Effect of Adding Drinking Water Treatment Sludge on Excess Activated Sludge Digestion Process
Abstract
1. Introduction
2. Materials and Methods
2.1. DWTS and EAS Preparation
2.2. Aerobic and Anaerobic Digestion Experiment
2.3. Batch Tests Procedure
2.4. Analytical Methods
2.4.1. Chemical and Physical Analysis
2.4.2. Fluorescence EEM Analysis
2.4.3. PARAFAC Analysis
2.4.4. Fluorescence Regional Integration Analysis (FRI) Analysis
2.4.5. Determination of Triphenyl-Tetrazolium Chloride (TTC)-Dehydrogenase Activity (DHA) Test
3. Results and Discussion
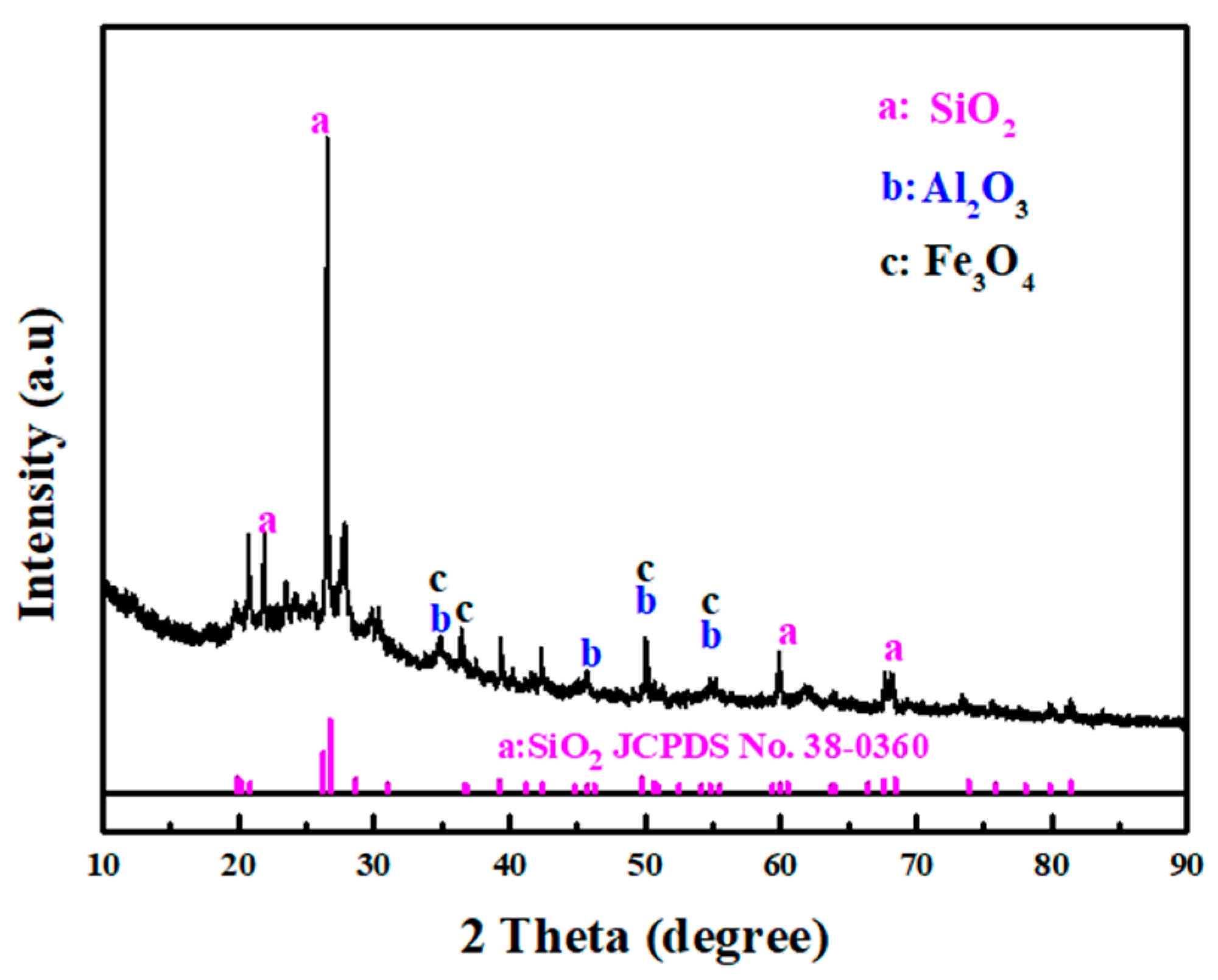
3.1. Characteristics of DWTS and EAS
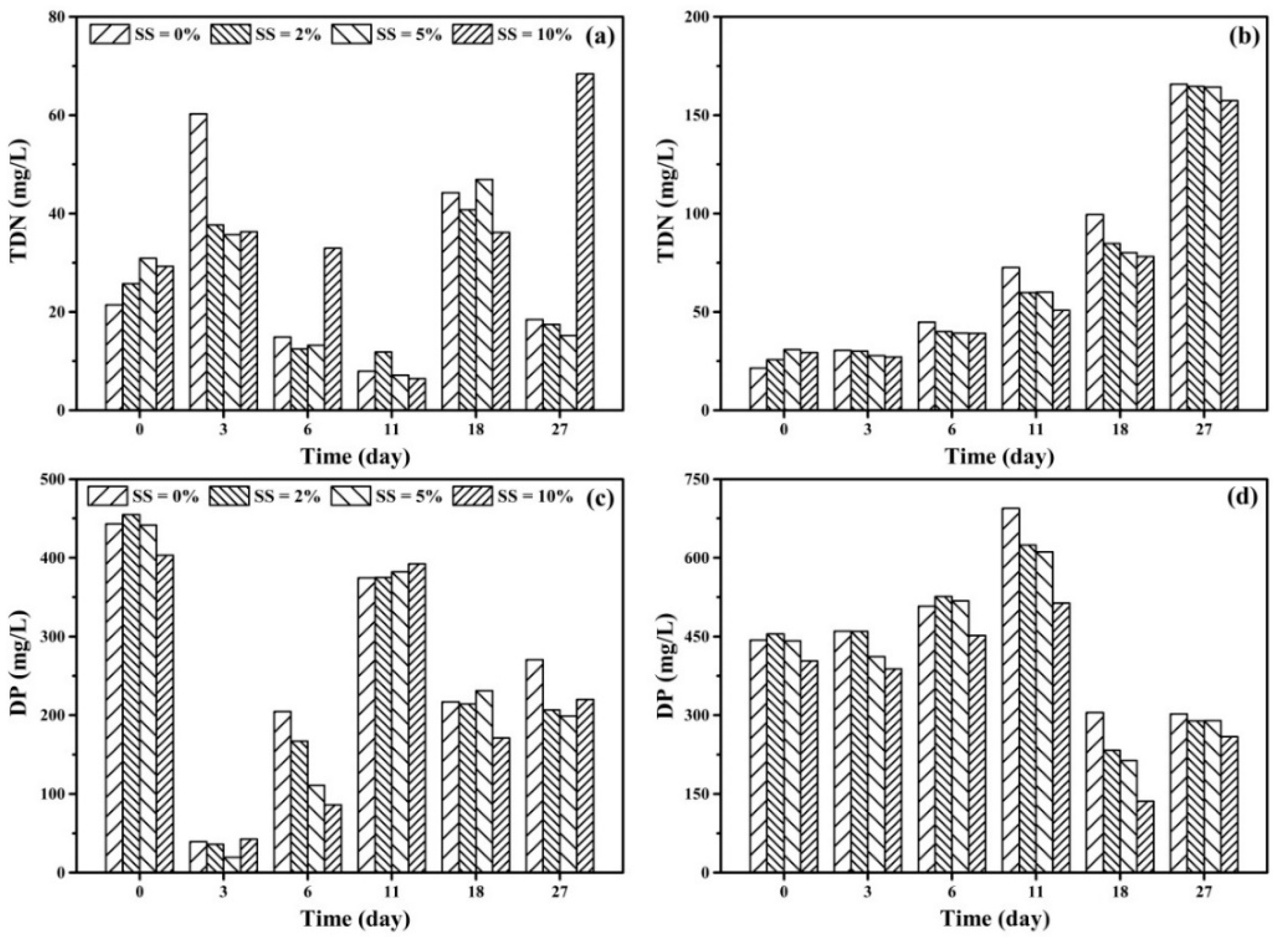
3.2. Effect of DWTS on the Removal of Nitrogen and Phosphorus
3.3. Variations of DOM Content and Composition
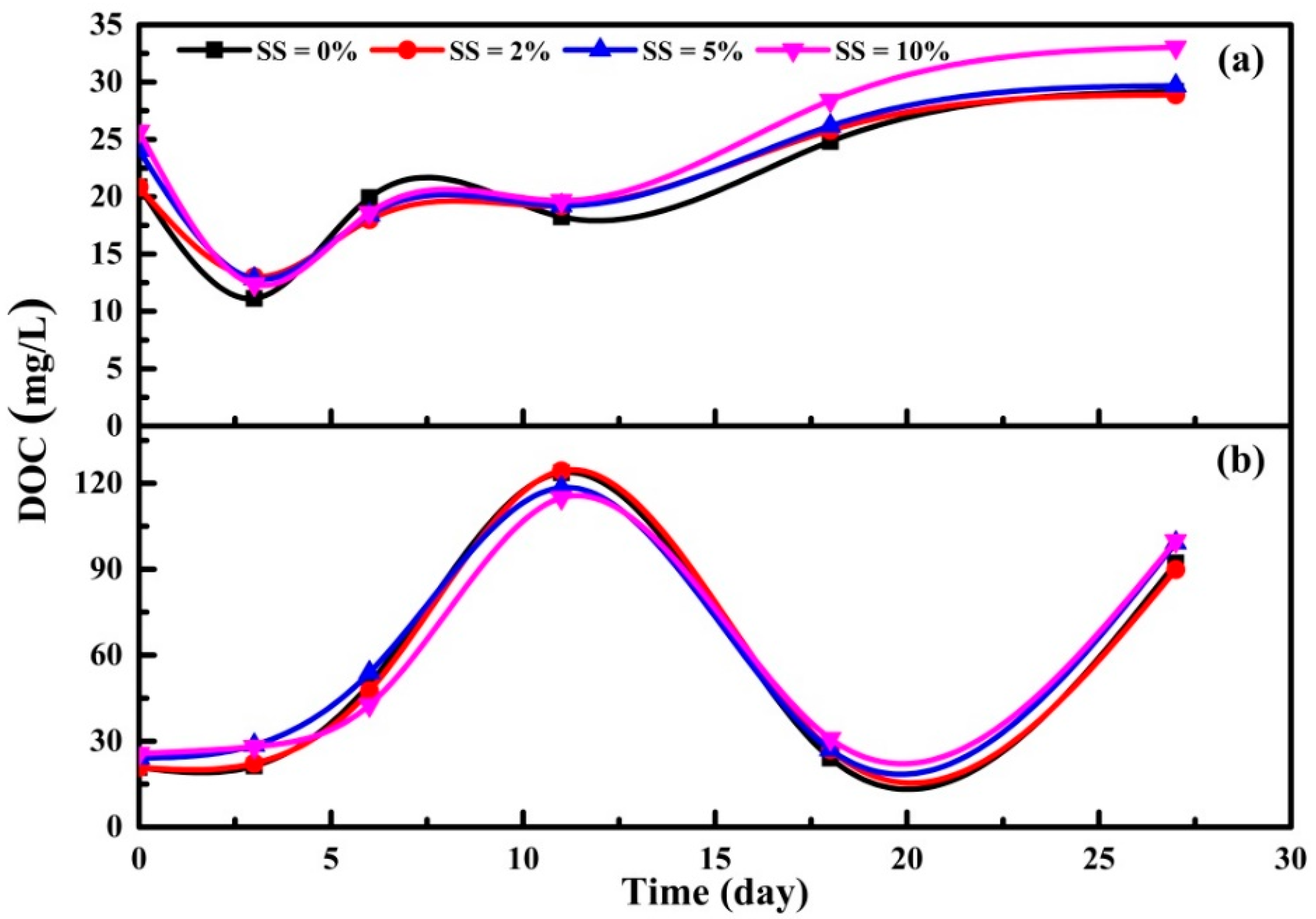
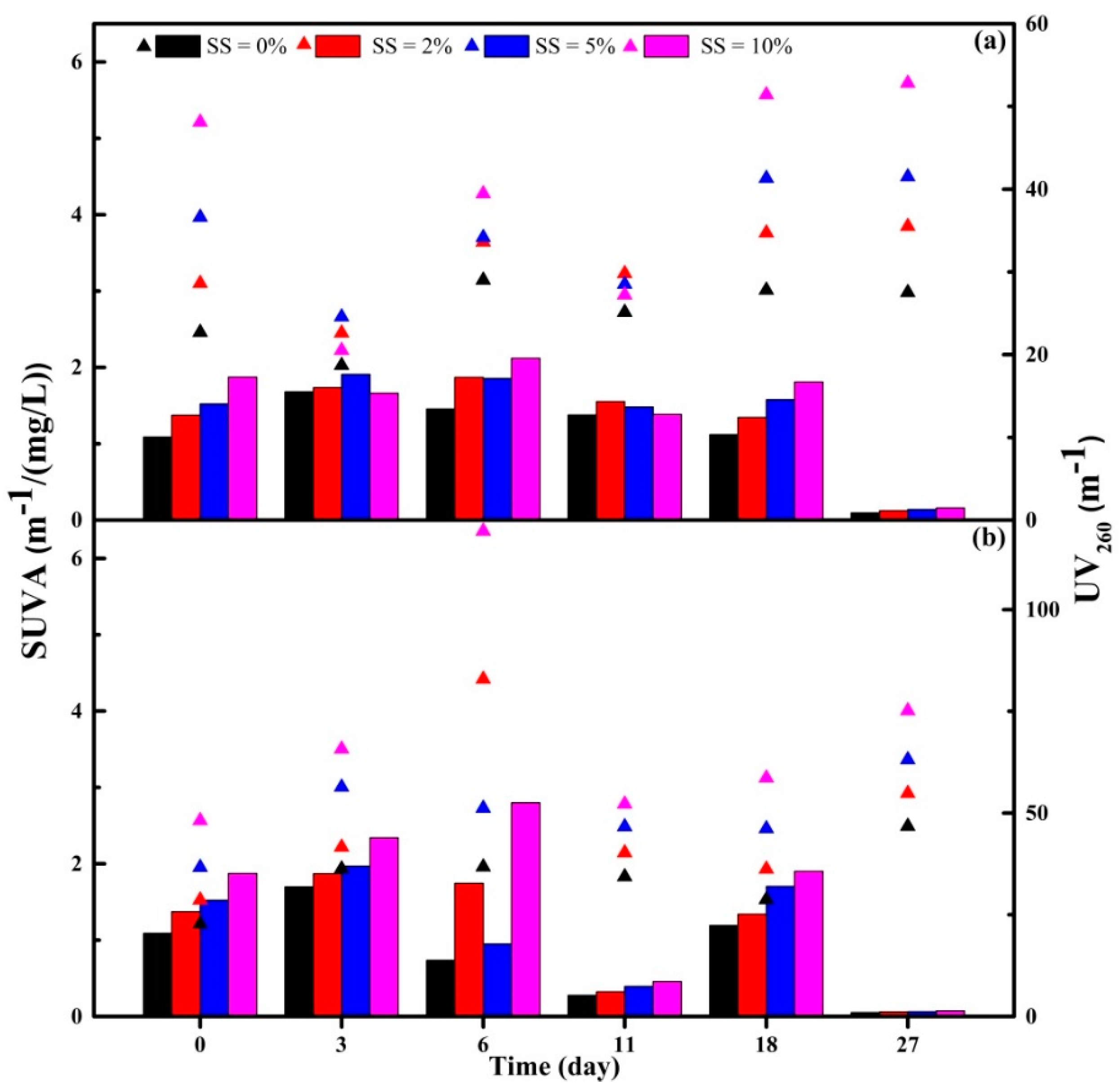
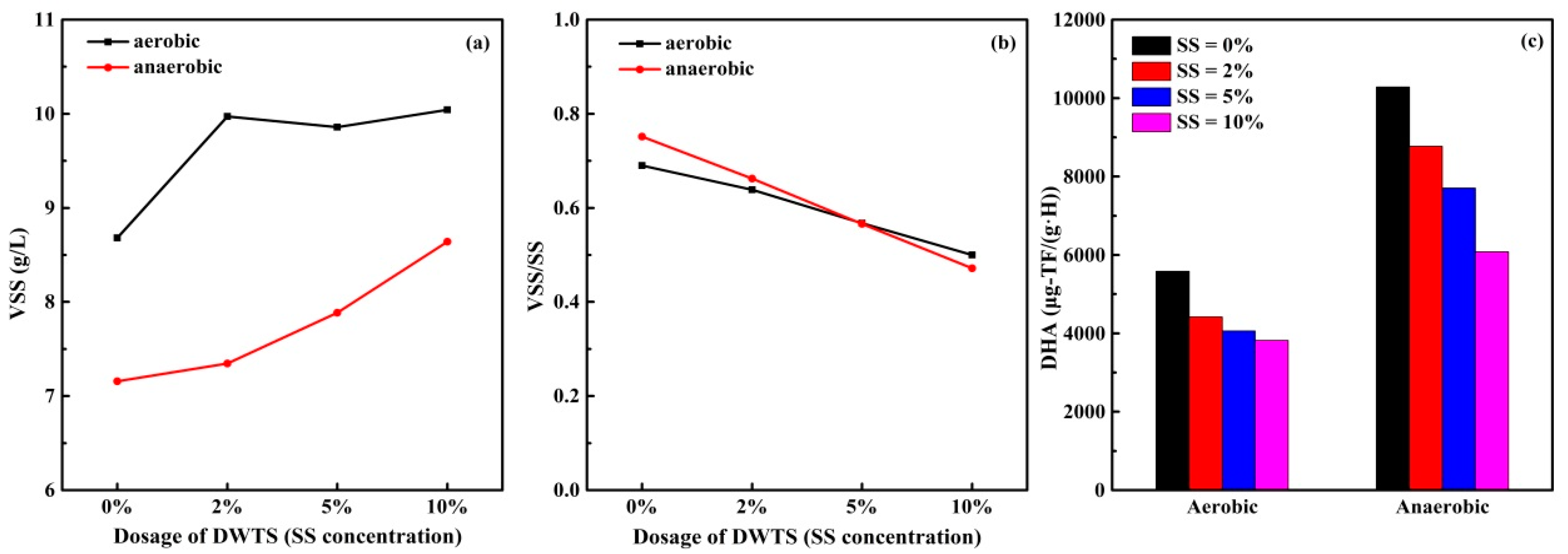
3.3.1. DOC, UV260 and SUVA Indexes of Supernatant
3.3.2. PARAFAC Component Characteristics
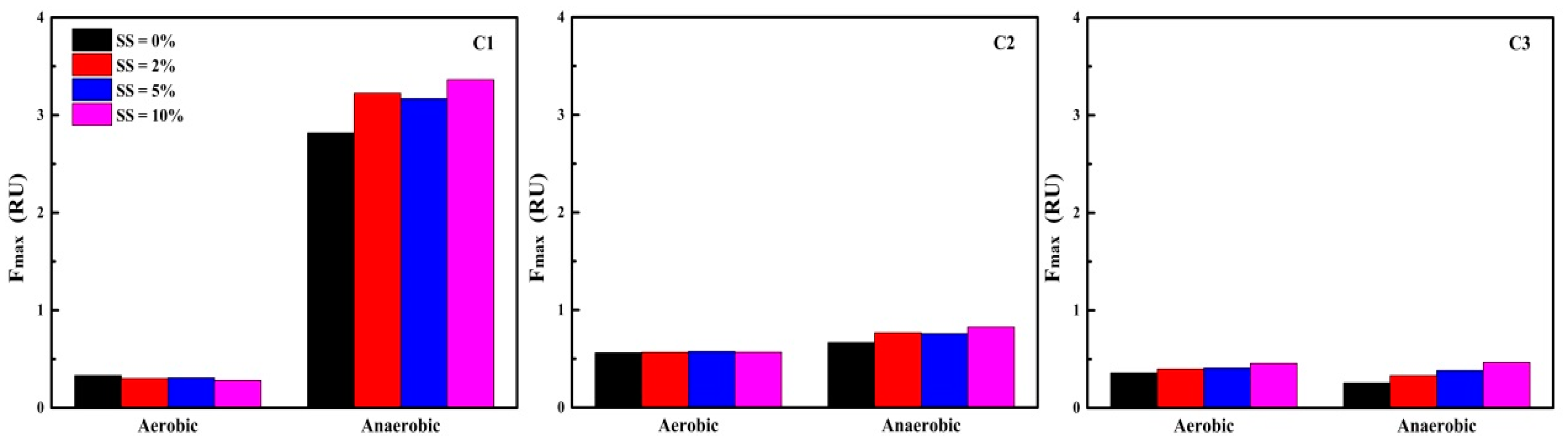
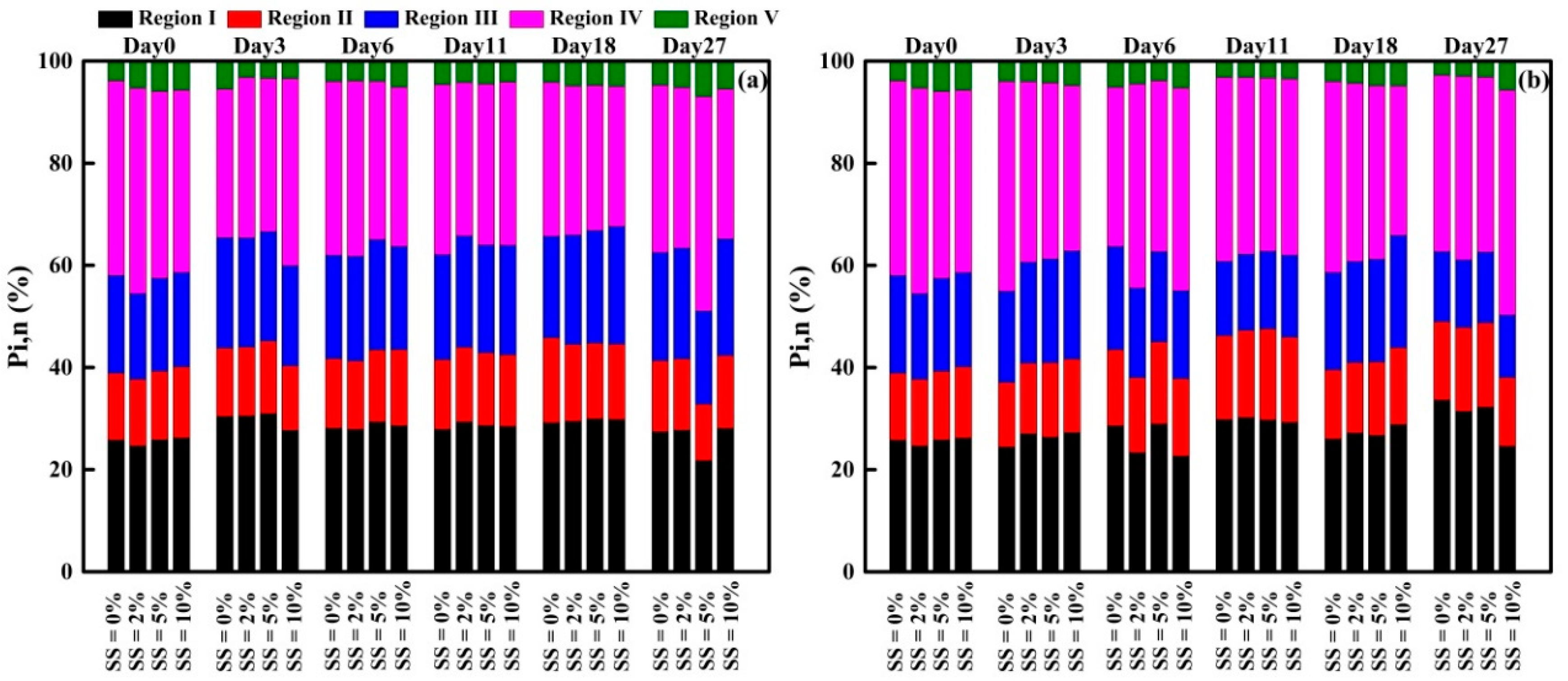
3.3.3. FRI Analysis of EEM Spectra
3.4. Study on Sludge Dehydrogenase Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meng, X.; Huang, Q.; Gao, H.; Tay, K.; Yan, J. Improved utilization of phosphorous from sewage sludge (as Fertilizer) after treatment by Low-Temperature combustion. Waste Manag. 2018, 80, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, V.W.-C.; Lim, T.-T. Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 2018, 226, 721–744. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, C.; Zhu, N. Disinhibition of the ammonium nitrogen in autothermal thermophilic aerobic digestion for sewage sludge by chemical precipitation. Bioresour. Technol. 2014, 169, 686–691. