Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China
Abstract
:1. Introduction
2. Methodology
2.1. Field Description and Experimental Design
2.2. Measurement of Ecosystem Function
2.3. Measurement of Plant Functional Traits
2.4. Calculation Method
2.5. Data Analyses
3. Results
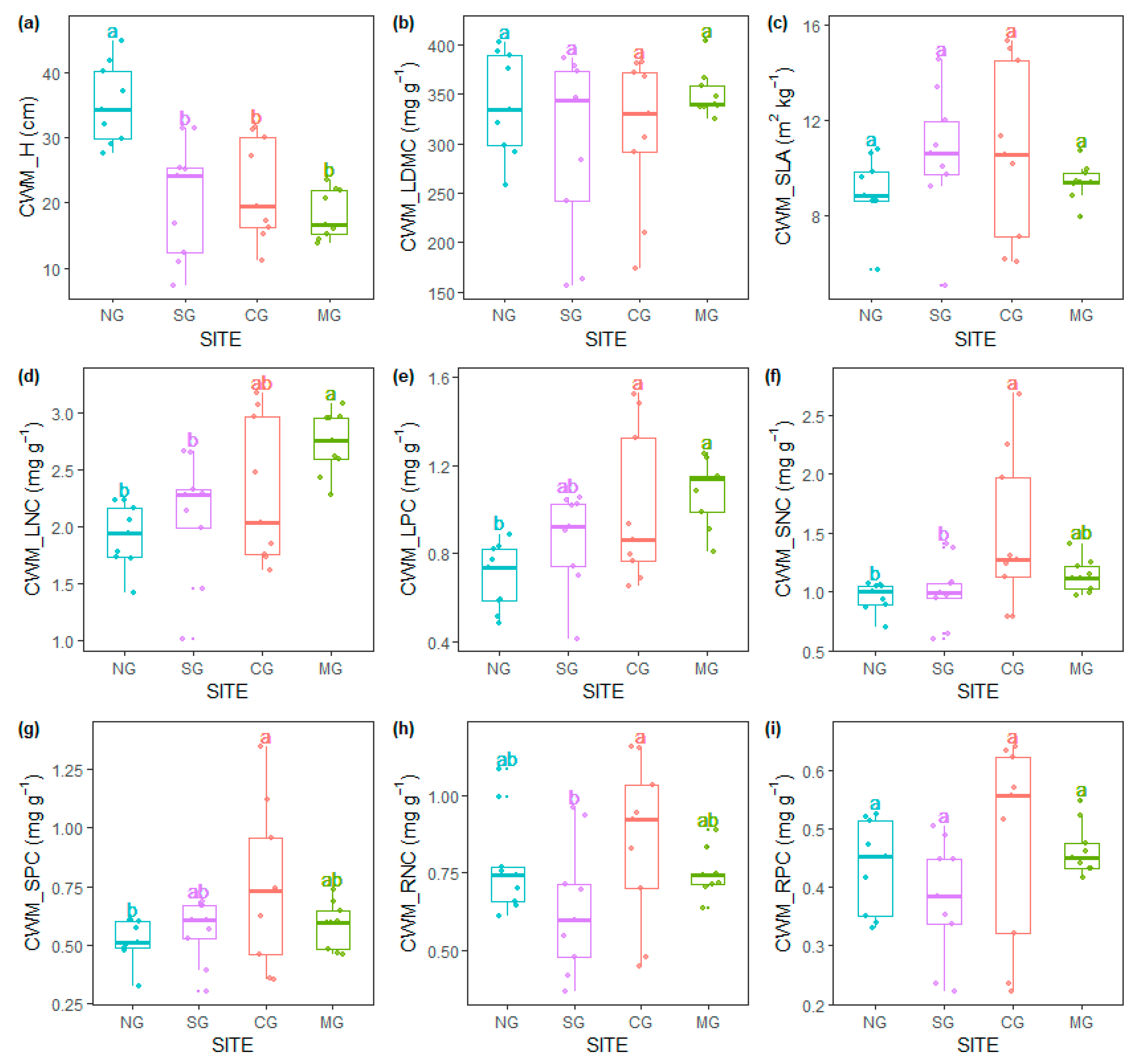
3.1. Community-Weighted Mean Traits
3.2. Ecosystem Functions
3.3. Relationship between Community-Weighted Mean Traits and Ecosystem Functions
4. Discussion
4.1. Effect of Grazing Types on Community-Weighted Mean Values
4.2. Effect of Grazing Types on Ecosystem Functions
4.3. Linking CWM Traits to Explain the Effect of Grazing Types on Ecosystem Functions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- RöMermann, C.; Bucher, S.F.; Hahn, M.; Bernhardt-RöMermann, M. Plant functional traits—Fixed facts or variable depending on the season? Folia Geobot. 2016, 51, 143–159. [Google Scholar] [CrossRef]
- Garcia, C.A.M.; Schellberg, J.; Ewert, F.; Brueser, K.; Canales-Prati, P.; Linstaedter, A.; Oomen, R.J.; Ruppert, J.C.; Perelman, S.B. Response of community-aggregated plant functional traits along grazing gradients: Insights from African semi-arid grasslands. Appl. Veg. Sci. 2014, 17, 470–481. [Google Scholar] [CrossRef]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [Green Version]
- He, J.-S.; Wang, L.; Flynn, D.F.B.; Wang, X.; Ma, W.; Fang, J. Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; Dolezal, J.; Eriksson, O.; Fortunel, C.; Freitas, H.; Golodets, C.; et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: A standardized methodology and lessons from an application to 11 European sites. Ann. Bot. 2007, 99, 967–985. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing—a global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M. Functional diversity affects decomposition processes in experimental grasslands. Funct. Ecol. 2008, 22, 547–555. [Google Scholar] [CrossRef]
- Majekova, M.; de Bello, F.; Dolezal, J.; Leps, J. Plant functional traits as determinants of population stability. Ecology 2014, 95, 2369–2374. [Google Scholar] [CrossRef]
- Zheng, S.X.; Ren, H.Y.; Lan, Z.C.; Li, W.H.; Wang, K.B.; Bai, Y.F. Effects of grazing on leaf traits and ecosystem functioning in Inner Mongolia grasslands: Scaling from species to community. Biogeosciences 2010, 7, 1117–1132. [Google Scholar] [CrossRef] [Green Version]
- Garnier, E.; Cortez, J.; Billes, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Marti, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Suding, K.N.; Goldstein, L.J. Testing the Holy Grail framework: Using functional traits to predict ecosystem change. New Phytol. 2008, 180, 559–562. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional. Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Diaz, S.; Lavorel, S.; de Bello, F.; Quetier, F.; Grigulis, K.; Robson, M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, J.; Roscher, C. Differential effects of functional traits on aboveground biomass in semi-natural grasslands. Oikos 2009, 118, 1659–1668. [Google Scholar] [CrossRef]
