Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview
Abstract
1. Introduction
2. Development and Application of Neonics in Farmland Systems
2.1. History of the Development of Neonics
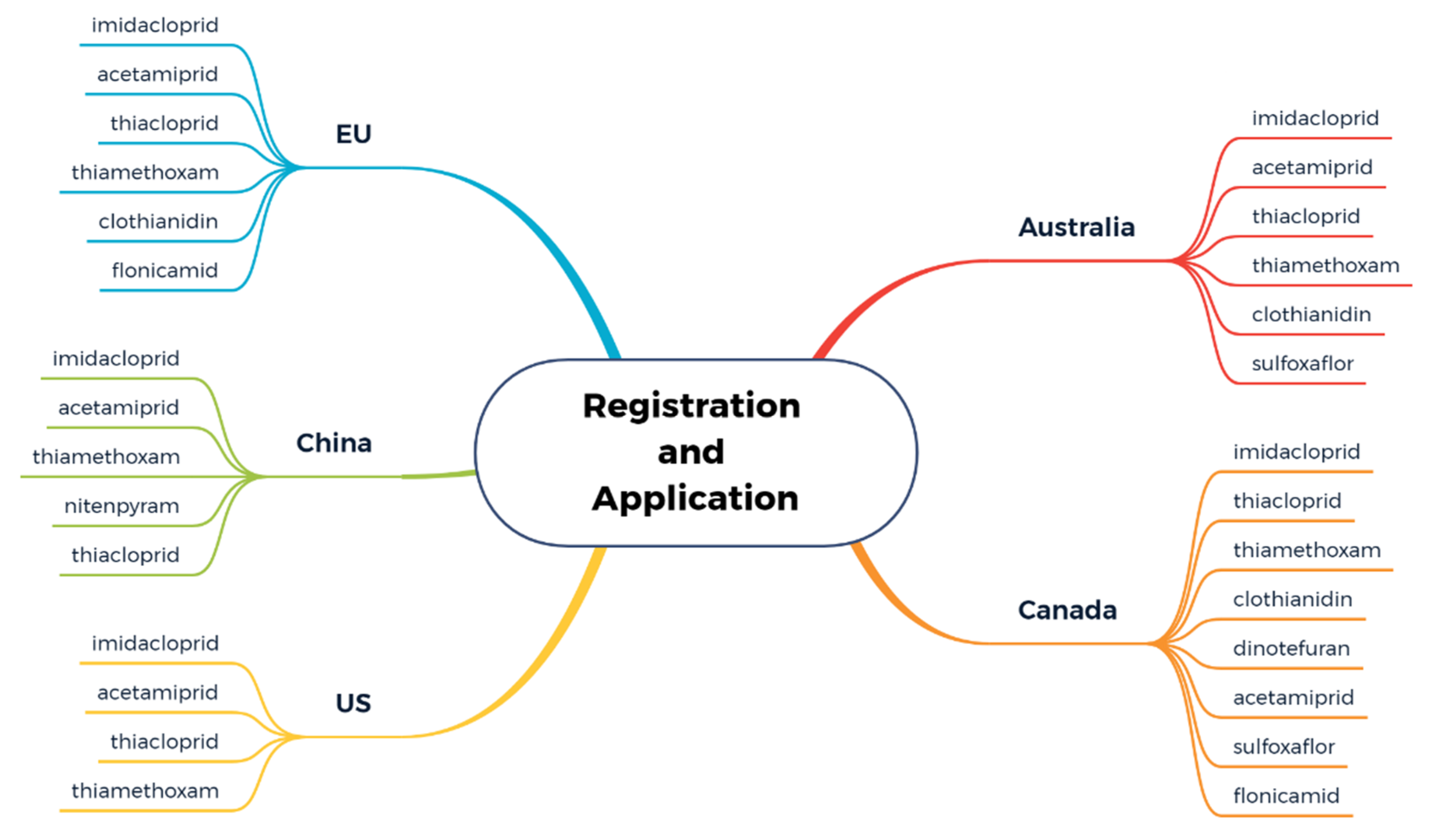
2.2. Application and Prohibition of Neonics in Farmland Systems of Different Regions
2.3. Analysis of the Similarities and Differences in Toxicity Mechanisms among Traditional Neonics, Novel Neonics, and Other Insecticides
3. Exposure Level and Potential Hazards of Neonics in the Food Chain
3.1. Exposure Level and Potential Hazards of Neonics in Producers
3.2. Exposure Level and Potential Hazards of Neonics in Primary Consumers
3.2.1. Exposure Level and Potential Hazards of Neonics in Bees
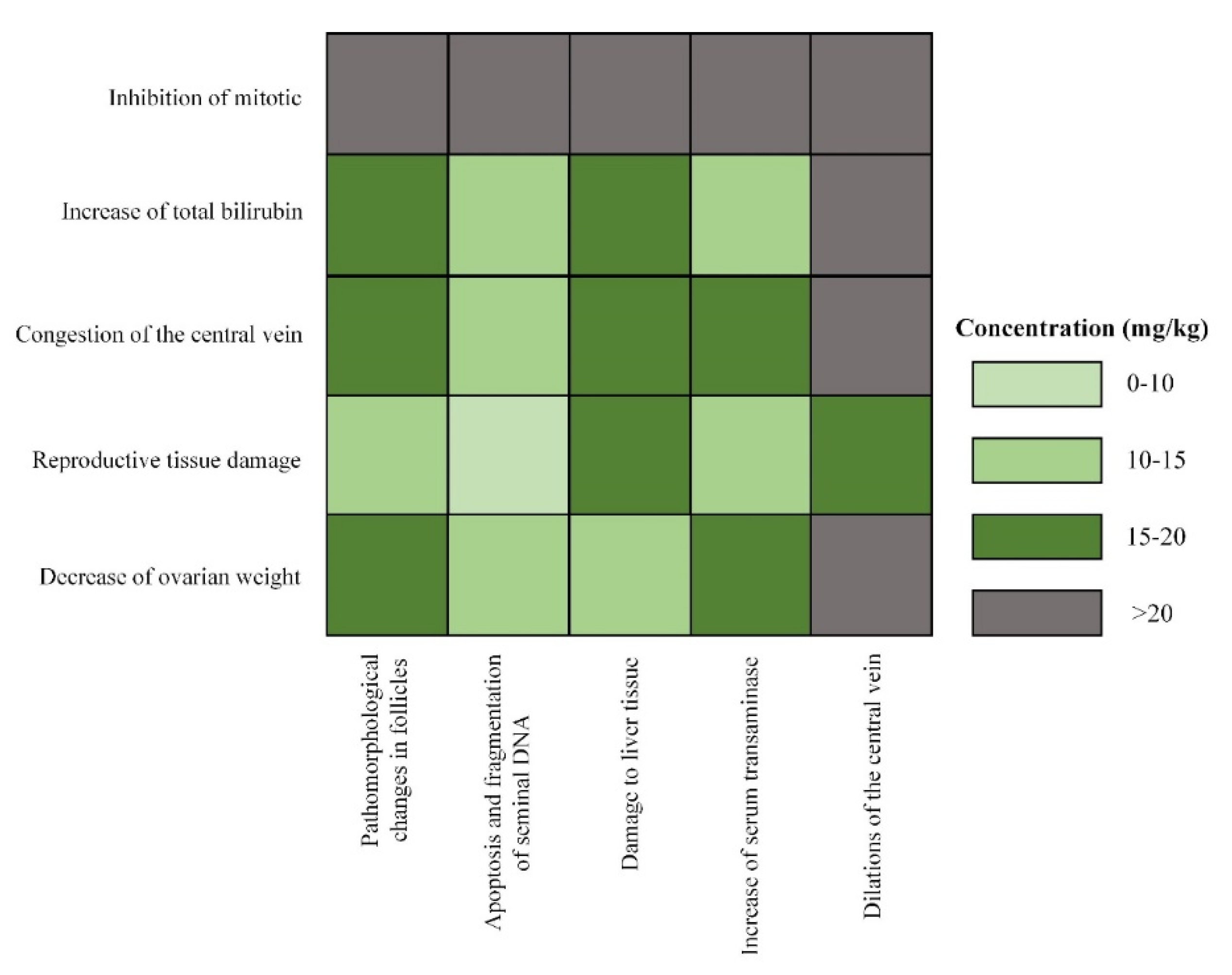
3.2.2. Exposure Level and Potential Hazards of Neonics in Rats
3.3. Exposure Level and Potential Hazards of Neonics in Secondary Consumers
3.4. Exposure Level and Potential Hazards of Neonics in Top-Level Consumers
4. Assessment of the Human Health Impact and Environmental Risk of Neonics
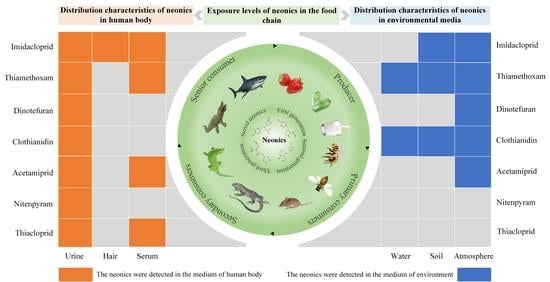
4.1. Distribution Characteristics and Assessment of the Health Impact of Neonics on the Human Body
4.1.1. Distribution Characteristics of Neonics in the Human Body
4.1.2. Assessment of the Impact of Neonics on Human Health
4.2. Distribution Characteristics and Risk Assessment of Neonics in Environmental Media
4.2.1. Distribution Characteristics of Neonics in Environmental Media
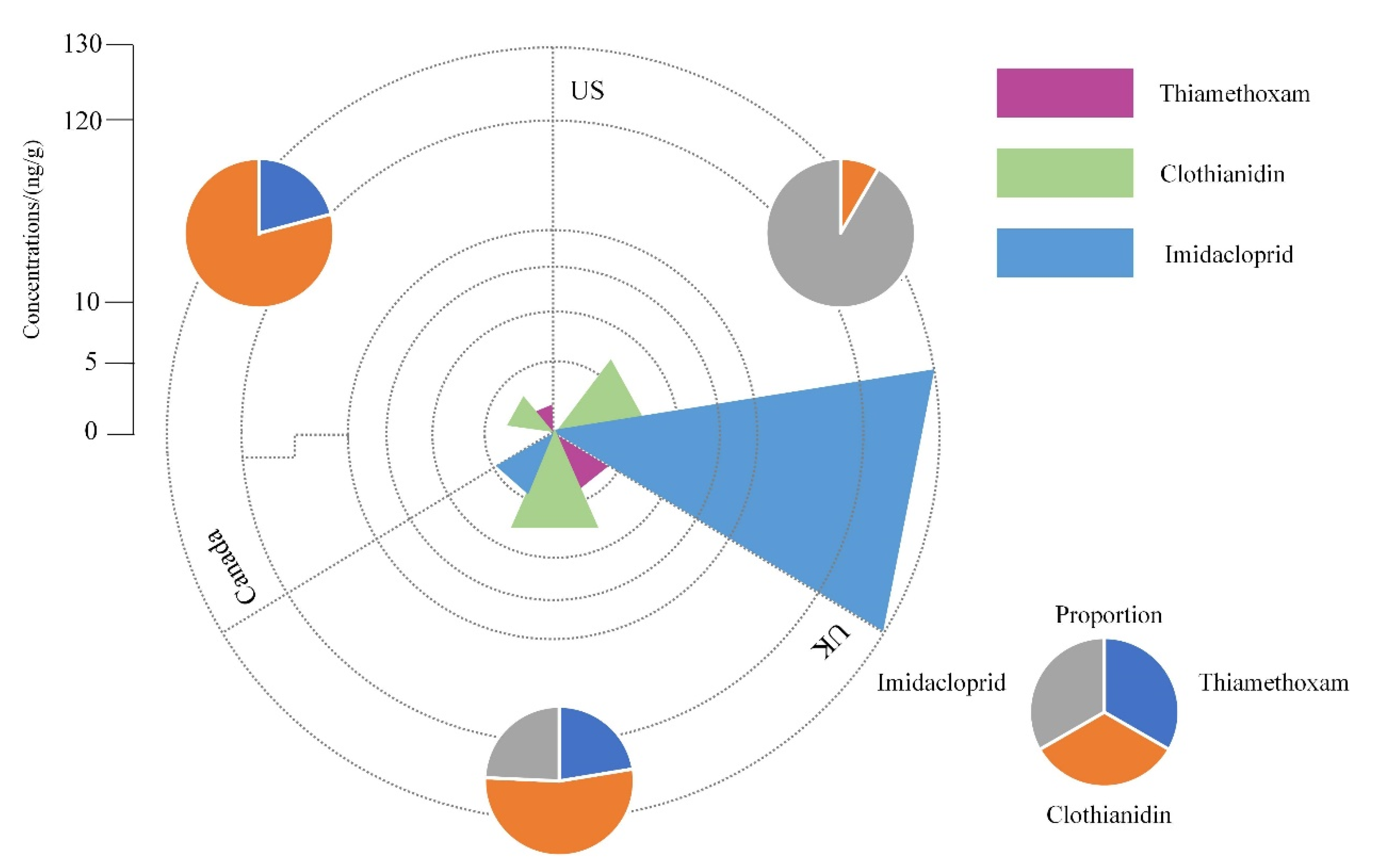
Distribution Characteristics of Neonics in Water
Distribution Characteristics of Neonics in Soil
Distribution Characteristics of Neonics in the Atmosphere
4.2.2. Environmental Risk Assessment of Neonics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Neonics | Neonicotinoid Insecticides |
| nAChRs | Nicotinic Acetylcholine Receptors |
| UN | European Union |
| CAs | Chromosome Aberrations |
| SCEs | Sister Chromatid Exchanges |
| MN | Micronucleus |
| IUCN | International Union for Conservation of Nature |
| CR | Critically Endangered |
| EN | Endangered |
| VU | Vulnerable |
| ESI | Electrospray Ionization |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
References
- Zhao, Y.Y.; Li, Y. Modified neonicotinoid insecticide with bi-directional selective toxicity and drug resistance. Ecotoxicol. Environ. Saf. 2018, 164, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; He, J.; Wu, W.Z. UPLC-MS/MS detection of 9 insecticides in honey. Environ. Chem. 2016, 35, 1921–1927. [Google Scholar]
- Li, M.; Li, Z.G.; He, J.F.; Su, S.K. Advance in effect of neonicotinoid insecticides on behavior and physiology of honey bees. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2016, 45, 490–495. [Google Scholar]
- Jeschke, P.; Nauen, R.; Beck, M.E. Nicotinic acetylcholine receptor agonists: A milestone for modern crop protection. Angew. Chem. 2013, 52, 9464–9485. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Denholm, I. Nicotinic acetylcholine receptors: Targets for commercially important insecticides. Invertebr. Neurosci. 2007, 7, 53–66. [Google Scholar] [CrossRef]
- Barbara, G.S.; Grünewald, B.; Paute, S.; Gauthier, M.; Raymond-Delpech, V. Study of nicotinic acetylcholine receptors on cultured antennal lobe neurones from adult honeybee brains. Invertebr. Neurosci. 2008, 8, 19–29. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Stivaktakis, P.D.; Kavvalakis, M.P.; Tzatzarakis, M.N.; Alegakis, A.K.; Panagiotakis, M.N.; Fragkiadaki, P.; Vakonaki, E.; Ozcagli, E.; Hayes, W.A.; Rakitskii, V.N.; et al. Long-term exposure of rabbits to imidacloprid as quantified in blood induces genotoxic effect. Chemosphere 2016, 149, 108–113. [Google Scholar] [CrossRef]
- Geoffrey, R.W.; Aline, T.; Gina, R.; Kaspar, R.; Orlando, Y.; Dave, S.; Peter, N.; Laurent, G. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar]
- Zhang, X.; Liao, X.; Mao, K.; Zhang, K.X.; Li, J.H. Insecticide resistance monitoring and correlation analysis of insecticides in field populations of the brown planthopper Nilaparvata lugens (stål) in China 2012–2014. Pestic. Biochem. Phys. 2016, 132, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Iwanaga, Y.; Shono, T.; Narahashi, T. Modulation of the Neuronal Nicotinic Acetylcholine Receptor Channel by Imidacloprid and Cartap. Pestic. Biochem. Phys. 1997, 59, 119–128. [Google Scholar] [CrossRef]
