Harnessing Opportunities Provided by the Invasive Chromolaena odorata to Keep It under Control
Abstract
1. Introduction
2. Materials and Methods
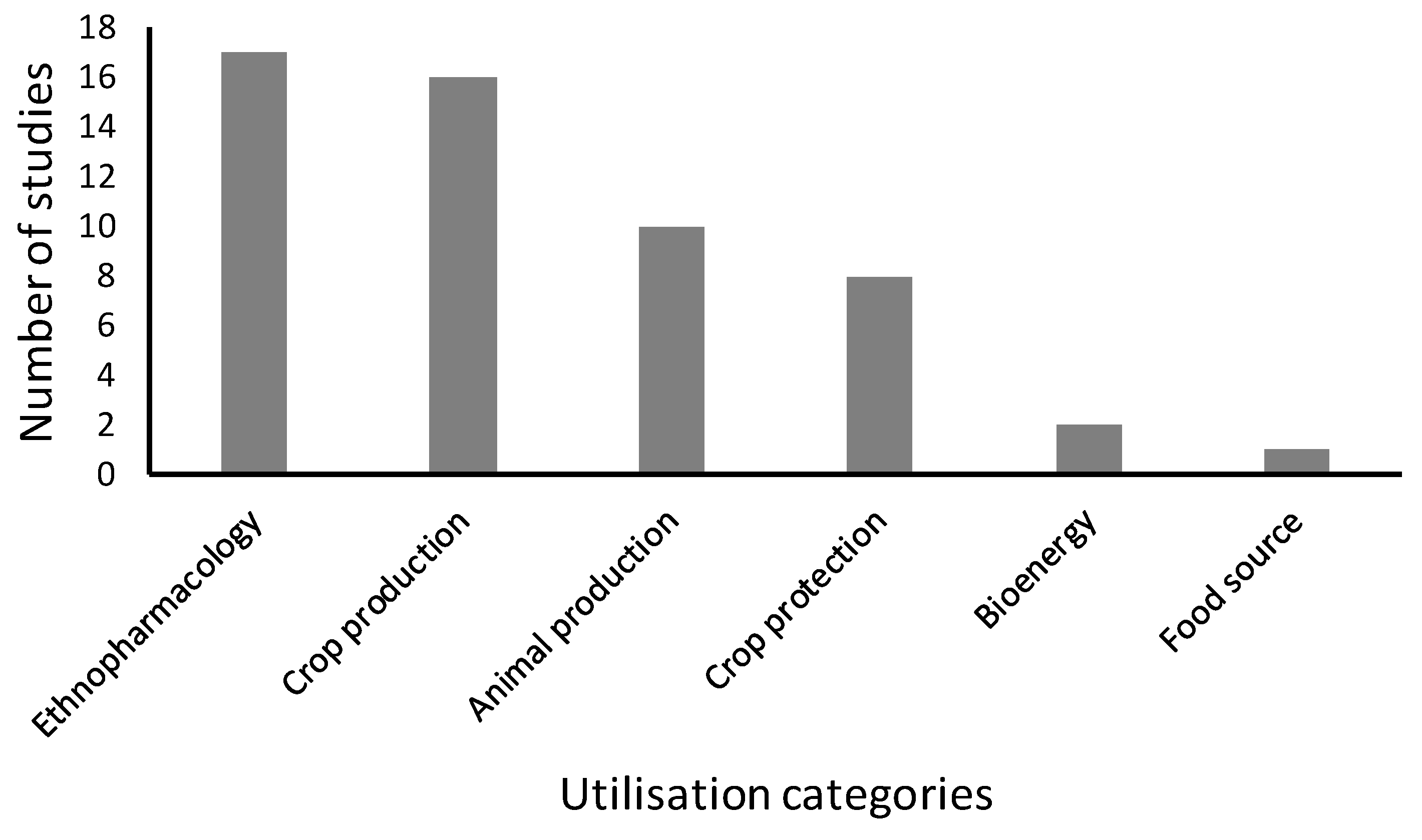
3. Results and Discussion
3.1. Ethnopharmacological Uses
3.2. Crop Production
3.3. Animal Production
3.4. Crop Protection
3.5. Bioenergy
3.6. Food Source
3.7. Chromolaena Odorata Management
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human wellbeing. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Kannan, R.; Shackleton, C.M.; Shaanker, R.U. Invasive alien species as drivers in socio-ecological systems: Local adaptations towards use of Lantana in Southern India. Environ. Dev. Sustain. 2014, 16, 649–669. [Google Scholar] [CrossRef]
- Vilà, M.; Ibàñez, I. Plant invasions in the landscape. Landsc. Ecol. 2011, 26, 461–472. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Shackleton, C.M.; Kull, C.A. The role of invasive alien species in shaping local livelihoods and human well-being: A review. J. Environ. Manag. 2019, 229, 145–157. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Broadhurst, L.M. The economic cost of managing invasive species in Australia. NeoBiota 2016, 31, 1–18. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Wilson, J.R.U. The Status of Biological Invasions and Their Management in South Africa in 2017; South African National Biodiversity Institute: Pretoria, South Africa, 2018; Available online: https://www.sanbi.org/wp-content/uploads/2018/11/National-Status-Report-web-6MB.pdf (accessed on 11 February 2020).