- Nicolas, G.; Nash, S.K.; Sandra, L. Leaf dry matter content and lateral spread predict response to land use change for six subalpine grassland species. J. Veg. Sci. 2007, 18, 289–300. [Google Scholar]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Vile, D.; Shipley, B.; Garnier, E. Ecosystem productivity can be predicted from potential relative growth rate and species abundance. Ecol. Lett. 2006, 9, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Pontes, L.D.S.; Soussana, J.F.; Louault, F.; Andueza, D.; Carrere, P. Leaf traits affect the above-ground productivity and quality of pasture grasses. Funct. Ecol. 2007, 21, 844–853. [Google Scholar] [CrossRef]
- Fortunel, C.; Garnier, E.; Joffre, R.; Kazakou, E.; Quested, H.; Grigulis, K.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 2009, 90, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Weigel, J.R.; Mcpherson, B.G.R. Trampling Effects from Short-Duration Grazing on Tobosagrass Range. J. Range Manag. 1990, 43, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Yang, Y.; Ma, W.; Mohammat, A.; Shen, H. Ecosystem carbon stocks and their changes in China’s grasslands. Sci. China Life Sci. 2010, 53, 757–765. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, S.; Shan, Y.; Taube, F.; Bai, Y. Vertebrate herbivore-induced changes in plants and soils: Linkages to ecosystem functioning in a semi-arid steppe. Funct. Ecol. 2013, 27, 273–281. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Pan, Q.; Huang, J.; Wang, Q.; Li, F.; Buyantuyev, A.; Han, X. Positive linear relationship between productivity and diversity: Evidence from the Eurasian Steppe. J. Appl. Ecol. 2007, 44, 1023–1034. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Posse, G.; Collantes, M.B. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. J. Appl. Ecol. 2005, 42, 50–59. [Google Scholar] [CrossRef]
- Nishizuka, Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988, 334, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhong, H.; Harris, W.; Yu, G.; Wang, S.; Hu, Z.; Yue, Y. Carbon storage in the grasslands of China based on field measurements of above- and below-ground biomass. Clim. Chang. 2008, 86, 375–396. [Google Scholar] [CrossRef]
- Qasim, S.; Gul, S.; Shah, M.H.; Hussain, F.; Ahmad, S.; Islam, M.; Rehman, G.; Yaqoob, M.; Shah, S.Q. Influence of grazing exclosure on vegetation biomass and soil quality. Int. Soil Water Conserv. Res. 2017, 5, 62–68. [Google Scholar] [CrossRef]
- Na, Y.; Li, J.; Hoshino, B.; Bao, S.; Qin, F.; Myagmartseren, P. Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes. Sustainability 2018, 10, 4753. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Liu, L.-P.; Zheng, L.-L.; Yu, F.-H.; Song, M.-H.; Zhang, X.-Z. Long-term grazing affects relationships between nitrogen form uptake and biomass of alpine meadow plants. Plant Ecol. 2017, 218, 1035–1045. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, P.; Wu, N.; Yi, S.; Chen, H. Biomass and nitrogen responses to grazing intensity in an alpine meadow on the eastern Tibetan Plateau. Pol. J. Ecol. 2007, 55, 469–479. [Google Scholar]
