Chemosphere 2007, 68, 893–906. [Google Scholar] [CrossRef]
- White, P.A.; Claxton, L.D. Mutagens in contaminated soil: A review. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef]
- Haaper, P.; Tuhkanen, T. Integrated treatment of PAH contaminated soils by soil washing, Ozonation and biological treatment. J. Hazard. Mater. 2006, B136, 244–250. [Google Scholar] [CrossRef]
- Perfumo, A.; Banat, I.M.; Marchant, R.; Vezzulli, L. Thermally enhanced approaches for bioremediation of hydrocarbon-contaminated soils. Chemosphere 2007, 66, 179–184. [Google Scholar] [CrossRef]
- Ossai, I.C.; Aziz, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Jelusic, M.; Grcman, H.; Vodnik, D.; Suhadolc, M.; Lestan, D. Functioning of metal contaminated garden soil after remediation. Environ. Pollut. 2013, 174, 63–70. [Google Scholar] [CrossRef]
- Banwart, S. Save our soils. Nature 2011, 474, 151–152. [Google Scholar] [CrossRef]
- Sierra, C.; Martínez-Blanco, D.; Blanco, J.A.; Gallego, J.R. Optimization of magnetic separation: A case study for soil washing at a heavy metal polluted site. Chemosphere 2014, 107, 290–296. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. Pb, Zn and Cd mobility, availability and fractionation in aged soil remediated by EDTA leaching. Chemosphere 2009, 74, 1367–1373. [Google Scholar] [CrossRef]
- Villa, R.D.; Trovó, A.G.; Nogueira, R.F.P. Soil remediation using a coupled process: Soil washing with surfactant followed by photo-Fenton oxidation. J. Hazard. Mater. 2010, 174, 770–775. [Google Scholar] [CrossRef]
- Tang, X.; He, L.Y.; Dang, Z.; Guo, C.L.; Lu, G.N.; Yi, X.Y. Construction of an artificial microalgal-bacterial consortium that efficiently degrades crude oil. J. Hazard. Mater. 2010, 181, 1158–1162. [Google Scholar] [CrossRef]
- Al-Mutairi, N.; Bufarsan, A.; Al-Rukaibi, F. Ecorisk evaluation and treatability potential of soils contaminated with petroleum hydrocarbon-based fuels. Chemosphere 2008, 74, 142–148. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef]
- Mremner, J.M. Nitrogen-total. In Method of Soil Analysis: Part III-Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Method of Soil Analysis: Part III-Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Method of Soil Analysis: Part III-Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1947, 59, 39–45. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soil. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- OECD. Terrestrial plant test: Seedling emergence and seedling growth test. In OECD/OCDE Guidelines for the Testing of Chemical Section 2. Effects on Biotic Systems; Test No. 208.; Organization for Economic and Cooperation Development (OECD): Paris, France, 2006. [Google Scholar]
- OECD. Earthworm acute toxicity test. In OECD/OCDE Guidelines for the Testing of Chemical Section 2. Effects on Biotic Systems; Test No. 207.; Organization for Economic and Cooperation Development (OECD): Paris, France, 1984. [Google Scholar]
- Jurelevicius, D.; Alvarez, V.M.; Marques, J.M.; Fonseca de Sousa Lima, L.R.; Dias, F.D.A.; Seldin, L. Bacterial community response to petroleum hydrocarbon amendments in freshwater, marine, and hypersaline water-containing microcosms. Appl. Environ. Microbiol. 2013, 79, 5927–5935. [Google Scholar] [CrossRef]
- Barcenas-Moreno, G.; Baath, E. Bacterial and fungal growth in soil heated at different temperatures to simulate a range of fire intensities. Soil Biol. Biochem. 2009, 41, 2517–2526. [Google Scholar] [CrossRef]
- Guerrero, C.; Mataix-Solera, J.; Gomez, I.; Garcia-Orenes, F.; Jordan, M.M. Microbial recolonization and chemical changes in a soil heated at different temperatures. Int. J. Wildland Fire 2005, 14, 385–400. [Google Scholar] [CrossRef]
- Pape, A.; Switzer, C.; McCosh, N.; Knapp, C.W. Impacts of thermal and smouldering remediation on plant growth and soil ecology. Geoderma 2015, 243–244, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, X.; Zhou, L.; Lin, Y. Biological indicators capable of assessing thermal treatment efficiency of hydrocarbon mixture-contaminated soil. Chemosphere 2010, 80, 837–844. [Google Scholar] [CrossRef]
