Abstract
The biochar treated through several processes can be modified and utilized as catalyst or catalyst support due to specific properties with various available functional groups on the surface. The functional groups attached to the biochar surface can initiate active radical species to play an important role, which lead to the destruction of contaminants as a catalyst and the removal of adsorbent by involving electron transfer or redox processes. Centering on the high potential to be developed in field applications, this paper reviews more feasible and sustainable biochar-based materials resulting in efficient removals of environmental pollutants as catalyst or support rather than describing them according to the technology category. This review addresses biochar-based materials for utilization as catalysts, metal catalyst supports of iron/iron oxides, and titanium dioxide because the advanced oxidation process using iron/iron oxides or titanium dioxides is more effective for the removal of contaminants. Biochar-based materials can be used for the removal of inorganic contaminants such as heavy meals and nitrate or phosphate to cause eutrophication of water. The biochar-based materials available for the remediation of eutrophic water by the release of N- or P-containing compounds is also reviewed.
1. Introduction
Biochar obtained from the pyrolysis of various biomass under the control of an oxidant has been studied because of the reduced cost, utilization of waste, and eco-friendly method [1,2]. Biochar applications can be divided into several categories, of which the most prevalent utilization of biochar might be an application as a catalyst and catalyst support for environmental remediation [3] and electrode materials or membrane in electrochemical field. Ag/biochar component was fabricated as supercapacitor electrode [4]. In order to improve removal efficiency of contaminants, biochar needs to be pretreated by activation and functionalization processes before use [5,6,7,8]. Generally, biochar can be activated physically or chemically to improve the activity by adjusting the surface area and pore volume [9,10,11]. In physical activation, CO2 or steam gas are commonly applied to biochar, whereas an inorganic acid, base, or neutral salts, such as HNO3, H2SO4, H3PO4, KOH, and ZnCl2 are used in the chemical activation of biochar [12,13]. Compared to physical activation, the activation of biochar by chemical reagents could necessarily require a post-process, such as neutralization, to avoid damaging the facility [12]. The functional groups attached to the surface of biochar have also attracted attention because of being able to expand its utilization [14,15]. Depending on the order of treatment process, there are two types of processes to place functional groups on the biochar surface more efficiently. One is the impregnation of biochar with functional group precursor reagents after the pyrolysis of biomass, and the other is the pre-coating of biomass with reagents containing functional groups prior to pyrolysis or thermal processes [13]. In general, depending on the components and structure of biochar, the physical and chemical properties of modified biochar show difference in accordance with the order of pyrolysis. Biochar improved with multi-functional groups was used to oxidize a reducible compound through the transfer of electrons released from a photocatalyst or metal [16]. These surface functional groups on the biochar are responsible for both enhancing the removal efficiency of pollutants by improved adsorption capability [17,18,19,20]. In addition, modified biochar-based materials can also be used to mitigate the malodourous air caused by volatile organic compounds generated from municipal waste [21]. The biochar was also effective in diminishing the odorous compounds from livestock farming [22,23].
When there is an active oxygen-included functional group on the biochar surface, e.g., carboxylic, carbonyl, and hydroxyl groups, the site of surface can act as an electron transfer platform, such as an electron acceptor or donor. Although the electric charge distribution of biochar surface is dependent on the applied feedstock, various multifunctional groups attached on the surface can play a nucleophile or electrophile in the process of reaction. Therefore, a range of applications using biochar-based materials have been investigated for catalytic degradation and photolysis for the removal of harmful organic contaminants and heavy metals [24,25]. Recently, beyond the application of bioenergy and biorefinery process [26], biochar modifications and the application of metal oxides as catalyst or supports have been investigated widely because biochar-based materials are sustainable and safe in the remediation of contaminated water and soil [27]. However, although the biochar-based materials have high potential in terms of cost, the application to large-scale novel technology should be developed due to lack of feasibility [28]. This paper reviews the literature, focusing on the environmental biochar catalyst and catalyst support of iron/iron oxides and titanium dioxides relating the characteristics of biochar-based materials to the activity for the removal of contaminants and the remediation of eutrophic water using biochar-based materials. Various biochar derived from plant residuals and animal excrements and pollutants removal by modified biochar were described as shown in Figure 1.