- Wagle, P.; Kakani, V.G. Seasonal variability in net ecosystem carbon dioxide exchange over a young Switchgrass stand. Glob. Chang. Biol. Bioenergy 2014, 6, 339–350. [Google Scholar] [CrossRef]
- Peng, F.; Quangang, Y.; Xue, X.; Guo, J.; Wang, T. Effects of rodent-induced land degradation on ecosystem carbon fluxes in an alpine meadow in the Qinghai-Tibet Plateau, China. Solid Earth 2015, 6, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Hadden, D.; Grelle, A. Changing temperature response of respiration turns boreal forest from carbon sink into carbon source. Agric. For. Meteorol. 2016, 223, 30–38. [Google Scholar] [CrossRef]
- Guo, W.H.; Kang, S.Z.; Li, F.S.; Li, S.E. Variation of NEE and its affecting factors in a vineyard of arid region of northwest China. Atmos. Environ. 2014, 84, 349–354. [Google Scholar] [CrossRef]
- Shao, C.; Chen, J.; Chu, H.; Lafortezza, R.; Dong, G.; Abraha, M.; Batkhishig, O.; John, R.; Ouyang, Z.; Zhang, Y.; et al. Grassland productivity and carbon sequestration in Mongolian grasslands: The underlying mechanisms and nomadic implications. Environ. Res. 2017, 159, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, D.; Li, C.; Seastedt, T.R.; Liang, C.; Wang, L.; Sun, W.; Liang, M.; Li, Y. Feces nitrogen release induced by different large herbivores in a dry grassland. Ecol. Appl. 2018, 28, 201–211. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Bai, Y.; Schoenbach, P.; Gierus, M.; Taube, F. Effects of grazing management system on plant community structure and functioning in a semiarid steppe: Scaling from species to community. Plant Soil 2011, 340, 215–226. [Google Scholar] [CrossRef]
- Illius, A.I.; Gordon, I.J. Diet selection in mammalian herbivores: Constraints and tactics. In Diet Selection: An Interdisciplinary Approach to Foraging Behaviour; Hughes, R.N., Ed.; Blackwell Scientific: Oxford, UK, 1993; pp. 157–181. [Google Scholar]
- Rook, A.J.; Dumont, B.; Isselstein, J.; Osoro, K.; Wallisdevries, M.F.; Parente, G.; Mills, J. Matching type of livestock to desired biodiversity outcomes in pastures—A review. Biol. Conserv. 2004, 119, 137–150. [Google Scholar] [CrossRef]
- Phillips, C.J.C. Cattle Behaviour; Farming Press Books: Ipswich, UK, 1993. [Google Scholar]
- Catorci, A.; Gatti, R.; Cesaretti, S. Effect of sheep and horse grazing on species and functional composition of sub-Mediterranean grasslands. Appl. Veg. Sci. 2012, 15, 459–469. [Google Scholar] [CrossRef]
- Liang, M.; Liang, C.; Bai, X.; Miao, B.; Wang, Y.; Bao, G.; Wang, X. Effects of Annual Plants Functional Group on Biomass and Soil Respiration of Grazing Community in Typical Steppe Grassland. Pratacult. Sci. 2016, 33, 2407–2417. [Google Scholar]