- Millennium Ecosystem Assessment (MEA). Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Scoones, I. Sustainable Rural Livelihoods: A Framework for Analysis; Institute of Development Studies: Brighton, UK, 1998. [Google Scholar]
- Shackleton, C.M.; McGarry, D.; Fourie, S.; Gambiza, J.; Shackleton, S.E.; Fabricius, C. Assessing the effects of invasive alien species on rural livelihoods: Case examples and a framework from South Africa. Hum. Ecol. 2007, 35, 113–127. [Google Scholar] [CrossRef]
- Kull, C.A.; Shackleton, C.M.; Cunningham, P.J.; Ducatillon, C.; Dufour-Dror, J.M.; Esler, K.J.; Friday, J.B.; Gouveia, A.C.; Griffin, A.R.; Marchante, E.; et al. Adoption, use and perception of Australian acacias around the world. Divers. Distrib. 2011, 175, 822–836. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; A-Berthou, E.G.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; van Wilgen, B.W.; Richardson, D.M. Use of non-timber forest products from invasive alien Prosopis species (mesquite) and native trees in South Africa: Implications for management. For. Ecosyst. 2015, 2, 16. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Witt, A.B.R.; Aool, W.; Pratt, C.F. Distribution of the invasive alien weed, Lantana camara, and its ecological and livelihood impacts in eastern Africa. Afr. J. Range Forage Sci. 2017, 34, 1–11. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Genovesi, P.; Bellingham, P.J.; Costello, M.J.; McGrannachan, C.; Sheppard, A. Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion. Biol. Invasions 2016, 18, 299–314. [Google Scholar] [CrossRef]
- Turpie, J.; Marais, C.; Blignaut, J.N. The working for water programme: Evolution of a payments for ecosystem services mechanism that addresses both poverty and ecosystem service delivery in South Africa. Ecol. Econ. 2008, 65, 788–798. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Richardson, D.M. Challenges and trade-offs in the management of invasive alien trees. Biol. Invasions 2014, 16, 721–734. [Google Scholar] [CrossRef]
- Novoa, A.; Kaplan, H.; Wilson, J.R.U.; Richardson, D.M. Resolving a prickly situation: Involving stakeholders in invasive cactus management in South Africa. Environ. Manag. 2016, 57, 998–1008. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Gaertnera, M.; O’Farrell, P.J.; Richardson, D.M. Perceptions of impact: Invasive alien plants in the urban environment. J. Environ. Manag. 2019, 229, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zengeya, T.; Ivey, P.; Woordford, D.J.; Weyl, O.; Novoa, A.; Shackleton, R.; Richardson, D.; van Wilgen, B. Managing conflict-generating invasive species in South Africa: Challenges and trade-offs. Bothalia 2017, 47, 1–11. [Google Scholar] [CrossRef]
- Goodall, J.M.; Zacharias, P.J.K. Managing Chromolaena odorata in subtropical grasslands in KwaZulu-Natal, South Africa. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. [Google Scholar]
- Kriticos, D.C.; Yonow, T.; McFadyen, R.E. The potential distribution of Chromolaena odorata (Siam weed) in relation to climate. Weed Res. 2005, 45, 246–254. [Google Scholar] [CrossRef]
- Raimundo, R.L.G.; Fonseca, R.L.; Schachetti-Pereira, R.; Peterson, A.T.; Lewinsohn, T.M. Native and exotic distributions of siamweed (Chromolaena odorata) modeled using the genetic algorithm for rule-set production. Weed. Sci. 2007, 55, 41–48. [Google Scholar] [CrossRef]
- Goodall, J.M.; Erasmus, D.J. Review of the status and integrated control of the invasive alien weed, Chromolaena odorata, in South Africa. Agric. Ecosyst. Environ. 1996, 56, 151–164. [Google Scholar] [CrossRef]
- Mugwedi, L.F.; Rouget, M.; Egoh, B.; Sershen; Ramdhani, S.; Slotow, R.; Rentería, J.L. An assessment of a community-based, forest restoration programme in Durban (eThekwini), South Africa. Forests 2017, 8, 255. [Google Scholar] [CrossRef]
- McFadyen, R.E.C. Chromolaena in Asia and the Pacific: Spread continues but control prospects improve. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. [Google Scholar]
- Zachariades, C. Biotype matching of Chromolaena for more successful biological control. Proc. S. Afr. Sugarcane Technol. Assoc. 2003, 7, 216–219. [Google Scholar]