- Wang, K.; Pang, S.; Mu, X.Y.; Qi, S.Z.; Li, D.Z.; Cui, F.; Wang, C.J. Biological response of earthworm, Eisenia fetida, to five neonicotinoid insecticides. Chemosphere 2015, 132, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. The case of the empty hives. Science 2007, 316, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Tapparo, A.; Giorio, C.; Soldà, L. UHPLC-DAD method for the determination of neonicotinoid insecticides in single bees and its relevance in honeybee colony loss investigations. Anal. Bioanal. Chem. 2013, 405, 1007–1014. [Google Scholar] [CrossRef]
- Peng, J.H.; Liao, L.P.; Nie, S.Q.; Liang, J.; Fu, Q.M.; Wu, D.X.; Xu, W.J. Analysis of Triflumezopyrim Residues in Rice, Soil and Field Water. Agrochemicals 2018, 57, 50–53. [Google Scholar]
- Cao, L.D.; Zhu, P.; Zhao, Y.S.; Zhao, J.H. Using machine learning and quantum chemistry descriptors to predict the toxicity of ionic liquids. J. Hazard. Mater. 2018, 352, 17–26. [Google Scholar] [CrossRef]
- Cheng, X.; Yi, B. Development of thiamethoxam, a neonicotinoid of the second generation. World Pestic. 2001, 23, 17–25. (In Chinese) [Google Scholar]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid insecticides mode of action on insect nicotinic acetylcholine receptors using binding studies. Pestic. Biochem. Phys. 2018, 151, 59–66. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Leung, T.Y.G.; Calbick, S.; Metcalfe, C.; Kennedy, C. Sub-lethal effects of a neonicotinoid, clothianidin, on wild early life stage sockeye salmon (Oncorhynchus nerka). Aquat. Toxicol. 2019, 217, 105335. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. The counter attack way of nicotinic insecticide furosemide. Mark. Asp. 2020, 2, 36–37. (In Chinese) [Google Scholar]
- Mathews, M.J.; Mead, R.N.; Galizio, M. Effects of N-Methyl-D-aspartate (NMDA) antagonists ketamine, methoxetamine, and phencyclidine on the odor span test of working memory in rats. Exp. Clin. Psychopharmacol. 2018, 26, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hesselbach, H.; Scheiner, R. The novel pesticide flupyradifurone (Sivanto) affects honeybee motor abilities. Ecotoxicology 2019, 28, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Cordova, D.; Benner, E.A.; Schroeder, M.E. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. 2016, 74, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.F.; Fu, M.; Liu, Y.H. The European Union again tightened restrictions on the use of imidacloprid, thiamethoxam and thiamethoxam. Pestic. Sci. Manag. 2017, 38, 34–35. [Google Scholar]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2017, 192, 59–65. [Google Scholar] [CrossRef]
- Tian, J. Molecular Modeling Study on the Interaction Mechanism between the Neonicotinoid Insecticides and nAChR. Master’s Thesis, Lanzhou University, Lanzhou, China, May 2017. [Google Scholar]
- Zhang, J.; Wei, Y.; Li, H.; Zeng, E.Y.; You, J. Application of Box-Behnken design to optimize multi-sorbent solid phase extraction for trace neonicotinoids in water containing high level of matrix substances. Talanta 2017, 170, 392–398. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, C.; Liu, H.; Wu, R.; Tian, D.; Ruan, J.; Zhang, T.; Huang, M.; Ying, G. Occurrence and distribution of neonicotinoid insecticides in surface water and sediment of the Guangzhou section of the Pearl River, South China. Environ. Pollut. 2019, 251, 892–900. [Google Scholar] [CrossRef]
- Piao, X.; Ji, L.; Lin, R. Status analysis of neonicotinoid pesticide registration and management. China Plant Prot. 2015, 35, 70–74. [Google Scholar]
- Wintermantel, D.; Odoux, J.F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-induced mortality risk for bees foraging on oilseed rape nectar persists despite EU moratorium. Sci. Total Environ. 2020, 704, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Europe voted to ban neonicotinoids. Bee Craft 2018, 69, 9–13. [Google Scholar]
- Yu, L. France has become the first country in the European Union to ban the sale of five neonicotinoids. Pestic. Mark. Inf. 2018, 24, 45–53. [Google Scholar]
- Wang, Y.H.; Xu, P.; Chang, J.; Li, W.; Yang, L.; Tian, H.T. Unraveling the toxic effects of neonicotinoid insecticides on the thyroid endocrine system of lizards. Environ. Pollut. 2020, 258, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.L.; Qu, R.J.; Feng, M.B.; Chen, J.; Wang, L.S.; Wang, Z.Y. Photodegradation of Polyfluorinated Dibenzo-p-Dioxins (PFDDs) in Organic Solvents: Experimental and Theoretical Studies. Environ. Sci. Technol. 2016, 50, 8128–8134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Li, Y. Design of Environmentally Friendly Neonicotinoid Insecticides with Tuning of Bioconcentration and Bi-directional Selective Toxic Effects. J. Clean. Prod. 2019, 22, 113–121. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Hou, Y.L.; Li, Y. Design of Green Substitutes for Neonicotinoid Insecticides and Multi-Directional Selective Toxicity Effects on Farmland Ecosystems. J. Clean. Prod. 2020, accept. [Google Scholar] [CrossRef]
