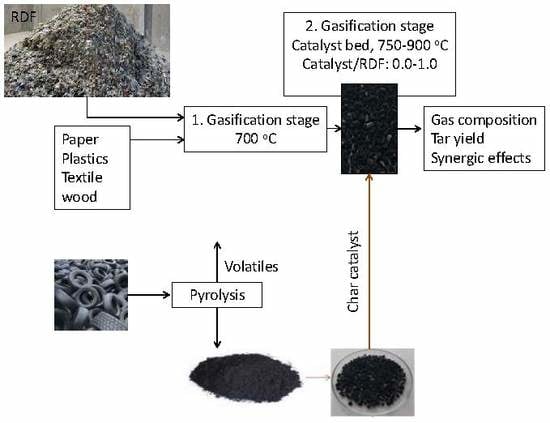
Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Characterization
- For i = H, O,
- TOD—theoretical oxygen demand,
- wi,dry—mass fraction of element i in dry feedstock,
- wi,raw—mass fraction of element i in raw feedstock,
- Mi—molecular weight of element i (g/mol).
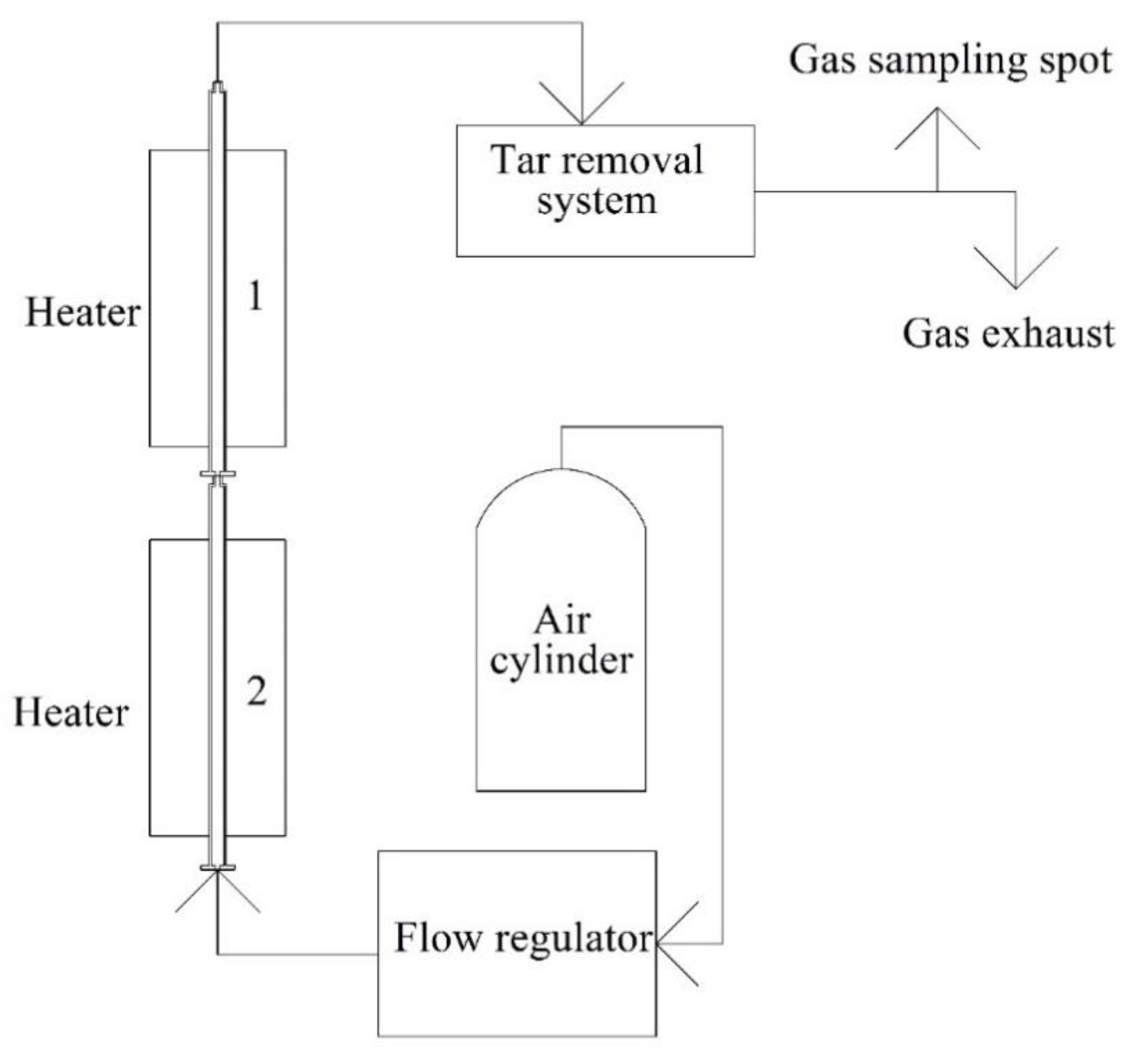
2.2. Experimental Apparatus
2.3. Experimental Procedure
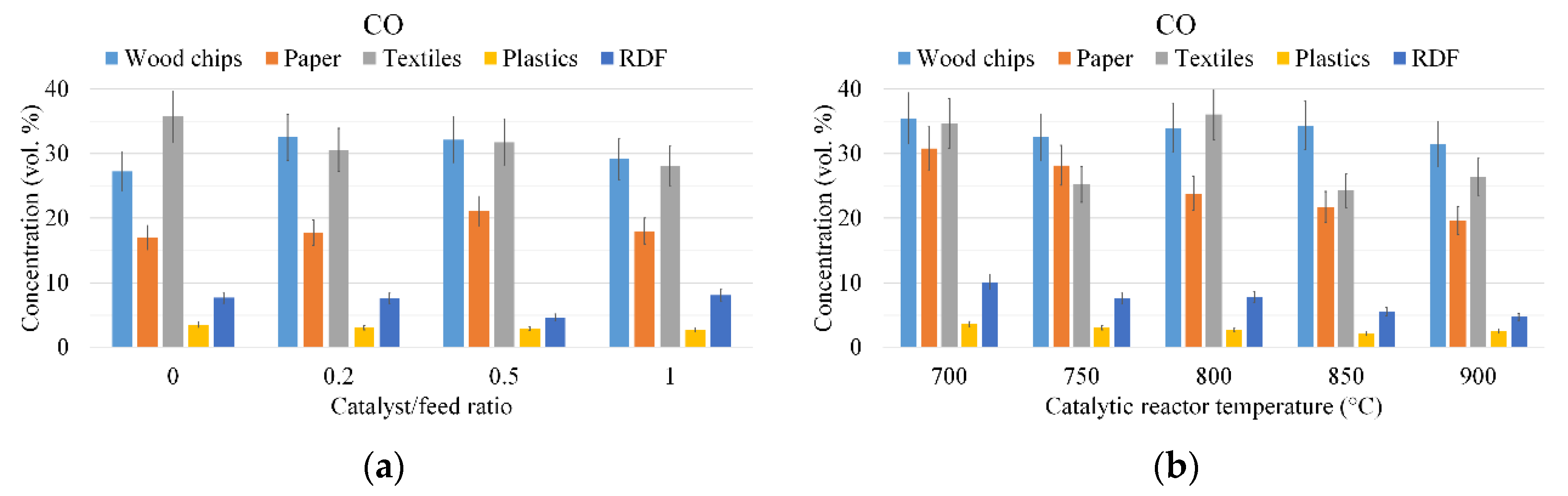
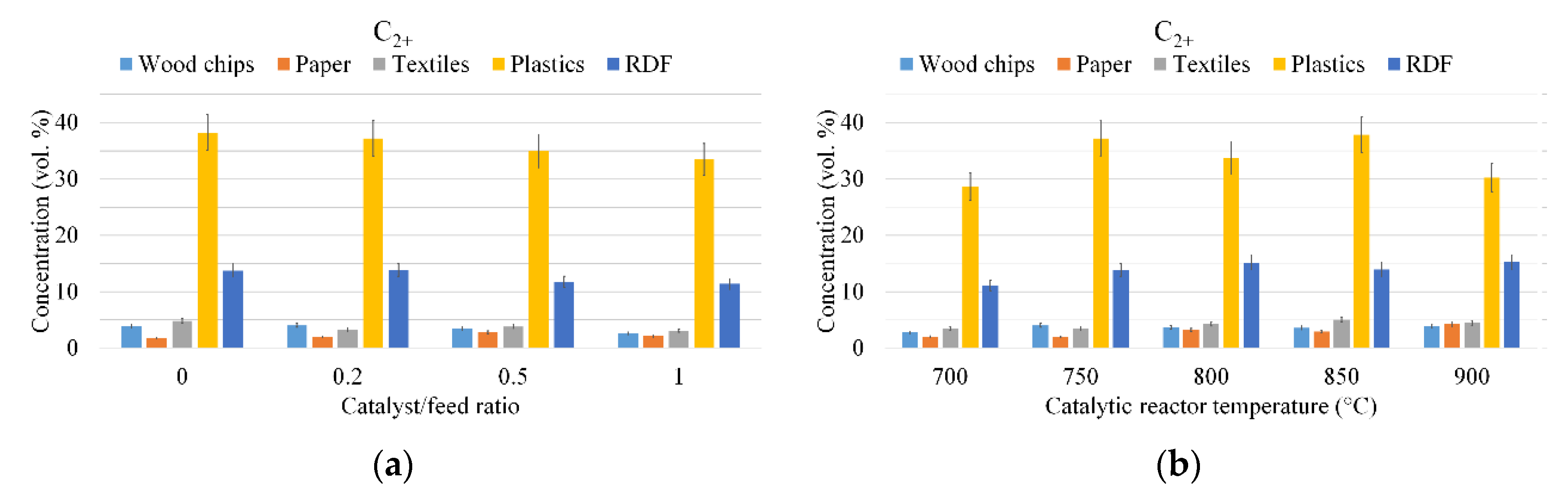
2.4. Experimental Conditions
2.5. Catalyst
2.6. Gas Analysis Procedure
- Initial temperature of the oven was 40 °C, hold time was 2 min.
- Increased temperature of the oven to 60 °C at a heating rate of 20 °C/min, hold time was 7.5 min.
- Increased temperature of the oven to 200 °C at a heating rate of 25 °C/min, hold time was 2 min.
2.7. Tar Analysis Procedure
3. Results and Discussion
3.1. Gas Composition
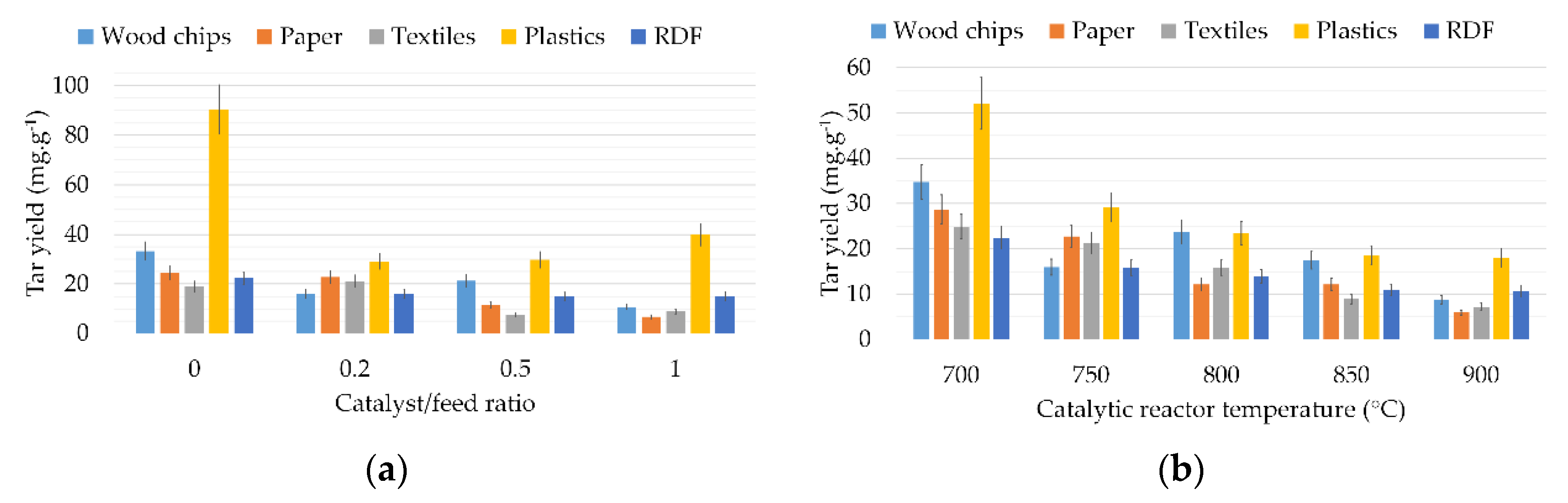
3.2. Tar Yields
3.3. Effect of Theoretical Oxygen Demand