- Codilla, L.T.; Metillo, E.B. Biotype of the invasive plant species Chromolaena odorata (Asteraceae: Eupatoriae) in the Zamboanga Peninsula, the Philippines. Philipp. J. Syst. Biol. 2012, 5, 28–42. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Mangla, S.; Callaway, I.M.; Callaway, R.M. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J. Ecol. 2008, 96, 58–67. [Google Scholar] [CrossRef]
- Zachariades, C.; Day, M.; Muniappan, R.; Reddy, G.V.P. Chromolaena odorata (L.) King and Robinson (Asteraceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Te Beest, M.; Mpandza, N.J.; Olff, H. Fire and simulated herbivory have antagonistic effects on resistance of savanna grasslands to alien shrub invasion. J. Veg. Sci. 2015, 26, 114–122. [Google Scholar] [CrossRef]
- MacWilliam, A. A Plague on your house? Some impacts of Chromolaena odorata on Timorese livelihoods. Hum. Ecol. 2000, 28, 451–469. [Google Scholar] [CrossRef]
- Zachariades, C.; Goodall, J.M. Distribution, impact and management of Chromolaena odorata in southern Africa. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. [Google Scholar]
- Sakuntaladewi, N.; Sumarhani; Suharti, S.; Widiarti, A.; Heriyanto, N.M.; Djaenudin, D. Socio-Economic Impacts of Chromolaena odorata on Communities around Merapi Mountain National Park, Indonesia; UNEP/GEF Project Fund No. 0515; United Nations Environment: Nairobi, Kenya, 2016. [Google Scholar]
- Witkowski, E.T.F.; Wilson, M. Changes in density, biomass, seed production and soil seedbanks of the non-native invasive plant, Chromolaena odorata, along a 15-year chronosequence. Plant Ecol. 2001, 152, 13–27. [Google Scholar] [CrossRef]
- Te Beest, M.; Cromsigt, J.P.G.M.; Ngobese, J.; Olff, H. Managing invasions at the cost of native habitat? An experimental test of the impact of fire on the invasion of Chromolaena odorata in a South African savanna. Biol. Invasions 2012, 14, 607–618. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Forsyth, G.G.; Le Maitre, D.C.; Wannenburgh, A.; Kotzé, J.D.F.; van den Berg, E.; Henderson, L. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biol. Conserv. 2012, 148, 28–38. [Google Scholar] [CrossRef]
- Zachariades, C.; Strathie, L.W.; Retief, E.; Dube, N. Progress towards the biological control of Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) in South Africa. Afr. Entomol. 2011, 19, 282–302. [Google Scholar]
- Semenya, S.S.; Tshisikhawe, M.P.; Potgieter, M.T. Invasive alien plant species: A case study of their use in the Thulamela Local Municipality, Limpopo Province, South Africa. Sci. Res. Essays 2012, 2363–2369. [Google Scholar]
- Aro, S.O.; Osho, I.B.; Aletor, V.A.; Tewe, O.O. Chromolaena odorata in livestock nutrition. J. Med. Plants Res. 2009, 3, 1253–1257. [Google Scholar]
- Omokhua, A.G.; McGaw, L.J.; Finnie, J.F.; van Stadden, J. Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016, 183, 112–122. [Google Scholar] [PubMed]
- Koutika, L.S.; Rainey, H.J. Chromolaena odorata in different ecosystems: Weed or fallow plant? Appl. Ecol. Environ. Res. 2010, 8, 131–142. [Google Scholar] [CrossRef]
- Akah, P.A. Mechanism of hemostatic activity of Eupatorium odoratum. Int. J. Crude Drug Res. 1990, 28, 253–256. [Google Scholar] [CrossRef]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W.; Le, T.T.; Pham, H.M. An aqueous extract of the leaves of Chromolaena odorata (formerly Eupatorium odoratum) (Eupolin) inhibits hydrated collagen lattice contraction by normal human dermal fibroblasts. J. Altern. Complement. Med. 1996, 2, 335–342. [Google Scholar] [CrossRef]
- Pandith, H.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. In vivo and in vitro hemostatic activity of Chromolaena odorata leaf extract. Pharm. Biol. 2012, 50, 1073–1077. [Google Scholar] [CrossRef]
- Onkaramurthy, M.; Veerapur, V.P.; Thippeswamy, B.S.; Madhusudana Reddy, T.N.; Rayappa, H.; Badami, S. Anti-diabetic and anti-cataract effects of Chromolaena odorata Linn., in streptozotocin-induced diabetic rats. J. Ethnopharm. 2013, 145, 363–372. [Google Scholar] [CrossRef]
- Triratana, T.; Suwannuraks, R.; Naengchomnong, W. Effect of Eupatorium odoratum on blood coagulation. J. Med. Assoc. Thail. 1991, 74, 283–286. [Google Scholar]
- Irobi, O.N. Activities of Chromolaena odorata (Compositae) leaf extract against Pseudomonas aeroginosa and Streptococcus faecalis. J. Ethnopharmacol. 1992, 37, 81–83. [Google Scholar] [CrossRef]