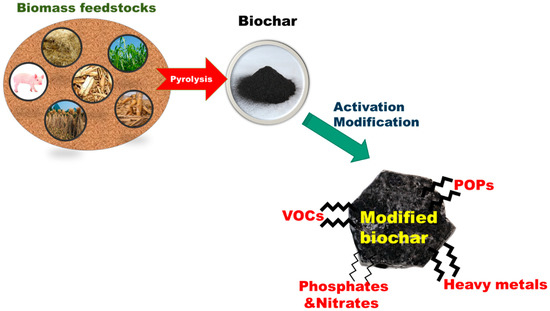
Figure 1.
Biochar by the pyrolysis of biomass derived from various feedstocks and application of modified biochar.
2. Modification and Application of the Biochar as an Environmental Catalyst
2.1. Active Oxidant Species Regarding the Reaction Mechanism
AOPs (advanced oxidation processes) are one of the most effective environmental remediations. In general, ozone, hydrogen peroxide (H2O2), and persulfate (PS, S2O82−) can produce active radical species, such as hydroxyl radicals (•OH) and the sulfate radical anion (SRA, SO4•−) by UV wavelength irradiation. These active radical species react with various functional groups on the biochar surface. What kinds of functional groups would be formed on the biochar surface is dependent on both the feedstock and the experimental conditions during pyrolysis of biomass such as temperature, residential time, and oxygen concentration. The biochar via modification and functionalization can be used as more effective catalyst for the removal of environmental pollutants compared to pristine biochar [13,24].
Also, bare biochar without modifications exhibited no catalytic activity, but CO2-activated biochar showed remarkably improved activity for phenolic pollutants. [29]. Cellulose biochar activated with CO2 under different temperatures (800 to 950 °C at 50 °C intervals) was evaluated for the removal of phenol and chlorinated phenolic compounds such as mono-chlorophenol, dichlorophenol, and trichlorophenol [29]. Based on the reaction mechanism of phenolic compounds degradation, among the prepared biochar samples, the biochar activated at 950 °C showed the highest efficiency for the degradation of phenolic compounds and its catalytic activity was improved by the addition of peroxymonosulfates (PMS). Without biochar, there was no degradation, even in the presence of PMS. In addition, regarding the active species involving degradation, singlet oxygen species 1O2 produced from the reaction of PMS with the biochar surface was a major oxidant responsible for the degradation of these phenolic compounds rather than hydroxyl radicals (•OH) or sulfate radicals (SO4•−). As a result, the more chlorinated phenol substituents showed a slower degradation rate. It seems to be higher electronegativity of phenolic compounds as the increase of chlorination level.
2.2. Removal of Environmental Pollutants
Among the biomass waste resources, the agricultural residues are abundantly produced from the worldwide farming countries. The biochar prepared from rice husk and corn straw could be used as an environmental catalyst for the removal of contaminants. Qin et al. [30] performed the degradation of both cis- and trans-1,3-dichloropropene using rice-husk biochar pyrolyzed at temperatures in the range of 300 to 700 °C. Among biochar samples prepared at different temperatures, the biochar pyrolyzed at 500 °C showed the best activity for 1,3-dichloropropene. Moreover, its catalytic activity was improved under the condition of reactor temperature in the range of 20 to 40 °C in the presence of moisture. The degradation mechanism of 1,3-dichloropropene was suggested to occur by OH radicals induced from a range of environmental free radicals on the biochar. In addition, the corn straw-derived biochar showed higher efficiency for the removal of Cr(VI) by reduction into Cr(III) compared to other biochar materials. Zhao et al. [31] carried out the saline water-mediated Cr(VI) removal by biochar synthesized with corn straw biomass pyrolyzed at different temperatures (300, 500, and 700 °C) to examine the influence of the biochar surface according to the pyrolysis temperature. The removal of Cr(VI) decreased significantly with increasing the pyrolysis temperature because of decreasing the number of oxygenated functional groups, such as hydroxyl or carbonyl groups, which transfer electrons to Cr(VI). Based on their results, the biochar pyrolyzed at 300 °C showed the highest efficiency for the removal of Cr(VI) due to the highest oxygen density on the surface. To examine the effect of the surface oxygenated functional groups, radical scavengers, e.g., H2O2 or ethanol-added biochar, were employed to remove Cr(VI). The removal efficiency using the scavenger-added biochar decreased significantly since the removal efficiency was related to the number of active species on the biochar surface. After treatment with radical scavengers, hydrogen peroxide, and ethanol, the concentration of EPFRs (environmentally persistent free radicals) on the surface of the biochar decreased significantly from 3.966 × 1018 spins/g to 2.695 × 1018 spins/g and 3.515 × 1018 spins/g, respectively. The active species on the biochar were identified as the semiquinone-type EPFRs, which was estimated to donate electrons to Cr(VI) reduced into Cr(III) and be transferred to quinone. The biochar surface contained quinone and hydroquinone [32].