- Chen, Y. Overview on 2014 World Insecticides Market. Mod. Agrochem. 2016, 15, 1–7. [Google Scholar]
- Duan, C.Q. The Technical Study of Thiacloprid and Bupirimate and the Synthesis and Activity of Neonicotinoid Insectides Thiazoles Ramification Compound. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, October 2015. [Google Scholar]
- Li, B.B.; Hou, C.S.; Diao, Q.Y. Neonicotinoid pesticides severely affect honey bee. Apic. China 2016, 67, 31–35. [Google Scholar]
- Ge, J.; Cui, K.; Yan, H.Q.; Li, Y.; Chai, Y.Y.; Liu, X.J.; Cheng, J.F.; Yu, X.Y. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 2017, 226, 479–485. [Google Scholar]
- Sánchez-Bayo, F. The trouble with neonicotinoids. Science 2014, 346, 806–807. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. Int. 2012, 22, 119–134. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Garrido, I.; Campillo, N.; Viñas, P.; Hellín, P.; Flores, P.; Fenoll, J. Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid−liquid microextraction. Food Chem. 2016, 202, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Z.; Cai, H.J.; Song, W.H. Determination of eight pesticide residues in tea by liquid chromatography-tandem mass spectrometry and its uncertainty evaluation. Chin. J. Chromatogr. 2012, 30, 889–895. (In Chinese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.Y.; Zheng, Z.T.; Wei, F.L.; Ren, Y.P.; Gui, W.J.; Wu, H.M.; Zhu, G.N. Simultaneous Determination of Seven Neonicotinoid Pesticide Residues in Food by Ultraperformance Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 3271–3278. [Google Scholar] [CrossRef]
- Chen, M.; Tao, L.; Mclean, J.; Lu, C.S. Quantitative analysis of neonicotinoid insecticide residues in foods: Implication for dietary exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Q.; Zhao, C.; Wang, X.Y.; Li, J.R.; Wang, D.; Zhou, Y.; Lu, X.X. Residues of neonicotinoid pesticides in vegetables and fruit and health risk assessment of human exposure via food intake. Asian J. Ecotoxicol. 2016, 11, 67–81. (In Chinese) [Google Scholar]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Botías, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016, 88, 169–178. [Google Scholar] [CrossRef]
- European Commission. Commission implementing regulation (EU) 2018/783 of 29 may 2018 amending implementing regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union 2018, 132, 31. [Google Scholar]
- European Commission. Commission implementing regulation (EU) 2018/784 of 29 may 2018 amending implementing regulation (EU) No 540/2011 as regards the conditions of approval of the active substance clothianidin. Off. J. Eur. Union 2018, 132, 35. [Google Scholar]
- European Commission. Commission implementing regulation (EU) 2018/785 of 29 may 2018 amending implementing regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam. Off. J. Eur. Union 2018, 132, 40. [Google Scholar]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Jones, A.; Harrington, P.; Turnbull, G. Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Manag. Sci. 2014, 70, 1780–1784. [Google Scholar] [CrossRef]
- Lima, M.A.P.; Martins, G.F.; Oliveira, E.E.; Guedes, R.N.C. Agrochemical-induced stress in stingless bees: Peculiarities, underlying basis, and challenges. J. Comp. Physiol. 2016, 202, 733–747. [Google Scholar] [CrossRef]
- La, N.; Lamers, M.; Bannwarth, M.; Nguyen, V.; Streck, T. Imidacloprid concentrations in paddy rice fields in northern Vietnam: Measurement and probabilistic modeling. Paddy Water Environ. 2014, 13, 191–203. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Wusmart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 2016, 6, 32108. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Genecque, E.; Menach, K.L.; Budzinski, H.; Cluzeau, S.; Pham-Delgue, M.H. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 2015, 48, 242–250. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2008, 54, 653–661. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Yang, E.C.; Chang, H.C.; Wu, W.Y.; Chen, Y.W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef]
- Tan, K.; Chen, W.; Dong, S.; Liu, X.W.; Wang, Y.C.; Nieh, J.C. Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS ONE 2014, 9, e102725. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederstrem, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed Coating with a Neonicotinoid Insecticide Negatively Affects Wild Bees. Nature 2015, 521, 77–94. [Google Scholar] [CrossRef]