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, L.; Wang, L.; Li, Y. Enhancing phosphorus release from waste activated sludge containing ferric or aluminum phosphates by EDTA addition during anaerobic fermentation process. Chemosphere 2017, 171, 601–608. [Google Scholar] [CrossRef]
- Du, H.; Li, F. Characteristics of dissolved organic matter formed in aerobic and anaerobic digestion of excess activated sludge. Chemosphere 2017, 168, 1022–1031. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; van Loosdrecht, M.C.M.; Yu, J.; Liu, R. Adaptation of semi-continuous anaerobic sludge digestion to humic acids. Water Res. 2019, 161, 329–334. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Zhao, J.; Wu, Y.; Wu, L.; Li, H.; Tang, M.; Sun, Y.; Guo, W.; Feng, Q.; et al. Potential influences of exogenous pollutants occurred in waste activated sludge on anaerobic digestion: A review. J. Hazard. Mater. 2020, 383, 121176. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Hwang, M.J.; Park, D.S.; Ahn, K.H. Comprehensive reuse of drinking water treatment residuals in coagulation and adsorption processes. J. Environ. Manag. 2016, 181, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization of Water Treatment Plant’s Sludge and its Safe Disposal Options. Procedia Environ. Sci. 2016, 35, 950–955. [Google Scholar] [CrossRef]
- Cremades, L.V.; Cusidó, J.A.; Arteaga, F. Recycling of sludge from drinking water treatment as ceramic material for the manufacture of tiles. J. Clean. Prod. 2018, 201, 1071–1080. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Murcia, M.D.; Gómez, M.; Gómez, E.; García-Izquierdo, C.; Solano, C. Possible Uses for Sludge from Drinking Water Treatment Plants. J. Environ. Eng. 2017, 143, 04016088. [Google Scholar] [CrossRef]