N-doped biochar can also be used for the removal of multi-contaminant wastewater. Zhu et al. [33] fabricated N-doped biochar by the pyrolysis of reed biomass to ammonium nitrate and evaluated the activity of N-doped biochar for the removal of phenol, orange G, sulfamethoxazole, and bisphenol A in an aqueous solution. The material fabricated at a high temperature (900 °C) was a multi-layered nanosheet structure with mesopores (2–50 nm) that showed good catalytic efficiency for the removal of organic pollutants. They suggested two different pathways for the oxidation of contaminants by the catalysts prepared at low and high temperature (>700 °C) because the biochar showed structural differences according to the pyrolysis temperature. The degradation of contaminants using the catalyst pyrolyzed at a low temperature (400 °C) was preceded by radical species from the activated persulfates. In contrast, the removal process using the catalyst prepared at temperatures higher than 700 °C with a well-structured nanosheet form proceeded through dominantly singlet oxygen and surface-confined persulfate complex formation rather than activated persulfate. In oxidation through a non-radical pathway, the adsorption of organic compounds on biochar should be a key step in N-doped biochar with a highly graphitic nanosheet structure [19].
Agricultural wastes and wastewater produced from livestock farming can also be used as a catalyst for pollutant removal. The wastewater and manure produced from livestock contain electron-rich functional groups, such as nitrogen or phosphate-containing groups that act as an electron donor or electron transfer. Huang et al. [34] prepared biochar from pyrolyzed coagulated sludge obtained from flocculated swine manure with the addition of Mg and Fe. They used this biochar for the removal of contaminants, tylosin, and rhodamine B, and obtained high removal efficiencies of 92.2% and 89.1% for tylosin and rhodamine B, respectively. They also carried out the degradation of the antibiotics component tylosin and dyestuff rhodamine B using swine manure biochar pyrolyzed at four different temperatures, 400, 500, 600, and 700 °C, and investigated the catalytic activity for contaminant removal according to the peroxymono sulfate (PMS) loading. Among the prepared catalysts, the biochar pyrolyzed at 700 °C showed the best activity for the removal of tylosin and rhodamine B. Regarding the reaction mechanism for the degradation of organic contaminants, the decomposition of tylosin and rhodamine B occurred faster in proportion to the amount of PMS due to the activation of PMS by the biochar catalyst. In the degradation of tylosin and rhodamine B using the biochar from swine wastewater, reactive oxygen radical species, such as hydroxyl radical, singlet oxygen molecule, and superoxide, were produced by a reaction of PMS and the surface functional groups on the biochar through electron transfer, which accelerated the removal of organic contaminants. No significant degradation of the contaminant was observed without the biochar despite adding PMS. There may occur competition among active radical species and paramagnetic oxygen species, but the reaction rate and mechanism would be up to the amount of available species produced from the reaction with biochar and environmental conditions such as pH and co-existing ions.