- McArt, S.H.; Fersch, A.A.; Milano, N.J.; Truitt, L.L.; Böröczky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Stanley, D.A.; Michael, P.; Garratt, D.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. Aworldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Christen, V.; Mittner, F.; Fent, K. Molecular effects of neonicotinoids in honey bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef]
- Babelova, J.; Šefčíková, Z.; Čikoš, S.; Špirková, A.; Kovaříková, V.; Koppel, J.; Makarevich, A.V.; Chrenek, P.; Fabian, D. Exposure to neonicotinoid insecticides induces embryotoxicity in mice and rabbits. Toxicology 2017, 392, 71–80. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Srivastava, L.P. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 3086–3089. [Google Scholar] [CrossRef]
- Bal, R.; Naziroglu, M.; Turk, G.; Yilmaz, O.; Kuloglu, T.; Etem, E.; Baydas, G. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem. Funct. 2012, 30, 492–499. [Google Scholar] [CrossRef]
- Bal, R.; Turk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Baydas, G.; Naziroglu, M.; Yener, Z.; Etem, E.; Tuzcu, Z. Effects of the neonicotinoid insecticide, clothianidin, on the reproductive organ system in adult male rats. Drug Chem. Toxicol. 2013, 36, 421–429. [Google Scholar] [CrossRef]
- Bal, R.; Turk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu, R.; Gur, S.; Agca, A.; Ulas, M.; Cambay, Z.; et al. Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. J. Environ. Sci. Health Part B 2012, 47, 434–444. [Google Scholar] [CrossRef]
- Bal, R.; Turk, G.; Yilmaz, O.; Etem, E.; Kuloglu, T.; Baydas, G.; Naziroglu, M. Effects of clothianidin exposure on sperm quality, testicular apoptosis and fatty acid composition in developing male rats. Cell Biol. Toxicol. 2012, 28, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yanai, S.; Omotehara, T.; Hashimoto, R.; Umemura, Y.; Kubota, N.; Minami, K.; Nagahara, D.; Matsuo, E.; Aihara, Y.; et al. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 2015, 77, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Srivastava, M.K.; Kapoor, U.; Srivastava, L.P. A 90 days oral toxicity of imidacloprid in female rats: Morphological, biochemical and histopathological evaluations. Food Chem. Toxicol. 2010, 48, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Toor, H.K.; Sangha, G.K.; Khera, K.S. Imidacloprid induced histological and biochemical alterations in liver of female albino rats. Pestic. Biochem. Phys. 2013, 105, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vohra, P.; Khera, K.S.; Sangha, G.K. Physiological, biochemical and histological alterations induced by administration of imidacloprid in female albino rats. Pestic. Biochem. Phys. 2014, 110, 50–56. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Trivedi, P.; Garg, V.; Srivastava, L.P. Disposition and acute toxicity of imidacloprid in female rats after single exposure. Food Chem. Toxicol. 2014, 68, 190–195. [Google Scholar] [CrossRef]
- Arfat, Y.; Mahmood, N.; Tahir, M.U.; Rashid, M.; Anjum, S.; Zhao, F.; Li, D.J.; Sun, Y.L.; Hu, L.; Zhihao, C.; et al. Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicol. Rep. 2014, 1, 554–561. [Google Scholar] [CrossRef]
- Calderon-Segura, M.E.; Gomez-Arroyo, S.; Villalobos-Pietrini, R.; Martinez-Valenzuela, C.; Carbajal-Lopez, Y.; Calderon-Ezquerro Mdel, C.; Cortes-Eslava, J.; Garcia-Martinez, R.; Flores-Ramirez, D.; Rodriguez-Romero, M.I.; et al. Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news. J. Toxicol. 2012, 2012, 612647. [Google Scholar] [CrossRef]
- Kocaman, A.Y.; Rencuzogullari, E.; Topaktas, M. In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes. Environ. Toxicol. 2014, 29, 631–641. [Google Scholar] [CrossRef]
- Galdikova, M.; Sivikova, K.; Holeckova, B.; Dianovsky, J.; Drazovska, M.; Schwarzbacherova, V. The effect of thiacloprid formulation on DNA/chromosome damage and changes in GST activity in bovine peripheral lymphocytes. J. Environ. Health Sci. Part B 2015, 50, 698–707. [Google Scholar] [CrossRef]
- Kataria, S.K.; Chhillar, A.K.; Kumar, A.; Tomar, M.; Malik, V. Cytogenetic and hematological alterations induced by acute oral exposure of imidacloprid in female mice. Drug Chem. Toxicol. 2016, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.J.; Bicho, R.C.; Carretero, M.A.; Sanchezhernandez, J.C.; Faustino, A.M.; Soares, A.M.; Mann, R.M. The use of a lacertid lizard as a model for reptile ecotoxicology studies: Part 2-biomarkers of exposure and toxicity among pesticide exposed lizards. Chemosphere 2012, 87, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A.; Anjum, M.N.; Butt, M.S.; Yasin, M.; Imran, M. Minimization of imidacloprid residues in cucumber and bell pepper through washing with citric acid and acetic acid solutions and their dietary intake assessment. Int. J. Food Prop. 2014, 17, 978–986. [Google Scholar] [CrossRef]