- Bamba, D.; Bessiere, J.M.; Marion, C.; Pelissier, Y.; Fouraste, I. Essential oil of Eupatorium odoratum. Planta Medica 1993, 59, 184–185. [Google Scholar] [CrossRef]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W. Effect of an aqueous extract from the leaves of Chromolaena odorata (Eupolin) on the proliferation of human keratinocytes and on the migration in an in vitro model of reepithelialization. Wound Repair Regen. 2001, 9, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; See, P.; Lee, S.T.; Chan, S.Y. Anti-oxidant effects of the extracts from the leaves of Chromolaena odorata on human dermal fibroblasts and epidermal keratinocytes against hydrogen peroxide and hypoxanthine–xanthine oxidase induced damage. Burns 2001, 27, 319–327. [Google Scholar]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Tirnsuksai, P.; Vimuttipong, S.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res. 2004, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ngono, N.A.; Ebelle Etame, R.; Ndifor, F.; Biyiti, L.; Amvam, Z.P.H.; Bouchet, P. Antifungal activity of Chromolaena odorata (L.) King & Robinson (Asteraceae) of Cameroon. Chemotherapy 2006, 52, 103–106. [Google Scholar]
- Kouamé, P.B.-K.; Jacques, C.; Bedi, G.; Silvestre, V.; Loquet, D.; Barillé-Nion, S.; Robins, R.J.; Tea, I. Phytochemicals isolated from leaves of Chromolaena odorata: Impact on viability and clonogenicity of cancer cell lines. Phytother. Res. 2013, 27, 835–840. [Google Scholar] [CrossRef]
- Ezenyi, I.C.; Salawu, O.A.; Kulkarni, R.; Emeje, M. Antiplasmodial activity-aided isolation and identification of quercetin-4’-methyl ether in Chromolaena odorata leaf fraction with high activity against chloroquine-resistant Plasmodium falciparum. Parasitol. Res. 2014, 113, 4415–4422. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Naidoo, K.K.; Coopoosamy, R.M.; Naidoo, G. Screening of Chromolaeana odorata (L.) King and Robinson for antibacterial and antifungal properties. J. Med. Plants Res. 2011, 5, 4859–4862. [Google Scholar]
- Reang, I.; Goswami, S.; Pala, N.A.; Kumar, M.; Bussmann, R.W. Ethnoveterinary applications of medicinal plants by traditional herbal healers in Reang Tribeo south District Tripura, India. Med. Aromat. Plants 2016, 5, 100023. [Google Scholar]
- Zahara, M. Description of Chromolaena odorata L. R.M King and H. Robinson as medicinal plant: A Review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Politeknik Aceh Selatan Campus, Kabupaten Aceh Selatan, Indonesia, 8–9 December 2018. [Google Scholar]
- Aba, P.E.; Joshua, P.E.; Ezeonugu, F.C.; Ezeja, M.I.; Omoja, V.U.; Umeakuana, P.U. Possible anti-diarrhoeal potential of ethanol leaf extract of Chromolaena odorata in castor oil-induced rats. J. Complement. Integr. Med. 2015, 12, 301–306. [Google Scholar] [CrossRef]
- Cácares, A.; Menéndez, H.; Méndez, E.; Cohobón, E.; Samayoa, B.E.; Jauregui, E.; Peralta, E.; Carrillo, G. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J. Ethnopharmacol. 1995, 48, 85–88. [Google Scholar] [CrossRef]
- Dangol, D.R. Traditional uses of plants of common land habitats in western Chitwan, Nepal. J. Inst. Agric. Anim. Sci. 2008, 29, 71–78. [Google Scholar] [PubMed]
- Ayensu, E.S. Medicinal Plants of West Africa; Reference Publication Inc.: Algonac, MI, USA, 1978. [Google Scholar]
- Prusti, A.B. Ethnobotanical exploration of Malkangiri Ditrict of Orissa, India. Ethnobot. Leafl. 2007, 11, 122–140. [Google Scholar]
- Agyare, C.; Asase, A.; Lechtenberg, M.; Niehues, M.; Deters, A.; Hensel, A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009, 125, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Erhenhi, A.H.; Obadoni, B.O. Known medicinal and aphrodisiac plants of Urhonigbe forest reserve, Edo State, Nigeria. J. Med. Plants Stud. 2015, 3, 101–106. [Google Scholar]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W. Enhanced proliferation of fibroblast and endothelial cells treated with an extract of leaves of Chromolaena odorata (Eupolin) an herbal remedy for treating wounds. Plast. Reconstr. Surg. 1998, 101, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Owoyele, V.B.; Adediji, J.O.; Soladoye, A.O. Anti-inflammatory activity of aqueous leaf extract of Chromolaena odorata. Inflammopharmacology 2005, 13, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Sinkevičienė, A.; Jodaugienė, D.; Pupalienė, R.; Urbonienė, M. The influence of organic mulches on soil properties and crop yield. Agron. Res. 2009, 7, 485–491. [Google Scholar]