- Bai, Y.; Wu, J.; Xing, Q.; Pan, Q.; Huang, J.; Yang, D.; Han, X. Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef]
- Lin, B.; Tan, Z.L.; Tang, S.X.; Sun, Z.H.; Wang, M. Research progress in methodologies for carrying capacity and proper stocking rate in grassland ecological system. Pratacult. Sci. 2008, 25, 91–99. [Google Scholar]
- Bai, Y.F.; Han, X.G.; Wu, J.G.; Chen, Z.Z.; Li, L.H. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Liang, C.; Tang, Y.; Harker-Schuch, I.; Porter, J.R. Effects and relationships of grazing intensity on multiple ecosystem services in the Inner Mongolian steppe. Sci. Total Environ. 2019, 675, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Martens, D. Methods of Soil Analysis Part 3—Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Lavorel, S.; Grigulis, K.; Mcintyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2010, 22, 134–147. [Google Scholar] [CrossRef]
- Leoni, E.; Altesor, A.; Paruelo, J.M. Explaining patterns of primary production from individual level traits. J. Veg. Sci. 2009, 20, 612–619. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; Volume 14, pp. 12–21. [Google Scholar]
- Zhang, Z.; Wang, S.P.; Nyren, P.; Jiang, G.M. Morphological and reproductive response of Caragana microphylla to different stocking rates. J. Arid Environ. 2006, 67, 671–677. [Google Scholar] [CrossRef]
- Takada, M.; Asada, M.; Miyashita, T. Regional differences in the morphology of a shrub Damnacanthus indicus: An induced resistance to deer herbivory? Ecol. Res. 2001, 16, 809–813. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and Lignin Control of Hardwood Leaf Litter Decomposition Dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Bodegom, P.M.V.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Cruz, P.; De Quadros, F.L.F.; Theau, J.P.; Frizzo, A.; Jouany, C.; Duru, M.; Carvalho, P.C.F. Leaf Traits as Functional Descriptors of the Intensity of Continuous Grazing in Native Grasslands in the South of Brazil. Rangel. Ecol. Manag. 2010, 63, 350–358. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Han, G.; Schellenberg, M.P.; Wu, Q.; Gu, C. Grazing induced changes in plant diversity is a critical factor controlling grassland productivity in the Desert Steppe, Northern China. Agric. Ecosyst. Environ. 2018, 265, 73–83. [Google Scholar] [CrossRef]
- Yan, P.; Gong, J.; Baoyin, T.; Luo, Q.; Zhai, Z.; Sha, X.; Wang, Y.; Min, L.; Yang, L. Effect of Seasonal Grazing on Trade-off Among Plant Functional Traits in Root, Stem and Leaf of Leymus chinensis in the Temperate Grassland of Inner Mongolia, China. Chin. Bull. Bot. 2017, 52, 307–321. [Google Scholar]
- Hooper, D.U.; Vitousek, P.M. The effects of plant composition and diversity on ecosystem processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-H.; Li, X.; Bai, W.-M.; Wang, Q.-B.; Yan, Z.-D.; Yuan, Z.-Y.; Dong, Y.-S. Soil carbon budget of a grazed Leymus chinensis steppe community in the Xilin river basin of Inner Mongolia. Phytoecol. Sin. 2004, 28, 312–317. [Google Scholar]