- Hou, Q.; Meng, P.; Pei, H.; Hu, W.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Nair, A.T.; Ahammed, M.M. The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J. Clean. Prod. 2015, 96, 272–281. [Google Scholar] [CrossRef]
- Li, C.-W.; Lin, J.-L.; Kang, S.-F.; Liang, C.-L. Acidification and alkalization of textile chemical sludge: Volume/solid reduction, dewaterability, and Al(III) recovery. Sep. Purif. Technol. 2005, 42, 31–37. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Smith, A.D.; Judd, S.J. Coagulant recovery and reuse for drinking water treatment. Water Res. 2016, 88, 502–509. [Google Scholar] [CrossRef]
- Ooi, T.Y.; Yong, E.L.; Din, M.F.M.; Rezania, S.; Aminudin, E.; Chelliapan, S.; Rahman, A.A.; Park, J. Optimization of aluminium recovery from water treatment sludge using Response Surface Methodology. J. Environ. Manag. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- Suman, A.; Ahmad, T.; Ahmad, K. Dairy wastewater treatment using water treatment sludge as coagulant: A novel treatment approach. Environ. Dev. Sustain. 2018, 20, 1615–1625. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T.; Tanskanen, J.; Gao, B.; Yue, Q. Utilization of ferric groundwater treatment residuals for inorganic-organic hybrid biosorbent preparation and its use for vanadium removal. Chem. Eng. J. 2019, 361, 680–689. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Judd, S.J. An economic assessment of coagulant recovery from water treatment residuals. Desalination 2012, 287, 132–137. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Papelis, C.; Cath, T.Y.; Xu, P. Sorption of metals and metalloids from reverse osmosis concentrate on drinking water treatment solids. Sep. Purif. Technol. 2014, 134, 37–45. [Google Scholar] [CrossRef]