3.4. Process Mass Balance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, L.; Giannis, A.; Lam, W.Y.; Lin, S.X.; Yin, K.; Yuan, G.A.; Wang, J.Y. Characterization of Singapore RDF resources and analysis of their heating value. Sustain. Environ. Res. 2016, 26, 51–54. [Google Scholar] [CrossRef]
- Haydary, J. Gasification of Refuse-Derived Fuel (RDF). Geosci. Eng. 2016, 62, 37–44. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Sarc, R.; Lorber, K.E. Production, quality and quality assurance of Refuse Derived Fuels (RDFs). Waste Manag. 2013, 33, 1825–1834. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Choi, G.G.; Jung, S.H.; Oh, S.J.; Kim, J.S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Striūgas, N.; Zakarauskas, K.; Stravinskas, G.; Grigaitienė, V. Comparison of steam reforming and partial oxidation of biomass pyrolysis tars over activated carbon derived from waste tire. Catal. Today 2012, 196, 67–74. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Y.; Song, Y.C.; Yi, Q.; Dong, L.; Li, T.T.; Zhang, L.; Feng, J.; Li, W.Y.; Li, C.Z. An advanced biomass gasification technology with integrated catalytic hot gas cleaning. Part III: Effects of inorganic species in char on the reforming of tars from wood and agricultural wastes. Fuel 2016, 183, 177–184. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Ko, D.C.K.; McKay, G. Production of active carbons from waste tyres—A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Husár, J.; Haydary, J.; Šuhaj, P.; Steltenpohl, P. Potential of tire pyrolysis char as tar cracking catalyst in solid waste and biomass gasification. Chem. Pap. 2019, 73, 2091–2101. [Google Scholar] [CrossRef]
- Steltenpohl, P.; Husár, J.; Šuhaj, P.; Haydary, J. Performance of Catalysts of Different Nature in Model Tar Component Decomposition. Catalysts 2019, 9, 894. [Google Scholar] [CrossRef]
- Ouadi, M.; Brammer, J.G.; Kay, M.; Hornung, A. Fixed bed downdraft gasification of paper industry wastes. Appl. Energy 2013, 103, 692–699. [Google Scholar] [CrossRef]
- Inayat, M.; Sulaiman, S.A.; Kurnia, J.C.; Shahbaz, M. Effect of various blended fuels on syngas quality and performance in catalytic co-gasification: A review. Renew. Sustain. Energy Rev. 2019, 105, 252–267. [Google Scholar] [CrossRef]
- Salavati, S.; Zhang, C.; Zhang, S.; Liu, Q.; Gholizadeh, M.; Hu, X. Cross-interaction during Co-gasification of wood, weed, plastic, tire and carton. J. Environ. Manag. 2019, 250, 109467. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Déparrois, N.; Singh, P.; Burra, K.G.; Gupta, A.K. Syngas production from co-pyrolysis and co-gasification of polystyrene and paper with CO2. Appl. Energy 2019, 246, 1–10. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Thermodynamic modelling and evaluation of a two-stage thermal process for waste gasification. Fuel 2013, 108, 356–369. [Google Scholar] [CrossRef]
- Van de Kamp, W.L.; de Wild, P.J.; Knoef, H.A.M.; Neeft, J.P.A.; Kiel, J.H.A. Tar Measurement in Biomass Gasification, Standardisation, and Supporting R&D. Available online: https://www.semanticscholar.org/paper/Tar-measurement-in-biomass-gasification%2C-and-R%26D-Kamp-Wild/8b2ee8f68ba439120043a781e6bcbfbbd77d1485 (accessed on 20 May 2020).