- Xu, J.G.; Johnson, R.L. Root growth, microbial activity and phosphatase activity in oil-contaminated, remediated and uncontaminated soils planted to barley and field pea. Plant Soil 1995, 173, 3–10. [Google Scholar] [CrossRef]
- Achuba, F.I. The effect of sublethal concentrations of crude oil on the growth and metabolism of cowpea (Vigna unguiculata) seedlings. Environmentalist 2006, 26, 17–20. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Influence of diesel fuel on seed germination. Environ. Pollut. 2002, 120, 363–370. [Google Scholar] [CrossRef]
- Douglas, G.S.; Hardenstine, J.H.; Liu, B.; Uhler, A.D. Laboratory and field verification of a method to estimate the extent of petroleum biodegradation in soil. Environ. Sci. Technol. 2012, 46, 8279–8287. [Google Scholar] [CrossRef]
- Maqbool, F.; Wang, Z.; JianZho, Y.X.; Gao, D.; Zhao, Y.G. Rhizodegradation of petroleum hydrocarbons by Sesbania cannabina in bioaugmented soil with free and immobilized consortium. J. Hazard. Mater. 2012, 237, 262–269. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Hund-Rinke, K.; Roembke, J. Assesment of ecotoxicity of contaminated soil using bioassays. In Manual for Soil Analysis-monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Berlin, Germany, 2005; pp. 321–359. [Google Scholar]
- Shakir Hanna, S.H.; Weaver, R. Earthworm survival in oil contaminated soil. Plant Soil 2002, 240, 127–132. [Google Scholar] [CrossRef]
- Ren, J.; Song, X.; Ding, D. Sustainable remediation of diesel-contaminated soil by low temperature thermal treatment: Improved energy efficiency and soil reusability. Chemosphere 2020, 241, 124951. [Google Scholar] [CrossRef]
| Parameter | LF 1 | HTTD 2 | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| pH | 8.53 ± 0.35a | 6.95 ± 0.08b | 9.75 ± 0.07a | 9.43 ± 0.03b |
| EC | 0.17 ± 0.05b | 0.45 ± 0.04a | 3.84 ± 0.14a | 3.89 ± 0.00a |
| OM 3 | 0.57 ± 0.18b | 1.04 ± 0.09a | 1.29 ± 0.06a | 0.76 ± 0.22b |
| TKN 4 | 0.03 ± 0.01b | 0.09 ± 0.01a | 0.04 ± 0.00a | 0.02 ± 0.00a |
| CEC 5 | 9.77 ± 0.08a | 9.84 ± 0.15a | 17.80 ± 0.10a | 15.72 ± 0.25b |
| Av.P. 6 | 3.85 ± 0.29b | 12.40 ± 0.48a | 0.76 ± 0.06a | 0.73 ± 0.04a |
| A.S. 7 | 10 ± 2b | 26 ± 1a | 17 ± 3a | 16 ± 1.1a |
| TPH 8 | 5491 ± 546a | 126 ± 9b | 528 ± 89a | N.D. 9 b |
| Phytotoxicity | LF 1 | HTTD 2 | ||||
|---|---|---|---|---|---|---|
| NCS 3 | Before | After | NCS | Before | After | |
| Germination (%) | 76.67 ± 4.71a | 23.33 ± 9.43b | 46.67 ± 9.43b | 76.67 ± 12.47a | 60.00 ± 8.16a | 36.67 ± 4.71b |
| Root length (cm plants−1) | 3.76 ± 0.15a | 3.43 ± 0.61a | 3.78 ± 0.10a | 2.70 ± 0.51c | 3.93 ± 0.53b | 4.34 ± 1.21a |
| Earthworm Toxicity | LF 1 | HTTD 2 | ||||
|---|---|---|---|---|---|---|
| NCS 3 | Before | After | NCS | Before | After | |
| Survival (%) | 96.67 ± 5.77a | 16.67 ± 5.77c | 86.67 ± 5.77b | 100 ± 0.00a | 96.67 ± 5.77a | 76.67 ± 5.28b |
| Body weight (g earthworm−1) | 0.22 ± 0.01a | 0.16 ± 0.01b | 0.21 ± 0.02a | 0.17 ± 0.01b | 0.15 ± 0.02c | 0.21 ± 0.01a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lee, J.H.; Jung, W.C.; Park, M.; Kim, M.S.; Lee, S.J.; Park, H. Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies. Sustainability 2020, 12, 10078. https://doi.org/10.3390/su122310078
Lee SH, Lee JH, Jung WC, Park M, Kim MS, Lee SJ, Park H. Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies. Sustainability. 2020; 12(23):10078. https://doi.org/10.3390/su122310078
Chicago/Turabian StyleLee, Sang Hwan, Jung Hyun Lee, Woo Chul Jung, Misun Park, Min Suk Kim, Seung Jae Lee, and Hyun Park. 2020. "Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies" Sustainability 12, no. 23: 10078. https://doi.org/10.3390/su122310078
APA StyleLee, S. H., Lee, J. H., Jung, W. C., Park, M., Kim, M. S., Lee, S. J., & Park, H. (2020). Changes in Soil Health with Remediation of Petroleum Hydrocarbon Contaminated Soils Using Two Different Remediation Technologies. Sustainability, 12(23), 10078. https://doi.org/10.3390/su122310078