- Mingo, V.; Lotters, S.; Wagner, N. Risk of pesticide exposure for reptile species in the European Union. Environ. Pollut. 2016, 215, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Amaral, M.J.; Faustino, A.M.; Power, D.M.; Rema, A.; Carretero, M.A.; Soares, A.M.; Mann, R.M. Thyroid disruption in the lizard Podarcis bocagei exposed to a mixture of herbicides: A field study. Ecotoxicology 2013, 22, 156–165. [Google Scholar] [CrossRef]
- Park, H.; Suk, H.Y.; Jeong, E.; Park, D.; Lee, H.; Min, M. Population genetic structure of endangered Mongolian racerunner (Eremias argus) from the Korean Peninsula. Mol. Biol. Rep. 2014, 41, 7339–7347. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, X.F.; Su, G.Y.; Yu, L.Q.; Robert, J.L.; Hou, J.; Yu, H.X.; John, P.G.; Liu, C. Environmentally Relevant Concentrations of the Flame-Retardant Tris (1,3-dichloro-2-propyl) Phosphate (TDCIPP) Inhibits Growth of Female Zebrafish and Decreases Fecundity. Environ. Sci. Technol. 2015, 49, 14579–14587. [Google Scholar] [CrossRef]
- Miles, J.C.; Hua, J.; Sepulveda, M.S.; Krupke, C.H.; Hoverman, J.T. Effects of clothianidin on aquatic communities: Evaluating the impacts of lethal and sublethal exposure to neonicotinoids. PLoS ONE 2017, 13, e0194634. [Google Scholar] [CrossRef]
- Pereira, A.S.; Cerejeira, M.J.; Daam, M.A. Ecological risk assessment of imidacloprid applied to experimental rice fields: Accurateness of the RICEWQ model and effects of ecosystem structure. Ecotoxicol. Environ. Saf. 2017, 142, 431–440. [Google Scholar] [CrossRef]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Neonicotinoid insecticides negatively affect performance measures of non-target terrestrial arthropods: A meta-analysis. Ecol. Appl. 2018, 28, 1232–1244. [Google Scholar] [CrossRef]
- Riens, J.R.; Schwarz, M.S.; Mustafa, F.; Hoback, W.W. Aquatic macroinvertebrate communities and water quality at buffered and non-buffered wetland sites on federal waterfowl production areas in the Rainwater Basin, Nebraska. Wetlands 2013, 33, 1025–1036. [Google Scholar] [CrossRef]
- Smit, C.E.; Posthuma-Doodeman, J.A.M.; van Vlaardingen, P.L.A.; de Jong, F.M.W. Ecotoxicity of imidacloprid to aquatic organisms: Derivation of water quality standards for peak and long-term exposure. Hum. Ecol. Risk Assess. 2015, 21, 1608–1630. [Google Scholar] [CrossRef]
- Anderson, T.A.; Salice, C.J.; Erickson, R.A.; McMurry, S.T.; Cox, S.B.; Smith, L.M. Effects of landuse and precipitation on pesticides and water quality in playa lakes of the southern high plains. Chemosphere 2013, 92, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 2016, 4, 291–303. [Google Scholar] [CrossRef]
- Evelsizer, V.; Skopec, M. Pesticides, including neonicotinoids, in drained wetlands of Iowa’s prairie pothole region. Wetlands 2018, 38, 221–232. [Google Scholar] [CrossRef]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Schepker, T.J.; Webb, E.B.; Tillitt, D.; LaGrange, T. Neonicotinoid insecticide concentrations in agricultural wetlands and associations with aquatic invertebrate communities. Agric. Ecosyst. Environ. 2020, 287, 106–109. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef]
- Ueyama, J.; Nomura, H.; Kondo, T.; Saito, I.; Ito, Y.; Osaka, A.; Kamijima, M. Biological monitoring method for urinary neonicotinoid insecticides using LCMS/MS and its application to Japanese adults. J. Occup. Health 2014, 56, 461–468. [Google Scholar] [CrossRef]
- Ueyama, J.; Harada, K.H.; Koizumi, A.; Sugiura, Y.; Kondo, T.; Saito, I.; Kamijima, M. Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environ. Sci. Technol. 2015, 49, 14522–14528. [Google Scholar] [CrossRef]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japanneonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y. Exposure Characteristics of Neonicotinoid Imidacloprid towards Human Body Based on Metabolomics. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, May 2019. [Google Scholar]
