Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea
Abstract
1. Introduction
2. Materials and Methods
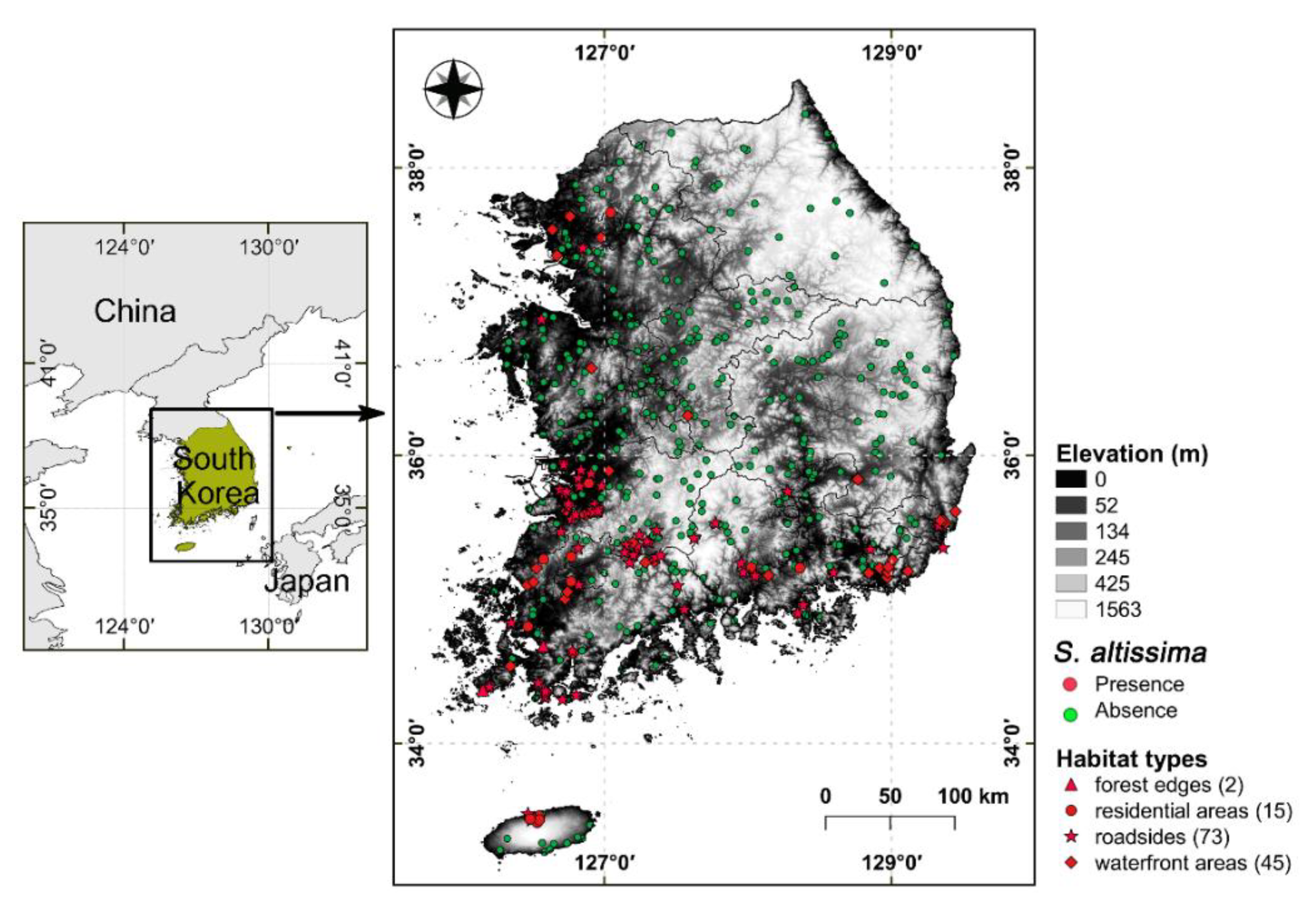
2.1. Study Area
2.2. Study Species
2.3. Model Variables
2.4. Species Distribution Modeling
3. Results
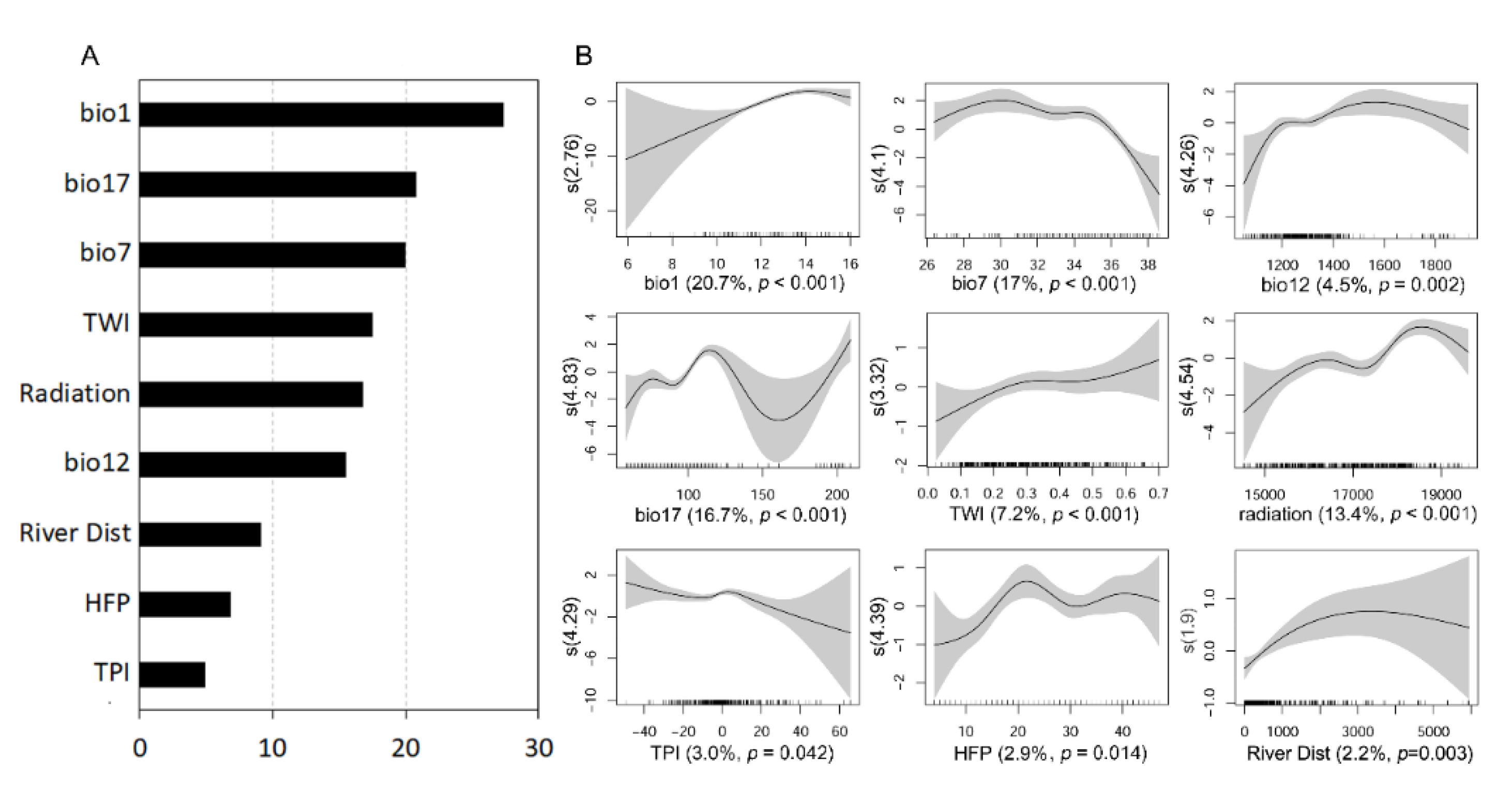
3.1. Variable Importance
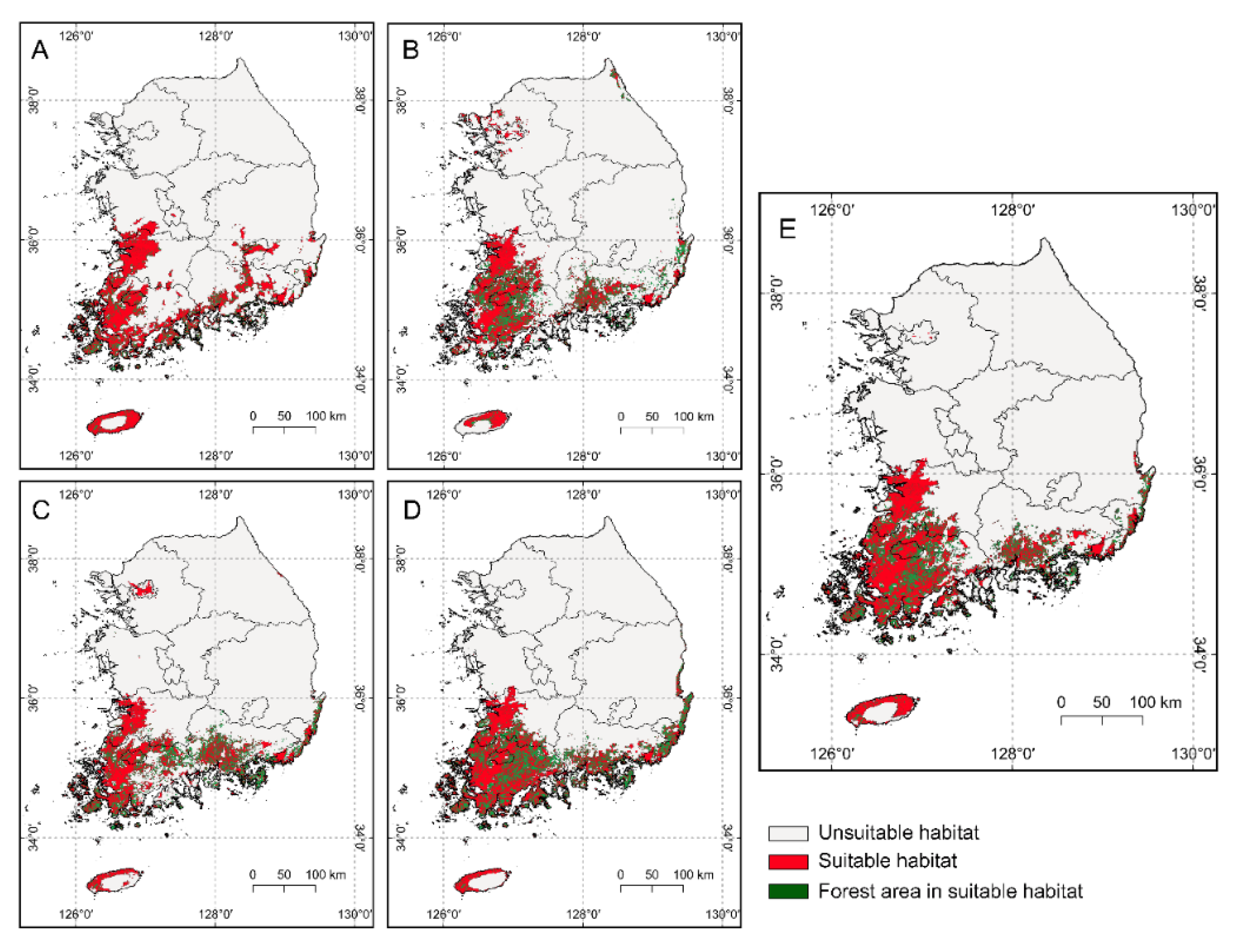
3.2. Current Habitat Suitability