- Haydary, J.; Jelemenský, Ľ. Design of Biomass Gasification and Combined Heat and Power Plant Based on Laboratory Experiments. In International Congress on Energy Efficiency and Energy Related Materials (ENEFM2013); Springer: Antalya, Turkey, 2014; pp. 171–178. [Google Scholar]
- Sansaniwal, S.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Ahmed, I.; Gupta, A.K. Evolution of syngas from cardboard gasification. Appl. Energy 2009, 86, 1732–1740. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of steam input to the pyrolysis-gasification of waste plastics for improved production of hydrogen or carbon nanotubes. Appl Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Aluri, S.; Syed, A.; Flick, D.W.; Muzzy, J.D.; Sievers, C.; Agrawal, P.K. Pyrolysis and gasification studies of model refuse derived fuel (RDF) using thermogravimetric analysis. Fuel Process. Technol. 2018, 179, 154–166. [Google Scholar] [CrossRef]
- Šuhaj, P.; Haydary, J.; Husár, J.; Steltenpohl, P.; Šupa, I. Catalytic gasification of refuse-derived fuel in a two-stage laboratory scale pyrolysis/gasification unit with catalyst based on clay minerals. Waste Manag. 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Hervy, M.; Berhanu, S.; Weiss-Hortala, E.; Chesnaud, A.; Gerente, C.; Vilot, C.; Pham Minh, D.; Thorel, A.; Le Coq, L.; Nzihou, A. Multi-scale characterization of chars mineral species for tar cracking. Fuel 2017, 189, 88–97. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Syngas production by catalytic steam gasification of municipal solid waste in fixed-bed reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Guan, Y.; Luo, S.; Liu, S.; Xiao, B.; Cai, L. Steam catalytic gasification of municipal solid waste for producing tar-free fuel gas. Int. J. Hydrog. Energy 2009, 34, 9341–9346. [Google Scholar] [CrossRef]




| Material | N (wt.%) | C (wt.%) | H (wt.%) | S (wt.%) | O 1 (wt.%) |
|---|---|---|---|---|---|
| Paper | 0.250 ± 0.110 | 35.08 ± 0.19 | 5.28 ± 0.04 | 0.000 | 43.91 ± 0.20 |
| Plastics | 0.533 ± 0.061 | 78.40 ± 1.80 | 12.56 ± 0.25 | 0.003 ± 0.010 | 2.70 ± 2.00 |
| Wood chips | 0.303 ± 0.036 | 46.02 ± 0.21 | 6.38 ± 0.11 | 0.027 ± 0.080 | 45.35 ± 0.20 |
| Textiles | 0.597 ± 0.010 | 45.48 ± 0.32 | 6.59 ± 0.17 | 0.083 ± 0.027 | 43.23 ± 0.22 |
| RDF | 1.970 ± 0.700 | 50.60 ± 3.00 | 7.22 ± 0.82 | 0.310 ± 0.250 | 17.80 ± 4.10 |
| Material | Moisture (wt.%) | Volatiles (wt.%) | Fixed Carbon (wt.%) | Ash (wt.%) | HHV (MJ/kg) | TOD (Mass Ratio) |
|---|---|---|---|---|---|---|