- Wang, S.; Wilkes, A.; Zhang, Z.; Chang, X.; Lang, R.; Wang, Y.; Niu, H. Management and land use change effects on soil carbon in northern China’s grasslands: A synthesis. Agric. Ecosyst. Environ. 2011, 142, 329–340. [Google Scholar] [CrossRef]
- Ford, H.; Garbutt, A.; Jones, L.; Jones, D.L. Methane, carbon dioxide and nitrous oxide fluxes from a temperate salt marsh: Grazing management does not alter Global Warming Potential. Estuar. Coast. Shelf Sci. 2012, 113, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhang, Z.; Wang, S.; Hu, Y.; Xu, G.; Luo, C.; Chang, X.; Duan, J.; Lin, Q.; Xu, B.; et al. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agric. For. Meteorol. 2011, 151, 792–802. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. REVIEW: Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Wang, M.J.; Han, G.D.; Zhao, M.L.; Chen, H.J.; Wang, Z.; Hao, X.L.; Tao, B.O. The effects of different grazing intensity on soil organic carbon content in meadow steppe. Pratacult. Sci. 2007, 24, 1. [Google Scholar]
- Louault, F.; Pillar, V.D.; Aufrere, J.; Garnier, E.; Soussana, J.F. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Veg. Sci. 2005, 16, 151–160. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]

| Species | Mean ± Std. Error | p-Value | |||
|---|---|---|---|---|---|
| Site NG | Site SG | Site CG | Site MG | ||
| Stipa grandis | 9.50 ± 1.90 c,* | 50.40 ± 9.00 b | 44.50 ± 7.00 b | 70.20 ± 3.6 a | <0.001 |
| Leymus chinensis | 46.00 ± 7.00 a | 8.10 ± 2.60 c | 22.00 ± 4.00 b | 6.40 ± 1.40 c | <0.001 |
| Anemarrhena asphodeloides | 29.40 ± 6.50 a | 1.40 ± 0.60 b | 8.60 ± 2.00 b | 2.90 ± 0.80 b | <0.001 |
| Cleistogenes squarrosa | 5.30 ± 1.20 b | 13.30 ± 4.20 a | 7.50 ± 2.40 a,b | 8.60 ± 1.60 a,b | 0.189 |
| Agropyrom cristatum | 6.90 ± 3.80 | 1.30 ± 1.30 | 6.40 ± 3.80 | 0.30 ± 0.30 | 0.243 |
| Salsola collina | 2.40 ± 1.30 | 5.30 ± 1.70 | 2.50 ± 1.70 | 4.50 ± 1.50 | 0.476 |
| Chenopodiaceae aristatum | 0 b | 3.00 ± 0.80 a | 0.10 ± 0.10 b | 2.20 ± 0.60 a | <0.001 |
| Allium mongolicum | 0.20 ± 0.20 | 0.90 ± 0.50 | 0.40 ± 0.20 | 1.00 ± 0.60 | 0.411 |
| Allium condensatum | 0.20 ± 0.10 | 0 | 1.20 ± 0.90 | 0 | 0.212 |
| Allium tenuissimum | 0 | 0 | 0.30 ± 0.30 | 0.30 ± 0.20 | 0.466 |
| Allium polyrhizum | 0 b | 7.00 ± 4.40 a | 0.40 ± 0.40 b | 2.00 ± 1.00 a,b | 0.134 |
| Allium bidentatum | 0 b | 3.80 ± 2.00 a | 0.70 ± 0.60 b | 0 b | 0.046 |
| Setaria viridis | 0 b | 1.10 ± 0.50 a | 0 b | 0.10 ± 0.10 b | 0.010 |
| Eragrostis pilosa | 0 b | 2.80 ± 1.80 a | 0 b | 0.60 ± 0.20 a,b | 0.114 |
| Portulaca oleracea | 0 b | 0.70 ± 0.40 a | 0 b | 0.40 ± 0.20 a,b | 0.049 |
| Astragalus galactites | 0 | 0.50 ± 0.50 | 1.00 ± 0.80 | 0.40 ± 0.30 | 0.643 |
| Convolvulus ammannii | 0 | 0 | 1.10 ± 0.80 | 0 | 0.201 |
| Caragana microphylla | 0 | 0 | 3.50 ± 3.50 | 0 | 0.405 |
| Thalictrum petaloideum | 0 | 0.30 ± 0.30 | 0 | 0.10 ± 0.10 | 0.569 |
| Carex korshinskyi | 0 | 0 | 0 | 0.04 ± 0.04 | 0.405 |