- Kumar, P.; Pant, M.; Negi, G.S.C. Lantana mulching for soil fertility improvement, soil and water conservation and crop yield enhancement in rainfed rice in the Kumaun Hills. In Invasive Plants: An Ecological Appraisal for the Indian Subcontinent; Bhatt, J.R., Singh, J.S., Singh, S.P., Tripathi, R.S., Kohli, R.K., Eds.; CABI: Wallingford, UK, 2012. [Google Scholar]
- Tondoh, J.E.; Koné, A.W.; N’Dri, J.K.; Tamene, L.; Brunet, D. Changes in soil quality after subsequent establishment of Chromolaena odorata fallows in humid savannahs, Ivory Coast. Catena 2013, 101, 99–107. [Google Scholar] [CrossRef]
- Teame, G.; Tsegay, A.; Abrha, B. Effect of organic mulching on soil moisture, yield, and yield contributing components of sesame (Sesamum indicum l.). Int. J. Agron 2017, 1–6. [Google Scholar] [CrossRef]
- Kanmegne, J.; Duguma, B.; Henrot, J.; Isirimah, N.O. Soil fertility enhancement by planted tree-fallow species in the humid lowlands of Cameroon. Agrofor. Syst. 1999, 46, 239–249. [Google Scholar] [CrossRef]
- Koutika, L.S.; Ndango, R.; Hauser, S. Nutrient concentrations and NH4-N mineralization under different soil and fallow types in southern Cameroon. Plant Nutr. Soil Sci. 2004, 167, 591–595. [Google Scholar] [CrossRef]
- Tian, G.; Kang, B.T.; Kolawole, G.O.; Idinoba, P.; Salako, F.K. Long-term effects of fallow systems and lengths on crop production and soil fertility maintenance in West Africa. Nutri. Cycl. Agroecosyst. 2005, 71, 139–150. [Google Scholar] [CrossRef]
- Mboukou-Kimbatsa, I.; Bernhard-Reversat, F.; Loumeto, J.-J.I.; Ngao, J.; Lavelle, P. Understory vegetation, soil structure and soil invertebrates in Congolese eucalypt plantations, with special reference to the invasive plant Chromolaena odorata and earthworm populations. Eur. J. Soil Biol. 2007, 43, 48–56. [Google Scholar] [CrossRef]
- Koné, A.W.; Edoukou, E.F.; Gonnety, J.T.; N’Dri, A.N.A.; Assémien, L.F.E.; Angui, P.K.T.; Tondoh, J.E. Can the shrub Chromolaena odorata (Asteraceae) be considered as improving soil biology and plant nutrient availability? Agroforest. Syst. 2012, 85, 233–245. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O.; Ogeh, J.S. Response of soil properties and yam yield to Chromolaena odorata (Asteraceae) and Tithonia diversifolia (Asteraceae) mulches. Arch. Agron. Soil Sci. 2014, 60, 209–224. [Google Scholar] [CrossRef]
- Fening, J.O.; Yeboah, E.; Adjei-Gyapong, T.; Gaizie, E. On farm evaluation of the contribution of three green manures to maize yield in the semi-deciduous forest zone of Ghana. Afr. J. Environ. Sci. Technol. 2009, 3, 234–238. [Google Scholar]
- Ngobo, M.P.; Weise, S.F.; Mcdonald, M.A. Revisiting the performance of natural fallows in Central Africa. Scand. For. Res. 2004, 19, 14–24. [Google Scholar] [CrossRef]
- Slaats, J.J.P.; Janssen, B.H.; Wessel, M. Crop production in relation to cultural practices in the Chromolaena odorata fallow system in South-West Côte d’Ivoire. Neth. J. Agric. Sci. 1998, 46, 305–317. [Google Scholar]
- Adjei-Nsiah, S.; Leeuwis, C.; Giller, K.E.; Sakyi-Dawson, O.; Cobbina, J.; Kuyper, T.W.; Abekoe, M.; Van Der Werf, W. Land tenure and differential soil fertility management practices among native and migrant farmers in Wenchi, Ghana: Implications for interdisciplinary action research. Wagening. J. Life Sci. 2004, 52, 331–348. [Google Scholar] [CrossRef]
- Roder, W.; Phengchanh, S.; Keoboualapha, B.; Maniphone, S. Chromolaena odorata in slash-and-burn rice systems of Northern Laos. Agroforest. Syst. 1995, 31, 79–92. [Google Scholar] [CrossRef]
- Ikuenobe, C.E.; Anoliefo, G.O. Influence of Chromolaena odorata and Mucuna pruriens fallow duration on weed infestation. Weed Res. 2003, 43, 199–207. [Google Scholar] [CrossRef]
- Yonghachea, P.F. Farmer’s Perceptions of Imperata cylindrica and Chromolaena odorata Fallows on the North West, South West and Littoral Provinces of Cameroon. Master’s Thesis, University of Hohenhein, Stuttgart, Germany, 2005. [Google Scholar]
- Jamilal; Mulyani, S.; Juniarti. The application of liquid organic fertilizer of Chromolaena odorata on ratooned rice plants cultivation. Asian J. Appl. Res. Community Dev. Empower. 2017, 1, 9–15. [Google Scholar]