| Paper | 2.69 ± 0.67 | 74.8 ± 2.8 | 7.40 ± 2.50 | 15.50 ± 1.80 | 10.2 ± 1.7 | 0.944 ± 0.006 |
| Plastics | 0.34 ± 0.09 | 93.7 ± 3.5 | 0.04 ± 0.02 | 5.90 ± 0.67 | 42.4 ± 4.1 | 3.079 ± 0.048 |
| Wood chips | 5.10 ± 1.30 | 77.3 ± 2.9 | 15.60 ± 5.20 | 1.92 ± 0.22 | 18.2 ± 0.4 | 1.353 ± 0.012 |
| Textiles | 3.05 ± 0.76 | 86.2 ± 3.2 | 6.70 ± 2.30 | 4.01 ± 0.45 | 18.4 ± 0.4 | 1.349± 0.020 |
| RDF | 2.00 ± 0.50 | 69.1 ± 2.6 | 6.80 ± 2.30 | 22.05 ± 2.50 | 21.0 ± 1.6 | 1.780 ± 0.100 |
| Name of the Component | Quantity (%) |
|---|---|
| Plastics | 75 |
| Wood | 5 |
| Paper | 5 |
| Cardboard | 2 |
| Rubber | 3 |
| Textile | 10 |
| Catalyst | SBET (m2/g) | νp (cm3/g) | Dp (nm) |
|---|---|---|---|
| Raw pyrolysis char | 28.30 | 0.279 | 34.05 |
| Compacted and carbonized pyrolysis char | 67.48 | 0.324 | 17.44 |
| Catalyst/Feed Ratio | 0 | 2 | 5 | 10 | 2 | 2 | 2 | 2 | 2 |
|---|---|---|---|---|---|---|---|---|---|
| Catalytic reactor temperature (°C) | 750 | 750 | 750 | 750 | 700 | 750 | 800 | 850 | 900 |
| H2/CO | |||||||||
| Wood chips | 0.269 | 0.273 | 0.256 | 0.390 | 0.123 | 0.273 | 0.197 | 0.222 | 0.343 |
| Paper | 0.648 | 0.706 | 0.673 | 1.110 | 0.827 | 0.706 | 0.867 | 0.873 | 0.811 |
| Textiles | 0.165 | 0.203 | 0.300 | 0.444 | 0.181 | 0.376 | 0.255 | 0.429 | 0.496 |
| Plastics | 2.240 | 3.210 | 4.020 | 5.650 | 2.450 | 3.210 | 3.820 | 5.420 | 6.120 |
| RDF | 0.911 | 1.110 | 2.250 | 1.850 | 0.688 | 1.110 | 1.176 | 2.630 | 3.440 |
| Net heating value (MJ/Nm3) | |||||||||
| Wood chips | 6.82 | 7.12 | 6.80 | 6.27 | 6.15 | 7.12 | 6.97 | 6.90 | 7.11 |
| Paper | 3.85 | 4.40 | 5.23 | 5.00 | 4.20 | 4.40 | 5.40 | 5.52 | 6.34 |
| Textiles | 6.31 | 5.57 | 5.81 | 5.57 | 5.61 | 5.50 | 6.40 | 6.34 | 6.32 |
| Plastics | 18.90 | 19.00 | 18.1 | 17.7 | 14.70 | 19.00 | 17.70 | 20.60 | 17.50 |
| RDF | 8.82 | 9.17 | 7.71 | 8.62 | 7.71 | 9.17 | 10.00 | 10.10 | 10.90 |
| Catalytic Reactor Temperature (°C) | 700 | 750 | 800 | 850 | 900 |
| Gas (wt.%) | |||||
| Wood chips | 85.6 ± 1.5 | 90.7 ± 0.8 | 88.5 ± 1.1 | 91.1 ± 0.9 | 93.0 ± 0.6 |
| Paper | 75.9 ± 1.6 | 77.2 ± 1.5 | 81.8 ± 1.0 | 79.7 ± 1.2 | 81.5 ± 1.0 |
| Textile | 89.3 ± 1.0 | 91.7 ± 0.5 | 90.7 ± 0.9 | 90.7 ± 0.9 | 93.2 ± 0.6 |
| Plastics | 78.7 ± 2.0 | 84.0 ± 1.4 | 84.3 ± 1.4 | 85.3 ± 1.2 | 87.2 ± 1.0 |
| RDF | 70.4 ± 2.0 | 70.8 ± 1.9 | 72.5 ± 1.7 | 72.8 ± 1.8 | 73.9 ± 1.5 |
| Liquid (wt.%) | |||||
| Wood chips | 12.4 ± 1.4 | 6.2 ± 0.7 | 8.6 ± 1.0 | 7.2 ± 0.8 | 4.6 ± 0.5 |
| Paper | 7.2 ± 0.8 | 7.1 ± 0.8 | 2.3 ± 0.3 | 4.4 ± 0.5 | 2.5 ± 0.3 |