| Euphorbia humifusa | 0.10 ± 0.10 | 0 | 0 | 0 | 0.405 |
| Relevant Variables | Abbreviations | Unit | Definition ([45,58]) |
|---|---|---|---|
| Community-weighted mean traits | |||
| Mean height | CWM_H | cm | Average plant height |
| Mean leaf dry matter content | CWM_LDMC | mg g−1 | Ratio of average leaf dry weight to saturated fresh weight |
| Mean specific leaf area | CWM_SLA | m2 kg−1 | Ratio of average leaf area to dry leaf weight |
| Mean root nitrogen concentration | CWM_RNC | mg g−1 | Average nitrogen content per root dry mass |
| Mean root phosphorus concentration | CWM_RPC | mg g−1 | Average phosphorus content per unit of root dry mass |
| Mean stem nitrogen concentration | CWM_SNC | mg g−1 | Average nitrogen content per stem mass |
| Mean stem phosphorus concentration | CWM_SPC | mg g−1 | Average phosphorus content per stem mass |
| Mean leaf nitrogen concentration | CWM_LNC | mg g−1 | Average nitrogen content of the leaf stem mass |
| Mean leaf phosphorus concentration | CWM_LPC | mg g−1 | Average phosphorus content per unit of leaf dry mass |
| Ecosystem function | |||
| Aboveground biomass | AGB | g m−2 | Total dry weight of plants on the ground per unit area |
| Below-ground biomass | BGB | g m−2 | Biomass of herbaceous roots and rhizomes under grassland vegetation per unit area |
| Net ecosystem CO2 exchange | NEE | µmol m−2 s−1 | Carbon absorbed or emitted by the ecosystem |
| Ecosystem respiration | ER | µmol m−2 s−1 | Sum of aboveground respiration and soil respiration in the ecosystem |
| Gross ecosystem productivity | GEP | µmol m−2 s−1S | Amount of photosynthetic products fixed by organisms through photosynthesis in a unit of time |
| Traits | ER (µmol m−2 s−1) | NEE (µmol m−2 s−1) | GEP (µmol m−2 s−1) | BGB (g m−2) | AGB (g m−2) |
|---|---|---|---|---|---|
| Site SG | |||||
| CWM_H (cm) | 0.687 * | ||||
| Site CG | |||||
| CWM_RNC (mg g−1) | −0.876 ** | −0.682 * | 0.862 ** | 0.948 *** | |
| CWM_RPC (mg g−1) | −0.961 *** | −0.897 ** | 0.863 ** | ||
| CWM_SNC (mg g−1) | 0.95 *** | 0.892 ** | |||
| CWM_SPC (mg g−1) | −0.765 * | 0.932 *** | 0.983 *** | ||
| CWM_LDMC (mg g−1) | −0.737 * | −0.801 ** | |||
| CWM_LNC (mg g−1) | −0.695 * | ||||
| CWM_LPC (mg g−1) | −0.794 * | ||||
| CWM_SLA (m2 kg −1) | −0.846 ** | −0.982 *** | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, H.; Zhang, J.; Li, Z.; Wang, L.; Wang, Z.; Wu, Y.; Wang, Y.; Liang, C. Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China. Sustainability 2020, 12, 7169. https://doi.org/10.3390/su12177169
Wang W, Liu H, Zhang J, Li Z, Wang L, Wang Z, Wu Y, Wang Y, Liang C. Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China. Sustainability. 2020; 12(17):7169. https://doi.org/10.3390/su12177169
Chicago/Turabian StyleWang, Wen, Huamin Liu, Jinghui Zhang, Zhiyong Li, Lixin Wang, Zheng Wang, Yantao Wu, Yang Wang, and Cunzhu Liang. 2020. "Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China" Sustainability 12, no. 17: 7169. https://doi.org/10.3390/su12177169