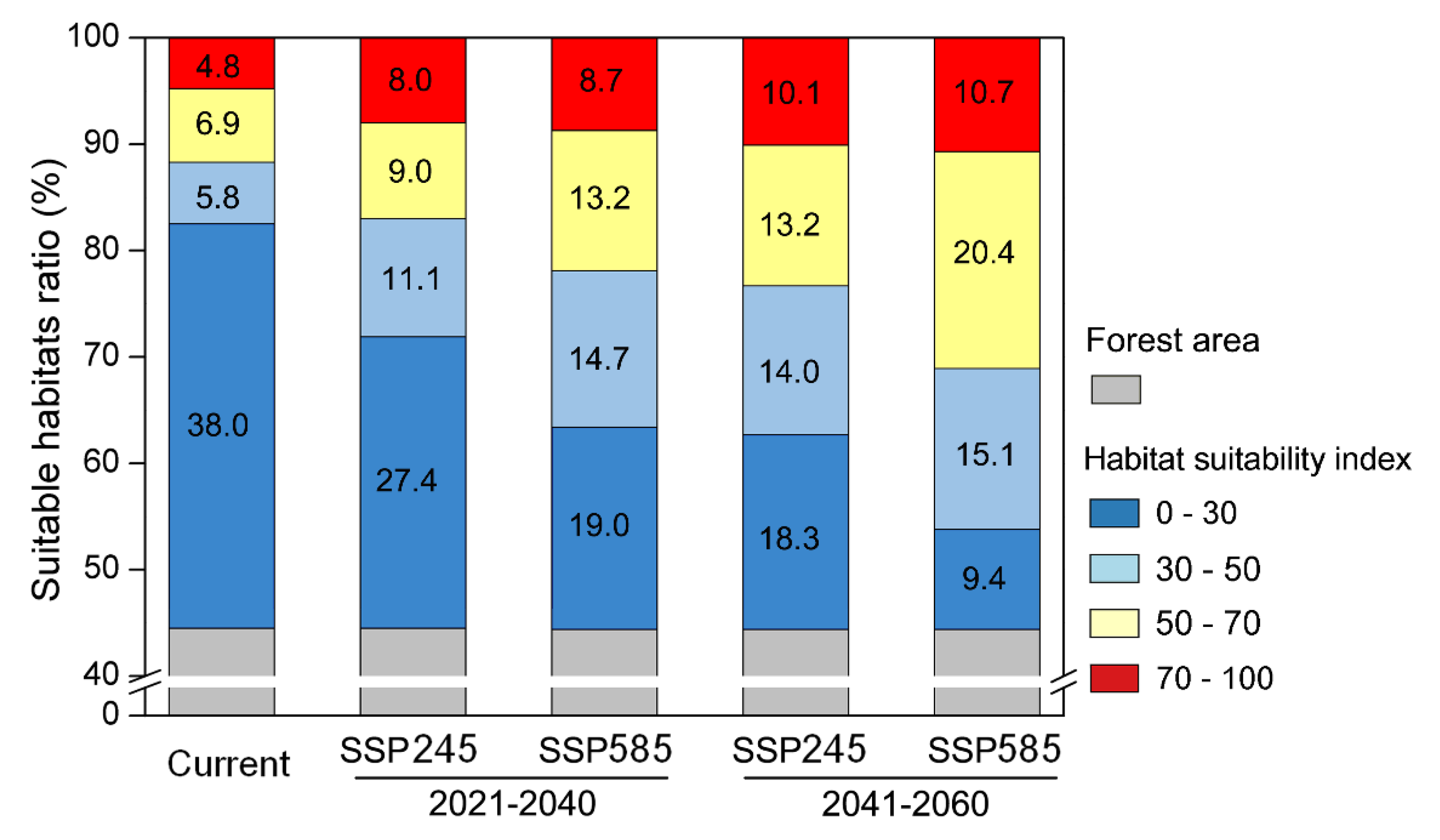
3.3. Future Habitat Suitability under Climate Change Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Szymura, M.; Szymura, T.H. Interactions between alien goldenrods (Solidago and Euthamia species) and comparison with native species in Central Europe. Flora-Morphol. Distrib. Funct. Ecol. Plants 2016, 218, 51–61. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 Habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111141. [Google Scholar]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Forcella, F. Final distribution is related to rate of spread in alien weeds. Weed Res. 1985, 25, 181–191. [Google Scholar] [CrossRef]
- Weber, E. The dynamics of plant invasions: A case study of three exotic goldenrod species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- Nakagawa, K.; Enomoto, T. The distribution of tall goldenrod (Solidago altissima L.) in Japan. Nogaku Kenkyu 1975, 55, 67–78. [Google Scholar]
- Weber, E. Biological flora of Central Europe: Solidago altissima L. Flora 2000, 195, 123–134. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility (GBIF). Available online: https://www.gbif.org/ (accessed on 10 June 2020).