3. Application of the Biochar as a Support of Fe/FeOx
3.1. Biochar Support Nanosized ZVI Catalysts
Recently, metal-impregnated biochar has been used widely for the degradation of organic contaminants and the removal of heavy metals owing to its large surface area and functional groups associated with the redox mechanism. In addition, compared to other support materials, biochar-supported ZVI (zero-valent iron Fe0) combination systems have been reported for the environmental contaminant removal because biochar-derived support can prohibit the aggregation of nanosized ZVI (nZVI). Based on the results of remediation of soil contaminated with aromatic compounds, iron-containing soils were more active for the removal of anthracene because of the capability of producing EPFRs (environmentally persistent free radicals) by the interaction of Fe and potential active functions on the biochar surface [35].
The recently published literature using the fabricated ZVI/biochar catalysts is listed below. One of the great merits of biochar is that it can generate resistant free radicals by oxygen-containing functional groups which react with ZVI.
Yan et al. examined the degradation of trichloroethylene (TCE) in the presence of Fe/biochar and persulfate (PS, S2O82−) to investigate the effects on the reaction mechanism [36]. The rice hulls biomass provided from Nanjing in China were used to prepare the biochar, and the nZVI particles were prepared by a reaction of ferrous sulfate heptahydrates and NaBH4. PS was used to remove organic or inorganic environmental contaminants by photolysis because PS generates the active oxidant, SRA, under UV irradiation. Based on the previously reported literature [37], PS was more effective for the removal of acetic acid rather than •OH without any significant by-product under the UV irradiation. The removal by SRA from PS/UV was superior compared to the process by •OH, whereas the pH condition should be neutral or slightly acidic to avoid inhibition of the SRA activity. Compared to the removal efficiency in the absence of biochar, TCE degradation reached 56.6%, whereas the degradation efficiency was improved to 99.4% within 5 min in the presence of the biochar-supported Fe. Active oxygen-included functional groups on the surface of biochar associated with the redox process generated the sulfate radical anion (SRA, SO4•−) to destroy TCE. On the other hand, ZVI was combined with biochar-derived materials to remove heavy metals. Zhang et al. fabricated the nanoscale ZVI stabilized by carboxymethyl cellulose (-CH2-COOH, CMC) with the biochar prepared from wheat straw-pyrolyzed at 600 °C and the catalytic activity was performed for the removal of Cr(VI) [38]. Based on the experimental results, the removal of Cr(VI) occurred through sorption on the biochar surface followed by the reduction of Cr(V) to Cr(III) in the form of CrxFe1-x(OH)3, CrxFeyO4, and complexes with a range of multiple groups on the biochar in the wake of partial oxidation and reduction of multivalent metals oxides. They fabricated three catalysts with different CMC concentrations, biochar-0.05CMC-nZVI, biochar-0.1CMC-nZVI, and biochar-0.2CMC-nZVI, in which the mass ratio of biochar: nZVI = 1: 1. Among the catalysts and the reference catalysts (nZVI, biochar, biochar-nXVI, and CMC-nZVI), biochar-0.1CMC-nZVI showed the best activity for the removal of Cr(VI), reaching 104.4 mg/g, which was approximately twice as high as biochar-0.05CMC-nZVI, 55.6 mg/g. In the presence of CMC, a stabilizer of nZVI, the catalytic efficiency was enhanced for the uptake of Cr(VI). They also described the reaction kinetics by fitting the reaction parameters to the adsorption of Cr(VI) on the biochar-CMC-nZVI and assigned the adsorption on the surface of the catalyst to the Elovich model rather than pseudo-first-order or pseudo-second-order kinetics. In addition, based on the adsorption isotherms of Cr(VI) on the catalyst, the sorption kinetics was well described by the SIPS isotherm model over the heterogeneous catalyst surface among the Langmuir, Freundlich, SIPS, and Redlich–Peterson models [38]. The oxygen-included functional groups are believed to involve electron-transfer regarding the oxidation and reduction for Cr(VI) uptake using biochar-CMC-nZVI [38].