- Guan, X.-H.; Chen, G.-H.; Shang, C. Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Res. 2005, 39, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Mazari, L.; Abdessemed, D.; Szymczyk, A. Evaluating Reuse of Alum Sludge as Coagulant for Tertiary Wastewater Treatment. J. Environ. Eng. 2018, 144, 04018119. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q. Equilibrium and kinetic analysis of phosphorus adsorption from aqueous solution using waste alum sludge. J. Hazard. Mater. 2010, 184, 746–752. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Li, X.; Li, P.; Zhang, T.; Lv, X.; Liu, L.; Dong, J.; Zheng, D. Optimized removal of natural organic matter by ultrasound-assisted coagulation of recycling drinking water treatment sludge. Ultrason. Sonochem. 2018, 48, 171–180. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, V.C.; Mandal, T.K.; Mall, I.D. Synthesis of different crystallographic Al2O3 nanomaterials from solid waste for application in dye degradation. RSC Adv. 2014, 4, 50801–50810. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Lo, S.-L.; Kumar, N.; Kazmi, A.A.; Fakour, H. Preparation and reuse of iron and aluminum oxides activated sewage sludge based coagulants for the post-treatment of up-flow anaerobic sludge blanket reactor effluent. J. Clean. Prod. 2017, 149, 1020–1032. [Google Scholar] [CrossRef]
- Wang, H.-F.; Hu, H.; Wang, H.-J.; Zeng, R.J. Combined use of inorganic coagulants and cationic polyacrylamide for enhancing dewaterability of sewage sludge. J. Clean. Prod. 2019, 211, 387–395. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Sibille, C.; Ren, B. Evaluation of natural organic matter release from alum sludge reuse in wastewater treatment and its role in P adsorption. Chem. Eng. J. 2016, 302, 120–127. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T.; Liu, Z.; Zhu, S.; Cui, F.; Shi, W. The impact of recycling alum-humic-floc (AHF) on the removal of natural organic materials (NOM): Behavior of coagulation and adsorption. Chem. Eng. J. 2016, 284, 1049–1057. [Google Scholar] [CrossRef]
- Lee, B.M.; Seo, Y.S.; Hur, J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence EEM-PARAFAC. Water Res. 2015, 73, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zepp, R.G.; Sheldon, W.M.; Moran, M.A. Dissolved organic fluorophores in southeastern US coastal waters: Correction method for eliminating Rayleigh and Raman scattering peaks in excitation–emission matrices. Mar. Chem. 2004, 89, 15–36. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods UK 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol. Oceanogr. 2005, 50, 1415–1426. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Feng, W.; Tang, Z.; Giesy, J.P.; Guo, F.; Shi, D.; Liu, X.; Qin, N.; Xing, B.; et al. Fluorescence regional integration and differential fluorescence spectroscopy for analysis of structural characteristics and proton binding properties of fulvic acid sub-fractions. J. Environ. Sci. 2018, 74, 116–125. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.; Li, Q.; Zhao, Y.; Gao, M.; She, Z.; Jin, C. Three-dimensional fluorescence excitation–emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis at different pretreated temperatures. Environ. Sci. Pollut. Res. 2016, 23, 24061–24067. [Google Scholar] [CrossRef] [PubMed]