- Kil, J.; Kim, C.G. Current status of naturalization by exotic ornamental plants in Korea. Weed Turfgrass Sci. 2014, 3, 206–214. [Google Scholar] [CrossRef]
- USDA Plants Database. Available online: https://plants.usda.gov (accessed on 12 June 2020).
- Weber, E.; Schmid, B. Das Neophytenproblem. Diss. Bot. 1993, 196, 207–227. [Google Scholar]
- Weber, E. Current and potential ranges of three exotic goldenrods (Solidago) in Europe. Conserv. Biol. 2001, 15, 122–128. [Google Scholar] [CrossRef]
- Zamora, D.L.; Thill, D.C.; Eplee, R.E. An eradication plan for plant invasions. Weed Technol. 1989, 3, 2–12. [Google Scholar] [CrossRef]
- Randall, J.M. Weed control for the preservation of biological diversity. Weed Technol. 1996, 10, 370–383. [Google Scholar] [CrossRef]
- Mooney, H.A.; Hofgaard, A.N. Biological invasions and global change. In Invasive Species and Biodiversity Management: Based On a Selection of Papers, Proceedings of the Norway/UN Conference on Alien Species, Trondheim, Norway, 1–5 July 1996; Sandlund, O.T., Schei, P.J., Viken, Å., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 24, pp. 139–148. [Google Scholar]
- Convention on Biological Diversity-Clearing House Mechanism Korea (CBD-CHM KOREA). Available online: http://www.kbr.go.kr/index.do (accessed on 5 June 2020).
- Korea Meteorological Administration (KMA). Available online: https://www.weather.go.kr (accessed on 12 June 2020).
- Kim, C.G.; Kil, J. Alien Flora of the Korean Peninsula. Boil. Invasions 2016, 18, 1843–1852. [Google Scholar] [CrossRef]
- Meyer, A.H.; Schmid, B. Experimental demography of the old-field perennial Solidago altissima: The dynamics of the shoot population. J. Ecol. 1999, 87, 17–27. [Google Scholar] [CrossRef]
- Socioeconomic Data and Applications Center (SEDAC). Available online: https://sedac.ciesin.columbia.edu (accessed on 10 May 2020).
- SAGA, System for Automated Geoscientific Analyses. Available online: http://www.saga-gis.org (accessed on 22 June 2020).
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology. Hydrol. Sci. Bull. 1979, 24, 43–69. [Google Scholar]
- WorldClim. Available online: https://worldclim.org (accessed on 2 May 2020).
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.4. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 10 April 2020).
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Araújo, M.B.; Whittaker, R.J.; Ladle, R.J.; Erhard, M. Reducing uncertainty in projections of extinction risk from climate change. Glob. Ecol. Biogeogr. 2005, 14, 529–538. [Google Scholar] [CrossRef]
- Gregory, A.W.; Smith, G.W.; Yetman, J. Testing for forecast consensus. J. Bus. Econ. Stat. 2001, 19, 34–43. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Crossman, N.D.; Bryan, B.A.; Summers, D.M. Identifying priority areas for reducing species vulnerability to climate change. Divers. Distrib. 2012, 18, 60–72. [Google Scholar] [CrossRef]
- Environmental Space Information Service. Available online: http://egis.me.go.kr/ (accessed on 29 July 2020).