| Textile | 7.9 ± 0.9 | 5.9 ± 0.7 | 6.9 ± 0.8 | 7.1 ± 0.8 | 4.6 ± 0.5 |
| Plastics | 15.6 ± 1.7 | 10.0 ± 1.1 | 9.8 ± 1.1 | 8.5 ± 0.9 | 6.7 ± 0.7 |
| RDF | 9.2 ± 1.0 | 8.4 ± 0.9 | 5.7 ± 0.6 | 5.2 ± 0.6 | 4.6 ± 0.5 |
| Solid (wt.%) | |||||
| Wood chips | 2.0 ± 0.1 | 3.1 ± 0.2 | 2.8 ± 0.2 | 1.7 ± 0.1 | 2.4 ± 0.2 |
| Paper | 17.0 ± 0.8 | 15.7 ± 0.8 | 15.9 ± 0.8 | 15.9 ± 0.8 | 16.1 ± 0.8 |
| Textile | 2.8 ± 0.2 | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 |
| Plastics | 5.7 ± 0.3 | 6.0 ± 0.3 | 5.9 ± 0.3 | 6.2 ± 0.3 | 6.1 ± 0.3 |
| RDF | 20.4 ± 1.0 | 20.8 ± 1.0 | 21.8 ± 1.0 | 22.0 ± 1.2 | 21.5 ± 1.0 |
| Catalyst/Feed ratio | 0 | 0.2 | 0.5 | 1 | |
| Gas (wt.%) | |||||
| Wood chips | 84.3 ± 1.6 | 90.7 ± 0.8 | 91.1 ± 0.9 | 93.0 ± 0.6 | |
| Paper | 77.3 ± 1.4 | 77.2 ± 1.5 | 80.2 ± 1.2 | 78.8 ± 1.3 | |
| Textile | 92.7 ± 0.6 | 91.7 ± 0.8 | 93.7 ± 0.6 | 93.7 ± 0.6 | |
| Plastics | 74.3 ± 2.4 | 84.0 ± 1.4 | 83.5 ± 1.5 | 83.0 ± 1.9 | |
| RDF | 67.0 ± 2.0 | 66.8 ± 1.9 | 67 ± 2.0 | 66.2 ± 2.1 | |
| Liquid (wt.%) | |||||
| Wood chips | 13.1 ± 1.4 | 6.2 ± 0.7 | 7.7 ± 0.9 | 4.7 ± 0.5 | |
| Paper | 4.8 ± 0.5 | 7.1 ± 0.8 | 4.5 ± 0.5 | 5.0 ± 0.6 | |
| Textile | 4.6 ± 0.5 | 5.9 ± 0.7 | 3.7 ± 0.4 | 3.7 ± 0.4 | |
| Plastics | 18.8 ± 2.1 | 10.0 ± 1.1 | 11.0 ± 1.2 | 11.2 ± 1.7 | |
| RDF | 6.7 ± 0.7 | 7.4 ± 0.9 | 7.3 ± 0.8 | 8.0 ± 0.9 | |
| Solid (wt.%) | |||||
| Wood chips | 2.7 ± 0.2 | 3.1 ± 0.2 | 1.2 ± 0.1 | 2.4 ± 0.2 | |
| Paper | 17.9 ± 0.9 | 15.7 ± 0.8 | 15.4 ± 0.7 | 16.2 ± 0.8 | |
| Textile | 2.7 ± 0.2 | 2.3 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.2 | |
| Plastics | 6.9 ± 0.4 | 6.0 ± 0.3 | 5.5 ± 0.3 | 5.9 ± 0.3 | |
| RDF | 26.3 ± 1.3 | 25.8 ± 1.0 | 25.8 ± 1.2 | 25.8 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuhaj, P.; Husár, J.; Haydary, J. Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst. Sustainability 2020, 12, 6647. https://doi.org/10.3390/su12166647
Šuhaj P, Husár J, Haydary J. Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst. Sustainability. 2020; 12(16):6647. https://doi.org/10.3390/su12166647
Chicago/Turabian StyleŠuhaj, Patrik, Jakub Husár, and Juma Haydary. 2020. "Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst" Sustainability 12, no. 16: 6647. https://doi.org/10.3390/su12166647
APA StyleŠuhaj, P., Husár, J., & Haydary, J. (2020). Gasification of RDF and Its Components with Tire Pyrolysis Char as Tar-Cracking Catalyst. Sustainability, 12(16), 6647. https://doi.org/10.3390/su12166647