As mentioned in the literature, zero-valent iron or iron oxides containing biochar can be used for the sustainable remediation of environmental pollutants because iron oxides produce metal iron or iron hydroxides via a range of complicated interactions with multi-functional groups in biochar. Recently, the biochar fabricated using corn stalk and corncobs were employed to control contaminant [39,40]. Li et al. [39] removed trichloroethylene environmental pollutants from groundwater in situ using the Fe/C biochar prepared from corn cobs and corn stalks. They utilized two different feedstocks, i.e., corn cobs (CB) and corn stalks (SB), and pyrolyzed them at low and high temperatures, 300 °C (CB300, SB300) and 600 °C (CB300, SB600). Biochar catalysts supporting nanoscale Fe were prepared by mixing ferrous sulfate heptahydrate with pyrolyzed biochar. Among the four types of biochar support iron catalysts, Fe-CB600 showed the highest catalytic activity for the removal of TCE. They also performed TCE removal in the presence of peroxymonosulfate (PMS) and examined the influence of co-existing compounds, such as chlorine anion, nitrates, and phosphates, on the removal efficiency of TCE. When Fe-CB600 (Fe:C= 1:1) was used for the removal of TCE, 0.1 mM TCE was removed completely within 20 min in the presence of 5.0 mM PMS, whereas only 44% of TCE was removed in 20 min in the absence of PMS. By the addition of PMS, the removal efficiency of TCE using nanoscale Fe on a biochar support, CB or SB, was enhanced. In general, aqueous solutions persulfate or PMS can generate active sulfate radicals to initiate the degradation of organic compounds by transition metals, such as iron, cobalt, and manganese. The adsorption and oxidation process would occur in the removal of TCE using n-Fe/C in the presence of PMS. In addition, the complicated interaction of oxygen-containing multi-functional groups on the biochar and active radical species, such as singlet oxygen and superoxide anionic radicals, may contribute to the removal of contaminants.
3.2. Removal of Endocrine Disruptor
In addition to heavy metals removal, the biochar can be used to remove resistant endocrine disruptors in sediment. Dong et al. fabricated modified biochar, including Fe/FeOx, for the removal of nonylphenol (NP) in real sediment sampled from the Jen-Gen River in Taiwan [41]. The modified Fe3O4-BB (bamboo biochar) was prepared by the coprecipitation of iron oxides. The prepared biochar was obtained by pyrolyzing Moso bamboo (Phyllostachys pubescens) biomass at 800 °C and calcined at 300 °C prior to use. The removal efficiency of NP was slightly different according to the pH: 85%, 83%, and 71% at pH 3.0, 6.0, and 9.0, respectively. At low pH, the Fe3O4-BB catalyst showed higher activity for the removal of NP. According to their report, the NP removal reaction by Fe3O4-BB in the presence of PS (persulfate, [Na2S2O8] = 2.3 × 10−5 M) was fitted to pseudo-first-order kinetics. More available electron transfer between Fe2+ from Fe3O4 and PS resulted in better activity for the removal of NP.
3.3. Multi-Pollutant Removal Catalysts
Cho et al. [42] examined the applications of red mud and lignin because they are abundant waste materials from iron industry and forestry or agricultural activity residues. Various multi-functional groups on the pyrolyzed biochar surface and the red mud residue, including iron oxides, can make a synergetic effect by acting as an oxidant or reductant for the destruction of contaminants [43]. Cho et al. [42] utilized lignin and red mud (RM) to fabricate iron oxides-containing biochar (RLB) for the removal of environmental contaminants, such as heavy metals (Pb, Ni, Cr, and As), inorganic oxalates, such as nitrate and phosphate, and benzene substituents derived from pharmaceutical products. In addition, they compared the catalytic activity of RLB prepared at two different pyrolysis temperatures, i.e., 380 °C (RLB-380) and 700 °C (RLB-700), for the removal of these contaminants. The RLB-700 showed higher activity for the removal of contaminants rather than RLB-300 or RM for the removal of Ni(II), As(V), Cr(VI), PO43−, NO3−, methylene blue, p-nitrophenol, and p-chlorobenzoic acid. Both catalysts showed 100% removal of Pb(II). Pb(II) and Ni(II) could be removed via adsorption on the biochar. In contrast, As(V) and Cr(VI) could be removed via adsorption and reduction by the multi-functional groups on the surface of biochar and iron reduced from iron oxides. In addition, the anionic nitrates and nitrophenol were removed through the reduction process by metal iron. On the other hand, the oxidative catalytic efficiencies of RLB-700 are not superior to RM. Based on the experimental results of XRD and XPS, iron metal, which was not detected in RM or RLB-380, was produced only in RLB-700. The reduction of Fe2O3 to Fe metal would occur via an interaction of lignin and RM during pyrolyzing at high temperatures. The zero-valent iron including biochar may be significant for the reductive remediation of organic or inorganic contaminants by electron release as a reductant.