- Nawaz, M.; Sansamma, G. Eupatorium [Chromolaena odorata (L.) King and Robinson] biomass as a source of organic manure in okra cultivation. J. Trop. Agric. 2004, 42, 33–34. [Google Scholar]
- Munda, G.C.; Islam, M.; Panda, B.B.; Patel, D.P. Performance of rice (Oryza sativa L.)-rapeseed (Brassica campestris L.) cropping sequence under system based nutrient management. Crop Prod. 2008, 45, 36–39. [Google Scholar]
- Awopegba, M.; Awodun, M.; Oladele, S. Maize (Zea mays) biomass and yield as influenced by leguminous and non-leguminous mulch types in southeastern Nigeria. Bulg. J. Soil Sci. 2016, 1, 154–169. [Google Scholar]
- Kumar, A.; Mohanty, A.K.; Borah, T.R.; Singh, A.K. Evaluation of indigenous forest plant leaves for bio-mulching in organic ginger production and income generation under rain-fed conditions of NEH region. Indian J. Trad. Knowl. 2012, 11, 487–492. [Google Scholar]
- Hai, P.V.; Everts, H.; Tien, D.V.; Schonewille, J.T.; Hendriks, W.H. Feeding Chromolaena odorata during pregnancy to goat dams affects acceptance of this feedstuff by their offspring. Appl. Anim. Behav. Sci. 2012, 137, 30–35. [Google Scholar] [CrossRef]
- Aro, S.O. The Effects of Siam Weed Leaf Meal (Chromolaena odorata) on the Performance, Egg Quality Characteristics, Nutrient Utilization, Haematological and Biochemical Indices of Layers. Master’s Thesis, University of Ibadan, Ibadan, Nigeria, 1990. [Google Scholar]
- Apori, S.O.; Long, R.J.; Castro, F.B.; Ørskov, E.R. Chemical composition and nutritive value of leaves and stems of tropical weed Chromolaena odorata. Grass Forage Sci. 2000, 55, 77–81. [Google Scholar] [CrossRef]
- Ngozi, I.M.; Jude, I.C.; Catherine, I.C. Chemical Profile of Chromolaena odorata L. (King and Robinson) Leaves. Pak. J. Nutr. 2009, 8, 521–524. [Google Scholar] [CrossRef]
- Nwinuka, N.; Nwiloh, B.; Eresama, J. Nutritional and potential medicinal value of Chromolaena odorata leaves. Int. J. Trop. Agric. Food Syst. 2009, 3, 122–129. [Google Scholar] [CrossRef]
- Fasuyi, S.O.; Fajemilehin, S.O.K.; Omojola, A.B. The egg quality characteristics of layers fed varying dietary inclusions of siam weed (Chromolaena odorata) leaf meal (SWLM). Int. J. Poult. Sci. 2004, 4, 752–757. [Google Scholar]
- Jiwuba, P.C.; Ogbuewu, I.P.; Nwachukwuguru, K. Performance and economy of production of broilers fed Siam weed (Chromolaena odorata) leaf meal (SWLM). Trop. Anim. Health Prod. 2018, 50, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Donkoh, A.; Atuahene, C.C.; Anang, D.M.; Badu-Botah, E.K.; Boakye, K.T. Response of broiler chickens to the dietary inclusion of Chromolaena odorata leaf meal. J. Anim. Feed Sci. 2002, 11, 309–319. [Google Scholar] [CrossRef]
- Ekenyem, B.U.; Obih, T.K.O.; Odo, B.I.; Mba, F.I.A. Performance of finisher broiler chicks fed varying replacement of Chromolaena odorata leaf meal for soyabean meal. Pak. J. Nutr. 2010, 558–561. [Google Scholar] [CrossRef]
- Bonsu, F.R.K.; Kagya-Agyemang, J.K.; Kwenin, W.K.J.; Hope, K.N.; Sekyere, F.O. Growth performance, haematological indices and carcass characteristics of broilers fed diet containing different levels of Chromolaena odorata leaf meal. Egerton J. Sci. Technol. 2013, 13, 115–125. [Google Scholar]
- Shao, J.K. Effect of Chromolaena odorata Leaf Meal on the Performance of Small East African Goats. Master’s Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2016. [Google Scholar]
- Ogadu, C.B.-J. In vitro and in vivo Evaluation of Siam Weed (Chromolaena odorata) Leaf Meal as Additive in the Diet of West African Dwarf Bucks. Master’s Thesis, Federal University of Agriculture, Makurdi, Nigeria, 2018. [Google Scholar]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Nicol, H.I.; Munyakazi, L.; Stevenson, P.C. Tri-trophic insecticidal effects of African plants against cabbage pests. PLoS ONE 2013, 8, e78651. [Google Scholar] [CrossRef]