- Kavvalakis, M.P.; Tzatzarakis, M.N.; Theodoropoulou, E.P.; Barbounis, E.G.; Tsakalof, A.K.; Tsatsakis, A.M. Development and application of LC-APCIMS method for biomonitoring of animal and human exposure to imidacloprid. Chemosphere 2013, 93, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Tadashi, Y.; Hikoto, O.; Mika, A.; Baisuke, W. Simultaneous determination of neonicotinoid insecticides in human serum and urine using diatomaceous earth-assisted extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2014, 969, 85–94. [Google Scholar]
- Sagiv, S.K.; Harris, M.H.; Gunier, R.B.; Kogut, K.R.; Harley, K.G.; Deardorff, J.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal Organophosphate Pesticide Exposure and Traits Related to Autism Spectrum Disorders in a Population Living in Proximity to Agriculture. Environ. Health Perspect. 2018, 126, 47–59. [Google Scholar]
- Mercadante, R.; Polledri, E.; Moretto, A.; Fustinoni, S. Long-term occupational and environmental exposure to penconazole and tebuconazole by hair biomonitoring. Toxicol. Lett. 2018, 298, 19–24. [Google Scholar] [CrossRef]
- Lozano-Paniagua, D.; Parron, T.; Alarcon, R.; Requena, M.; Gil, F.; López-Guarnido, O.; Lacasaña, M.; Hernández, A.F. Biomarkers of oxidative stress in blood of workers exposed to non- cholinesterase inhibiting pesticides. Ecotoxicol. Environ. Saf. 2018, 162, 121–128. [Google Scholar] [CrossRef]
- Wei, Y.; Carmichael, S.L.; Roberts, E.M.; Susan, E.K.; Amy, M.P.; Paul, B.E.; Gary, M.S. Residential Agricultural Pesticide Exposures and Risk of Neural Tube Defects and Orofacial Clefts Among Offspring in the San Joaquin Valley of California. Am. J. Epidemiol. 2014, 179, 740–748. [Google Scholar]
- Keil, A.P.; Daniels, J.L.; Hertz-Picciotto, I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: The CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Environ. Health 2014, 13, 3–13. [Google Scholar] [CrossRef]
- Koureas, M.; Tsezou, A.; Tsakalof, A.; Orfanidou, T.; Hadjichristodoulou, C. Increased levels of oxidative DNA damage in pesticide sprayers in Thessaly Region (Greece). Implications of pesticide exposure. Sci. Total Environ. 2014, 496, 358–364. [Google Scholar] [CrossRef]
- Marfo, J.T.; Fujioka, K.; Ikenaka, Y.; Nakayama, S.M.; Mizukawa, H.; Aoyama, Y.; Ishizuka, M.; Taira, K. Relationship between urinary N-Desmethyl-Acetamiprid and typical symptoms including neurological findings: A prevalence case-control study. PLoS ONE 2015, 10, e0142172. [Google Scholar] [CrossRef]
- Seltenrich, N. Catching Up with Popular Pesticides: More Human Health Studies Are Needed on Neonicotinoids. Environ. Health Perspect. 2017, 125, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Bisemi, M.; Genkova, D. Evaluation of neonicotinoid insecticides for oestrogenic, thyroidogenic and adipogenic activity reveals imidacloprid causes lipid accumulation. J. Appl. Toxicol. 2018, 38, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Main, A.R.; Headley, J.V.; Peru, K.M.; Michel, N.L.; Cessna, A.J.; Morrissey, C.A. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE 2014, 9, e92821. [Google Scholar] [CrossRef] [PubMed]
- Sadaria, A.; Supowit, S.D.; Halden, R.U. Mass balance assessment for six neonicotinoid insecticides during conventional wastewater and wetland treatment: Nationwide reconnaissance in United States wastewater. Environ. Sci. Technol. 2016, 50, 6199–6206. [Google Scholar] [CrossRef]
- Yamamoto, A.; Terao, T.; Hisatomi, H.; Kawasaki, H.; Arakawa, R. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionisation. J. Environ. Monit. 2012, 14, 2189–2194. [Google Scholar] [CrossRef]
- Moschet, C.; Wittmer, I.; Simovic, J.; Junghans, M.; Piazzoli, A.; Singer, H.; Stamm, C.; Leu, C.; Hollender, J. How a complete pesticide screening changes the assessment of surface water quality. Environ. Sci. Technol. 2014, 48, 5423–5432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, D.; Yi, X.H.; Zhang, T.; Ruan, J.J.; Wu, R.R.; Chen, C.; Huang, M.Z.; Ying, G.G. Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China. Chemosphere 2019, 217, 437–446. [Google Scholar] [CrossRef]
- Qi, Q.X.; Singer, H.; Berg, M.; Müller, B.; Pernet-Coudrier, B.; Liu, H.J.; Qu, J.H. Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 2015, 119, 1054–1061. [Google Scholar] [CrossRef]
- Chen, M.; Yi, Q.; Hong, J. Simultaneous determination of 32 antibiotics and 12 pesticides in sediment using ultrasonic-assisted extraction and high-performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2015, 7, 1896–1905. [Google Scholar] [CrossRef]
- Bradley, P.M.; Journey, C.A.; Romanok, K.M.; Barber, L.B.; Buxton, H.T.; Foreman, W.T.; Furlong, E.T.; Glassmeyer, S.T.; Hladik, M.L.; Iwanowicz, L.R.; et al. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. streams. Environ. Sci. Technol. 2017, 51, 4792–4802. [Google Scholar] [CrossRef]