Yang et al. [40] fabricated an efficient hydrophilic biochar/nZVI catalyst for the removal of Pb2+, Zn2+, and Cu2+. In addition, biochar codoped with N and Cu, N-Cu/biochar, was fabricated for the removal of tetracycline by activated persulfates [44]. The composite (weight) ratio of glucose, urea, and cupric nitrate trihydrate (Cu(NO3)2·3H2O) was 5:1:1 applied to prepare N-Cu/biochar and biochar, N/biochar, and Cu/biochar sample were also fabricated to compare the catalytic activity for the removal of tetracycline using glucose, urea and glucose, and cupric nitrate trihydrate and glucose, respectively. Based on the experimental results, the removal efficiency of tetracycline by the prepared catalysts, Cu-N/biochar showed the best activity and Cu/biochar showed slightly inferior to Cu-N/biochar. However, biochar and N/biochar activity were significantly smaller than those of Cu-N/biochar or Cu/biochar. As for Cu-N/biochar, tetracycline (20 mg/L) was completely decomposed within 2 h under the addition of PS (persulfate) while only less than 50% of tetracycline was decomposed in 3 h without PS. The activation of PS produces radical species such as hydroxyl radical and PMS radical, which are active compounds to decompose contaminants. Based on the EPR (electron paramagnetic resonance), the interaction of Cu-N/biochar and PS produced abundant •OH species, which significantly reduced after the addition of tetracycline. However, only small amount of SO4•− was detected from the PS addition into Cu-N/biochar. It means that •OH species will play a major role of decomposing tetracycline. A non-radical pathway for the decomposition of tetracycline was also proposed to occur by the electron transfer between PS and on the surface of Cu-N/biochar without radical species involving.
3.4. Reductive Removal of Pollutants
Not only catalytic oxidation of pollutant using biochar-based materials, but the catalytic reductive removal of contaminants was also performed using noble metals with magnetic biochar support. The removal of 4-nitrophenol by Pd, Pt, Ag nanoparticles with Fe-biochar prepared by the pyrolysis of Fe preloaded sawdust provided by a wood factory in Hefei, China, was performed in the presence of metal hydride, NaBH4 [45]. During the reduction of 4-nitrophenol, hydrogen gas was obtained from the oxidation of metal hydride. The electronic state of noble metal nanoparticles could be affected by the interaction with transition metals and the binding energy was observed shifted to slightly higher level in the presence of transition metals [46,47], which was considered to enhance the hydrogen evolution. Compared to commercial Pd/C catalysts, the synthesized Pd/Fe-biochar was superior to H2 production.
The biochar-based materials playing a role of a support or carrier of catalysts for the removal of contaminants were listed in Table 1.

Table 1.
The biochar-based applications as catalyst support or carrier for the removal of contaminants.