- Bamikole, M.A.; Ikhatua, U.J.; Osemwenkhae, A.E. Converting bush to meat: A case of Chromolaena odorata feeding to rabbits. Pak. J. Nutr. 2004, 3, 258–261. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Ezena, G.N. Exploiting the Insecticidal Potential of the Invasive Siam Weed, Chromolaena odorata L. (Asteraceae) in the Management of major pests of Cabbage, Brassica oleracea var capitata and Their Natural Enemies for Enhanced Yield in the Moist Semi-Deciduous Agro-Ecological Zone of Ghana. Master’s Thesis, University of Ghana, Accra, Ghana, 2015. [Google Scholar]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Degri, M.M.; Mailafiya, D.M.; Wabekwa, J.W. Efficacy of aqueous leaf extracts and synthetic insecticide on pod-sucking bugs infestation of cowpea (Vigna unguiculata (L.) Walp) in the Guinea Savanna Region of Nigeria. Adv. Entomol. 2013, 1, 10–14. [Google Scholar] [CrossRef]
- Acero, L.H. Fresh siam (Chromolaena odorata) weed leaf extract in the control of housefly (Musca domestica). Int. J. Food Eng. 2017, 3, 56–61. [Google Scholar] [CrossRef]
- Abugri, A.D. The Efficacy of Ethanolic Root and Leaf Extract of Chromolaena odorata in Controlling Sitophilus zeamais in Stored Maize. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2011. [Google Scholar]
- Cobbinah, J.R.; Moss, C.; Golob, P.; Belmain, S.R. Conducting Ethanobotanical Surveys: An Example from Ghana on Plants Used for the Protection of Stored Cereals and Pulses; NRI Bulletin 77, Working Paper; Natural Resources Institute, University of Greenwich: London, UK, 1999. [Google Scholar]
- Mbah, O.I.; Okoronkwo, M.O. An assessment of two plant product efficacy for the control of the maize weevil (Sitophilus zeamais Motschulsky) in stored maize. Afr. J. Agric. Res. 2008, 3, 494–498. [Google Scholar]
- Pimentel, D.; Herz, M.; Glickstein, M.; Zimmerman, M.; Allen, R.; Becker, K. Renewable energy: Current and potential issues. BioScience 2002, 52, 1111–1120. [Google Scholar] [CrossRef]
- Girardet, H.; Mendonça, M. A Renewable World: Energy, Ecology, Equality: A Report for the World Future Council; Green Books: Totnes, UK, 2009. [Google Scholar]
- Liao, R.; Gao, B.; Fang, J. Invasive plants as feedstock for biochar and bioenergy production. Bioresour. Technol. 2013, 140, 439–442. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, L.; Hu, G.; Wu, J. Integrating animal manure-based bioenergy production with invasive species control: A case study at Tongren Pig Farm in China. Biomass Bioenergy 2010, 34, 821–827. [Google Scholar] [CrossRef]
- Mugido, W.; Blignaut, J.; Joubert, M.; De Wet, J.; Knipe, A.; Joubert, S.; Cobbing, B.; Jansen, J.; Le Maitre, D.; van der Vyfer, M. Determining the feasibility of harvesting invasive alien plant species for energy. S. Afr. J. Sci. 2014, 110, 1–6. [Google Scholar]
- Amaducci, S.; Perego, A. Field evaluation of Arundo donax clones for bioenergy production. Ind. Crops Prod. 2015, 75, 122–128. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as potential feedstock for bioenergy production. Biofuel Bioprod. Biorefin. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Melane, M. Evaluation of the Potential of Non-Woody Invasive Plant Biomass for Electricity Generation. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2016. [Google Scholar]
- Dahunsi, S.O.; Oramusi, S.; Efeovbokhan, V.E.; Olayanju, A.; Zahedi, S.; Ojediran, J.O.; Izebere, J.O.; Aladegboye, O.J. Anaerobic conversion of Chromolaena odorata (Siam weed) to biogas. Energy Rep. 2018, 4, 691–700. [Google Scholar] [CrossRef]
- Das, K.; Duarah, P. Invasive alien plant species in the roadside areas of Jorhat, Assam: Their harmful effects and beneficial uses. Int. J. Eng. Res. Appl. 2013, 3, 353–358. [Google Scholar]
- Maroyi, A. Use of weeds as traditional vegetables in Shurugwi District, Zimbabwe. J. Ethnobiol. Ethnomed. 2013, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Atyosi, Z.; Ramarumo, J.F.; Maroyi, A. Alien plants in the Eastern Cape Province in South Africa: Perceptions of their contributions to livelihoods of local communities. Sustainability 2019, 11, 5043. [Google Scholar] [CrossRef]
- Ramaano, R. The Effects of Chromolaena odorata on Tree Growth Dynamics at Buffelsdraai Landfill Site. Master’s Thesis, University of KwaZulu-Natal, Durban, South Africa, 2020. [Google Scholar]
- Blackmore, A.C. Seed dispersal of Chromolaena odorata reconsidered. In Proceedings of the Fourth International Workshop on the Biological Control and Management of Chromolaena odorata, Bangalore, India, 14–18 October 1996. [Google Scholar]