- González-Brambila, M.; Monroy, O.; López-Isunza, F. Experimental and theoretical study of membrane-aerated biofilm reactor behavior under different modes of oxygen supply for the treatment of synthetic wastewater. Chem. Eng. Sci. 2006, 61, 5268–5281. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Chou, P.-H.; Hua, C.-R.; Chien, K.-L.; Cheeseman, C. Lightweight bricks manufactured from water treatment sludge and rice husks. J. Hazard. Mater. 2009, 171, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.G.G.; Rohden, A.B.; Garcez, M.R.; Costa, E.B.; Dalt, S.; Oliveira Andrade, J.J. Valorization of water treatment sludge waste by application as supplementary cementitious material. Constr. Build. Mater. 2019, 223, 939–950. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Zeng, G.; Zhao, J.; Liu, Y.; Wang, Q.; Chen, F.; Li, X.; Yang, Q. Understanding the impact of cationic polyacrylamide on anaerobic digestion of waste activated sludge. Water Res. 2018, 130, 281–290. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, D.; Liu, Y.; Wang, Q.; Ni, B.-J.; Chen, F.; Yang, Q.; Li, X.; Zeng, G.; et al. Free nitrous acid promotes hydrogen production from dark fermentation of waste activated sludge. Water Res. 2018, 145, 113–124. [Google Scholar] [CrossRef]
- Arnaldos, M.; Pagilla, K. Effluent dissolved organic nitrogen and dissolved phosphorus removal by enhanced coagulation and microfiltration. Water Res. 2010, 44, 5306–5315. [Google Scholar] [CrossRef]
- Park, T.; Ampunan, V.; Lee, S.; Chung, E. Chemical behavior of different species of phosphorus in coagulation. Chemosphere 2016, 144, 2264–2269. [Google Scholar] [CrossRef]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristic and mechanisms of phosphoate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic matter fluorescence in municipal water recycling schemes: Toward a unified PARAFAC model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.P.; Skeriotis, A.T.; Miller, C.M. Assessment of dissolved organic matter fluorescence PARAFAC components before and after coagulation–filtration in a full scale water treatment plant. Water Res. 2013, 47, 1679–1690. [Google Scholar] [CrossRef]