- de Perre, C.; Murphy, T.M.; Lydy, M.J. Fate and effects of clothianidin in fields using conservation practices. Environ. Toxicol. Chem. 2015, 34, 258–265. [Google Scholar] [CrossRef]
- Benton, E.P.; Grant, J.F.; Mueller, T.C.; Webster, R.J.; Nichols, R.J. Consequences of imidacloprid treatments for hemlock woolly adelgid on stream water quality in the southern Appalachians. For. Ecol. Manag. 2016, 360, 152–158. [Google Scholar] [CrossRef]
- Huseth, A.S.; Groves, R.L. Environmental fate of soil applied neonicotinoid insecticides in an irrigated potato agroecosystem. PLoS ONE 2014, 9, e97081. [Google Scholar] [CrossRef] [PubMed]
- Main, A.R. Snowmelt transport of neonicotinoid insecticides to Canadian prairie wetlands. Agric. Ecosyst. Environ. 2016, 215, 76–84. [Google Scholar] [CrossRef]
- Samson-Robert, O.; Labrie, G.; Chagnon, M.; Fournier, V. Neonicotinoid-contaminated puddles of water represent a risk of intoxication for honey bees. PLoS ONE 2014, 9, e108443. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.; Limay-Rios, V.; Baute, T.; Smith, J.; Xue, Y. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in southwestern Ontario. PLoS ONE 2015, 10, e0118139. [Google Scholar] [CrossRef]
- Dijk, T.C.V.; Staalduinen, M.A.V.; Sluijs, J.P.V.D. Macroinvertebrate decline in surface water polluted with imidacloprid. PLoS ONE 2015, 9, e89837. [Google Scholar]
- Englert, D.; Bakanov, N.; Zubrod, J.P.; Schulz, R.; Bundschuh, M. Modeling remobilization of neonicotinoid residues from tree foliage in streams—A relevant exposure pathway in risk assessment. Environ. Sci. Technol. 2017, 51, 1785–1794. [Google Scholar] [CrossRef]
- López-Doval, J.C.; Montagner, C.C.; de Alburquerque, A.F.; Moschini-Carlos, V.; Umbuzeiro, G.; Pompêo, M. Nutrients, emerging pollutants and pesticides in a tropical urban reservoir: Spatial distributions and risk assessment. Sci. Total Environ. 2016, 575, 1307–1324. [Google Scholar] [CrossRef]
- Gonzalez-Rey, M.; Tapie, N.; Le, M.K.; Dévier, M.H.; Budzinski, H.; Bebianno, M.J. Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar. Pollut. Bull. 2015, 96, 384–400. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef]
- Masiá, A.; Campo, J.; Vázquez-Roig, P.; Blasco, C.; Picó, Y. Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J. Hazard. Mater. 2013, 263, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kreutzweiser, D.P.; Thompson, D.G.; Scarr, T.A. Imidacloprid in leaves from systemically treated trees may inhibit litter breakdown by non-target invertebrates. Ecotoxicol. Environ. Saf. 2009, 72, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Dankyi, E.; Gordon, C.; Carboo, D.; Formsgaard, T.S. Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci. Total Environ. 2014, 499, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Limayrios, V.; Forero, L.G.; Xue, Y.; Smith, J.; Baute, T.; Schaafsma, A. Neonicotinoid insecticide residues in soil dust and associated parent soil in fields with a history of seed treatment use on crops in Southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 303–310. [Google Scholar] [CrossRef]
- Xu, T.; Dyer, D.G.; Mcconnell, L.L.; Bondarenko, S.; Allen, R.; Heinemann, O. Clothianidin in agricultural soils and uptake into corn pollen and canola nectar after multiyear seed treatment applications. Environ. Toxicol. Chem. 2016, 35, 311–321. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Hussein, L.A.; El Azab, N.F.; El-Khatib, A.H.; Linscheid, M.W. Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography–tandem mass spectrometry coupled with QuEChERS. J. Chromatogr. B 2016, 1031, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, X.X.; Fu, X.F.; Wang, D.; Zhao, C.; Zhang, Q.; Tan, Y.; Wang, X.Y. Development of a fast and sensitive method for measuring multiple neonicotinoid insecticide residues in soil and the application in parks and residential areas. Anal. Chim. Acta 2018, 1016, 19–28. [Google Scholar] [CrossRef]
- Stewart, S.D.; Lorenz, G.M.; Catchot, A.L. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 2014, 48, 9762–9769. [Google Scholar] [CrossRef]
- Li, T.T.; Zheng, S.S.; Wang, J. A review on occurence and transformation behaviors of neonicotinoid pesticides. Asian J. Ecotoxicol. 2018, 13, 9–21. [Google Scholar]
- Hoffmann, E.J.; Vandervoort, C.; Wise, J.C. Plum Curculio (Coleoptera: Curculionidae) adult mortality and associated fruit injury after exposure to field-aged insecticides on tart cherry branches. J. Econ. Entomol. 2010, 103, 1196–1205. [Google Scholar] [CrossRef]