- Day, M.D.; Bofeng, I.; Nabo, I. Successful biological control of Chromolaena odorata (Asteraceae) by the gall fly Cecidochares connexa (Diptera: Tephritidae) in Papua New Guinea. In Proceedings of the 13th International Symposium on Biological Control of Weeds, Waikoloa, HI, USA, 11–16 September 2011. [Google Scholar]
- Desmier de Chenon, R.; Sipayung, A.; Sudharto, P. A decade of biological control against Chromolaena odorata at the Indonesian Oil Palm Research Institute in Marihat. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. [Google Scholar]
- Uyi, O.O.; Igbinosa, I.B. The status of Chromolaena odorata and its biocontrol in West Africa. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. [Google Scholar]
| Plant Part | Uses | Country | Refrences |
|---|---|---|---|
| Leaves | Cholesterol, dyspepsia, hypertension, vertigo, and diet | Indonesia | [59] |
| Leaves | Cough | Thailand | [52] |
| Leaves | Diarrhea | Nigeria | [60] |
| Leaves | Diabetes and eye problems | India | [46] |
| Leaves | Gonorrhea | Guatemala | [61] |
| Leaves | Insecticide | Nepal | [62] |
| Leaves | Malaria | Benin and Ghana | [44,55,63] |
| Leaves | Skin infections | Vietnam | [44] |
| Leaves | Wound | Ghana, India, Nigeria, Thailand | [45,64,65,66,67] |
| Leaves | Wound | Vietnam | [50,51,67] |
| Leaves | Wound | Ivory Coast | [68] |
| Leaves | Wound | Nepal | [62] |
| Not mentioned | Not mentioned | Tanzania | [13] |
| Practice | Crop | Country | Reference |
|---|---|---|---|
| Fallow | Arachis hypogaea L. | Cameroon, Ghana, Nigeria | [75,80,81] |
| Fallow | Dioscorea rotundata Poir | Ghana | [81] |
| Fallow | Manihot esculenta Crantz | Cameroon, Ghana, Nigeria | [75,80,82] |
| Fallow | Oryza sativa L. | Laos | [83] |
| Fallow | Zea mays L. | Cameroon; Ghana, Ivory Coast; Nigeria | [75,76,81,84] |
| Fallow | Not mentioned | Cameroon | [85] |
| Fallow | Not mentioned | Ivory Coast | [71] |
| Liquid organic fertilizer | Oryza sativa L. | Indonesia | [86] |
| Mulch | Abelmoschus esculentus L. Moench | India | [87] |
| Mulch | Brassica campestris L. | India | [88] |
| Mulch | Dioscorea rotundata Poir | Nigeria | [78] |
| Mulch | Oryza sativa L. | India | [88] |
| Mulch | Zea mays L. | Nigeria, Ghana | [79,89] |
| Mulch | Zingiber officinale Rosc. | India | [90] |
| Plant Part | Livestock | Country | References |
|---|---|---|---|
| Leaf meal | Layer chickens | Nigeria | [40,92,96] |
| Leaf meal | Broiler chickens | Nigeria | [99] |
| Leaf meal | Broiler chickens | Ghana, Nigeria | [97,100] |
| Leaf meal | Goats | Nigeria, Tanzania, Vietnam | [91,101,102] |
| Leaf meal | Rabbits | Nigeria | [104] |
| Formulation | Crop | Pest Controlled | Country | References |
|---|---|---|---|---|
| Leaf extract | Brassica oleracea var. capitata L. | Plutella xylostella (L.) | Ghana | [103] |
| Leaf extract | Brassica oleracea var. capitata | Brevicoryne brassicae (L.) | Ghana | [103] |
| Leaf extract | Brassica oleracea var. capitata | Various | Ghana | [106] |
| Leaf extract | Vigna unguiculata (L.) Walp. | Anoplocnemis spp., Clavigralla spp., Nezara viridula L. and Riptortus spp. | Nigeria | [108] |
| Leaf extract | Various vegetables | Musca domestica L. | Philippines | [109] |
| Leaf extract | Zea mays L. grain | Sitophilus zeamais Motschulsky | Ghana | [110] |
| Leaf powder | Vigna unguiculata grain | Post-harvest pests | Ghana | [111] |
| Leaf powder | Zea mays grain | Post-harvest pests | Ghana | [111] |
| Leaves | Zea mays grain | Sitophilus zeamais Motschulsky | Nigeria | [112] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugwedi, L. Harnessing Opportunities Provided by the Invasive Chromolaena odorata to Keep It under Control. Sustainability 2020, 12, 6505. https://doi.org/10.3390/su12166505
Mugwedi L. Harnessing Opportunities Provided by the Invasive Chromolaena odorata to Keep It under Control. Sustainability. 2020; 12(16):6505. https://doi.org/10.3390/su12166505
Chicago/Turabian StyleMugwedi, Lutendo. 2020. "Harnessing Opportunities Provided by the Invasive Chromolaena odorata to Keep It under Control" Sustainability 12, no. 16: 6505. https://doi.org/10.3390/su12166505
APA StyleMugwedi, L. (2020). Harnessing Opportunities Provided by the Invasive Chromolaena odorata to Keep It under Control. Sustainability, 12(16), 6505. https://doi.org/10.3390/su12166505