- Liu, R.; Liu, N.; Liu, X.; Yu, H.; Li, B.; Song, Y. Spectroscopic and microscopic characteristics of natural aquatic nanoscale particles from riverine waters. J. Geochem. Explor. 2016, 170, 10–20. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Li, X.; Pan, H.; An, D.; Bai, S.; Li, D.; Cui, D. Fluorescence excitation–emission matrix spectra coupled with parallel factor and regional integration analysis to characterize organic matter humification. Chemosphere 2013, 93, 2208–2215. [Google Scholar] [CrossRef]
- Guo, L.; Lu, M.; Li, Q.; Zhang, J.; Zong, Y.; She, Z. Three-dimensional fluorescence excitation–emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis treated with multi-enzyme and thermophilic bacteria. Bioresour. Technol. 2014, 171, 22–28. [Google Scholar] [CrossRef]
- Ryssov-Nielsen, H. Measurement of the inhibition of respiration in activated sludge by a modified determination of the ttc-dehydrogenase activity. Water Res. 1975, 9, 1179–1185. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Yan, K.; Wang, Z.; Lu, G.; He, Y.; He, W. Differences in the response of soil dehydrogenase activity to Cd contamination are determined by the different substrates used for its determination. Chemosphere 2017, 169, 324–332. [Google Scholar] [CrossRef]
- Han, B.-C.; Jiang, W.-L.; Zhang, H.-L.; Wei, S.-L.; Liu, R.; Zhang, Y. Nitrogen removal enhancement and nitrous oxide emission reduction by activated sludge with pulse aeration. Process Saf. Environ. 2019, 129, 40–46. [Google Scholar] [CrossRef]