- Girolami, V.; Marzaro, M.; Vivan, L.; Mazzon, L.; Giorio, C.; Marton, D.; Tapparo, A. Aerial powdering of bees inside mobile cages and the extent of neonicotinoid cloud surrounding corn drillers. J. Appl. Entomol. 2013, 137, 35–44. [Google Scholar] [CrossRef]
- Xue, Y.; Limay-Rios, V.; Smith, J.; Baute, T.; Forero, L.G.; Schaafsma, A. Quantifying neonicotinoid insecticide residues escaping during maize planting with vacuum planters. Environ. Sci. Technol. 2015, 49, 13003–13011. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gan, J. Conversion of pesticides to biologically active products on urban hard surfaces. Sci. Total Environ. 2016, 556, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Roessink, I.; Merga, L.B.; Zweers, H.J. The Neonicotinoid Imidacloprid Shows High Chronic Toxicity to Mayfly Nymphs. Environ. Toxicol. Chem. 2013, 32, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, D.; Morrissey, C.; Mineau, P. A Review of the Direct and Indirect Effects of Neonicotinoids and Fipronil on Vertebrate Wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef]
- Chen, Z.L. Molecular Mechanism of Enantioselective Environmental Behavior and Toxicity Difference of Furosemide. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, May 2017. [Google Scholar]
- Kessler, S.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Stout, J.C.; Wright, G.A. Bees Prefer Foods Containing Neonicotinoid Pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef]
- Forrester, M. Neonicotinoid Insecticide Exposures Reported to Six Poison Centers in Texas. Hum. Exp. Toxicol. 2014, 33, 568–573. [Google Scholar] [CrossRef]
- Wei, Z.S.; Li, W.; Zhao, D.Y.; Seo, Y.; Spinney, R.; Dionysiou, D.D.; Wang, Y.; Zeng, W.Z.; Xiao, R.Y. Electrophilicity index as a critical indicator for the biodegradation of the pharmaceuticals in aerobic activated sludge processes. Water Res. 2019, 160, 10–17. [Google Scholar] [CrossRef]
| Region | Sampling Point | Sample Type | Compound | Concentration μg/L | Reference |
|---|---|---|---|---|---|
| China | Beijing | sewage treatment plant | imidacloprid | 0.045–0.11 | [131] |
| Fujian | sediment | imidacloprid | 141 | [132] | |
| acetamiprid | 162 | ||||
| US | - | surface water | imidacloprid | 0.005–0.10 | [133] |
| clothianidin | 0.003–0.07 | ||||
| dinotefuran | 0.005–0.11 | ||||
| acetamiprid | 0.03 | ||||
| Texas | wetland | thiamethoxam | 225 | [108] | |
| acetamiprid | |||||
| Farmland | surface water | clothianidin | 0.85 | [134] | |
| River | surface water | imidacloprid | 1.46 | [135] | |
| - | sewage treatment plant | imidacloprid | 0.059 | [127] | |
| acetamiprid | 0.0020 | ||||
| clothianidin | 0.07 | ||||
| Wisconsin | groundwater | imidacloprid | 0.26–3.34 | [136] | |
| clothianidin | 0.21–3.34 | ||||
| thiamethoxam | 0.20–8.93 | ||||
| Canada | Wetland | surface water | clothianidin | 3.10 | [126] |
| thiamethoxam | 1.50 | ||||
| Saskatchewan | surface water | clothianidin | 0.27 ± 0.072 | [137] | |
| thiamethoxam | |||||
| Farmland | surface water | clothianidin | 55.70 | [138] | |
| thiamethoxam | 63.40 | ||||
| Ontario, farmland | surface water | thiamethoxam | 1.12 | [139] | |
| Holland | - | surface water | imidacloprid | 320 | [140] |
| Germany | - | surface water | imidacloprid | 0.25 | [141] |
| Vietnam | Farmland | surface water | imidacloprid | 53 | [63] |
| Brazil | Reservoir | surface water | imidacloprid | 0.0021 | [142] |
| Portugal | River | surface water | imidacloprid | 0.0080 | [143] |
| Hungary | River | surface water | clothianidin | 0.017–0.040 | [144] |
| thiamethoxam | 0.0040–0.030 | ||||
| Spain | River | surface water | imidacloprid | 0.0023–0.019 | [145] |
| Australia | Sydney, river | surface water | clothianidin | 0.42 | [43] |
| imidacloprid | 4.56 | ||||
| thiamethoxam | 1.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, J.; Ren, J.; Hou, Y.; Han, Z.; Xiao, J.; Li, Y. Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability 2020, 12, 7523. https://doi.org/10.3390/su12187523
Zhao Y, Yang J, Ren J, Hou Y, Han Z, Xiao J, Li Y. Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability. 2020; 12(18):7523. https://doi.org/10.3390/su12187523
Chicago/Turabian StyleZhao, Yuanyuan, Jiawen Yang, Jinbo Ren, Yilin Hou, Zhenzhen Han, Jiapeng Xiao, and Yu Li. 2020. "Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview" Sustainability 12, no. 18: 7523. https://doi.org/10.3390/su12187523
APA StyleZhao, Y., Yang, J., Ren, J., Hou, Y., Han, Z., Xiao, J., & Li, Y. (2020). Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability, 12(18), 7523. https://doi.org/10.3390/su12187523