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence–absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Purves, R.D. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J. Pharmacokinet. Biopharm. 1992, 20, 211–226. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Sun, P.; Wang, T.; Wang, G.; Zhang, X.; Wang, L. Consensus forecasting of species distributions: The effects of niche model performance and niche properties. PLoS ONE 2015, 10, e0120056. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G. PresenceAbsence: An R package for presence absence analysis. J. Stat. Softw. 2008, 23, 31. [Google Scholar] [CrossRef]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models; CRC press: Boca Raton, FL, USA, 2018. [Google Scholar]
- U.S. Forest Service. Plant of the Week. Available online: https://www.fs.fed.us/wildflowers/plant-of-the-week/solidago_altissima.shtml (accessed on 10 May 2020).
- Chun, J.H.; Lee, C.B. Assessing the effects of climate change on the geographic distribution of Pinus densiflora in Korea using Ecological Niche Model. Korean J. Agric. Forest Meteorol. 2013, 15, 219–233. [Google Scholar] [CrossRef]
- Cornelius, R. The strategies of Solidago canadensis L. in relation to urban habitats. III. Conformity to habitat dynamics. Acta Oecol. 1990, 11, 301–310. [Google Scholar]
- Kil, J.H.; Kim, Y.H.; Kim, H.M.; Kim, D.E.; Lee, D.H.; Hwang, S.M.; Lee, J.C.; Shin, H.C.; Kim, S.Y. Field Guide to the Invasive Alien Species in Korea; National Institute of Environmental Research: Incheon, Korea, 2013.
- Bornkamm, R.; Hennig, U. Experimentell-ökologische untersuchungen zur sukzession von ruderalen pflanzengesellschaften auf unterschiedlichen böden: I. zusammensetzung der vegetation. Flora 1982, 172, 267–316. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Dobley, K.B.; Houseknecht, H.R.; Pecone, C.A. Ecological divergence among five co-occurring species of old-field goldenrods. Plant. Ecol. 2005, 177, 43–56. [Google Scholar] [CrossRef]
- Viciani, D.; Vidali, M.; Gigante, D.; Bolpagni, R.; Villani, M.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; Angiolini, C.; et al. A first checklist of the alien-dominated vegetation in Italy. Plant Sociol. 2020, 57, 29–54. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Václavík, T.; Meentemeyer, R.K. Invasive species distribution modeling (iSDM): Are absence data and dispersal constraints needed to predict actual distributions? Ecol. Model. 2009, 220, 3248–3258. [Google Scholar] [CrossRef]
- Martin, T.G.; Murphy, H.; Liedloff, A.; Thomas, C.; Chadès, I.; Cook, G.; Fensham, R.; McIvor, J.; van Klinken, R.D. Buffel grass and climate change: A framework for projecting invasive species distributions when data are scarce. Biol. Invasions 2015, 17, 3197–3210. [Google Scholar] [CrossRef]

| Models | Sensitivity | Specificity | AUC | Kappa | Threshold | |
|---|---|---|---|---|---|---|
| Single model | GLM | 0.70 | 0.77 | 0.80 | 0.42 | 56 |
| GAM | 0.89 | 0.81 | 0.90 | 0.61 | 57 | |
| RF | 0.92 | 0.95 | 0.98 | 0.85 | 33 | |
| ANN | 0.83 | 0.81 | 0.87 | 0.56 | 62 | |
| Ensemble Model | 0.85 | 0.87 | 0.95 | 0.67 | 49 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Choi, D.; Kim, Y. Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability 2020, 12, 6710. https://doi.org/10.3390/su12176710
Park JS, Choi D, Kim Y. Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability. 2020; 12(17):6710. https://doi.org/10.3390/su12176710
Chicago/Turabian StylePark, Jeong Soo, Donghui Choi, and Youngha Kim. 2020. "Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea" Sustainability 12, no. 17: 6710. https://doi.org/10.3390/su12176710
APA StylePark, J. S., Choi, D., & Kim, Y. (2020). Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability, 12(17), 6710. https://doi.org/10.3390/su12176710