| Excess Activated Sludge (EAS) Condition | Day | DWTS Dosage | |||
|---|---|---|---|---|---|
| SS = 0% | SS = 2% | SS = 5% | SS = 10% | ||
| Aerobic | 0 | 6.45 | 6.43 | 6.44 | 6.48 |
| 3 | 5.73 | 5.78 | 5.95 | 5.65 | |
| 6 | 5.92 | 6.03 | 6.06 | 6.16 | |
| 11 | 5.90 | 5.99 | 5.93 | 5.88 | |
| 18 | 5.20 | 5.24 | 5.43 | 5.66 | |
| 27 | 5.61 | 5.68 | 5.77 | 5.93 | |
| Anaerobic | 0 | 6.45 | 6.43 | 6.44 | 6.48 |
| 3 | 6.49 | 6.41 | 6.41 | 6.41 | |
| 6 | 6.34 | 6.43 | 6.38 | 6.27 | |
| 11 | 6.28 | 6.21 | 6.18 | 6.14 | |
| 18 | 6.56 | 6.56 | 6.57 | 6.54 | |
| 27 | 6.56 | 6.66 | 6.59 | 6.61 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Du, H.; Li, F.; Su, H.; Bhat, S.A.; Hudori, H.; Rosadi, M.Y.; Arsyad, F.; Lu, Y.; Wu, H. Effect of Adding Drinking Water Treatment Sludge on Excess Activated Sludge Digestion Process. Sustainability 2020, 12, 6953. https://doi.org/10.3390/su12176953
Wu Y, Du H, Li F, Su H, Bhat SA, Hudori H, Rosadi MY, Arsyad F, Lu Y, Wu H. Effect of Adding Drinking Water Treatment Sludge on Excess Activated Sludge Digestion Process. Sustainability. 2020; 12(17):6953. https://doi.org/10.3390/su12176953
Chicago/Turabian StyleWu, Yanxia, Haixia Du, Fusheng Li, Haoning Su, Sartaj Ahmad Bhat, Hudori Hudori, Maulana Yusup Rosadi, Faisal Arsyad, Yanqiu Lu, and Huifang Wu. 2020. "Effect of Adding Drinking Water Treatment Sludge on Excess Activated Sludge Digestion Process" Sustainability 12, no. 17: 6953. https://doi.org/10.3390/su12176953
APA StyleWu, Y., Du, H., Li, F., Su, H., Bhat, S. A., Hudori, H., Rosadi, M. Y., Arsyad, F., Lu, Y., & Wu, H. (2020). Effect of Adding Drinking Water Treatment Sludge on Excess Activated Sludge Digestion Process. Sustainability, 12(17), 6953. https://doi.org/10.3390/su12176953