4. Applications of Biochar as TiO2 Support
For the abatement of persistent organic pollutants (POPs), photocatalytic degradation is considered one of the most effective treatments because of the high activity and low cost. On the other hand, metals, particularly transition metal oxides, used as photocatalysts, can be transformed into more harmful and form intermediate complexes according to the environmental conditions or dissolved impurities in the medium. Among the various metal oxides, TiO2 is widely used as a photocatalyst for environmental remediation because of its non-toxicity, low cost, and high stability. The activity of TiO2 as a photocatalyst can be improved by various surface modifications, such as doping and the addition of effective components to TiO2. These modifications increase the activity by reducing the recombination of electrons and holes or by narrowing the bandgap of bare TiO2 [48,49,50]. Among them, the physicochemical properties of the support or carrier of TiO2 affect the photocatalytic activity. A carbon-based support was applied to photocatalysis as a catalyst support. On the other hand, disturbance by carbon particles, such as blocking UV absorption, deteriorates the activity of TiO2. At the same time, biochar-based support can help enhance the photocatalytic activity of TiO2 by preventing the recombination of excitons, such as electrons and holes, and allowing electron transfer. Because of the active functional groups and persistent free radicals on the biochar surface, the biochar-based support could improve the catalytic activity of TiO2 while involving an electrochemical reaction for the degradation of pollutant molecules. Bare carbon-containing materials, such as activated carbon or carbon black, have been used as the TiO2 support for environmental remediation [51,52]. Mao and Weng [52] performed the photocatalytic degradation of methyl orange using carbon black and activated carbon support and compared the efficiency for the removal of organic pollutants. Compared to the activated carbon support TiO2, the carbon black supported TiO2 performed better for the removal of methyl orange than activated carbon because of the improved capability of carbon black to restrict the recombination of holes and electrons released from TiO2 and relatively larger pore size. Although a carbon-structured support generally has a larger surface area and pore structure and is an effective adsorbent, there are critical unavoidable limitations. These include the inabilities to produce highly reactive species, stabilize radical intermediates, and prevent electron and hole recombination on the photocatalyst. On the other hand, biochar-based materials can overcome the shortcomings of only carbon-component supports. Reactive free radicals on the biochar surface can produce powerful oxidants, such as •OH and SO4•−. Moreover, various functional groups attached to biochar can act as an electron donor and initiate or participate in the decomposition of pollutants by activating potential oxidative radical species.
Removal of Pollutant Using TiO2/Biochar
Zhang et al. [35] applied biochar-TiO2 to remove sulfamethoxazole and compared the catalytic efficiency with bare TiO2. Compared to bare TiO2, the biochar support TiO2 showed improved activity for the degradation of sulfamethoxazole, 91.27% from 58.47% in the system using TiO2. The feedstock of employed biochar was reed straws supplied near the Yellow River in China, and the biochar/TiO2 was fabricated using sol-gel methods. They prepared the biochar/TiO2 samples pyrolyzed at different temperatures, 300, 400, and 500 °C. Based on their analysis, the structure of biochar pyrolyzed at 300 °C retained the shape of the vessels, and the external and internal walls of biochar were attached to TiO2 particles. The removal efficiency of biochar/TiO2 increased with decreasing calcination temperature. For example, the removal using the biochar/TiO2 pyrolyzed at 300 °C was approximately twice as effective as that prepared at 500 °C. Although the surface area and pore volume of biochar were reduced by the attached TiO2 metal oxides to the internal tube and external wall of the biochar, the catalytic activity for the removal of sulfamethoxazole was enhanced by the improved adsorption capability of biochar and the lack of agglomeration of TiO2 granules immobilized on the biochar surface. The specific surface area of the biochar-support TiO2 was increased more than 200 times, i.e., the surface area of the bare TiO2 was 0.39 m2/g, whereas the biochar support TiO2 was 102 m2/g, leading to enhanced adsorption ability toward contaminants. The photocatalysis by TiO2 after adsorption contaminants on biochar was assigned to effectively enhance the removal of them.
Biochar derived from various biomass feedstocks, such as agricultural and forestry residues, could be used as a photocatalyst support for TiO2 for the removal of harmful contaminants because of the low cost and adsorbent capability toward organic molecules. Lu et al. examined the photocatalytic oxidation of methyl orange using TiO2 with the biochar derived from walnut shells in Guangzhou [50]. They reported significantly improved removal efficiency for the decolorization and mineralization of methyl orange using the walnut shells biochar under UV (500 W, 360 nm) irradiation because of the many functional groups on the biochar surface. The removal efficiency of methyl orange was measured by observing the absorption of methyl orange at 464 nm through decolorization using a spectrophotometer (Lambda 750, Perkin Elmer) and by a TOC (total organic carbon) analyzer (Vario TOC, Elementar) for mineralization. Walnut shells biochar was fabricated according to both pyrolysis temperatures (500, 600, 700, and 800 °C) and the composition ratio of biochar toward TiO2 (0.1:1, 0.2:1, 0.5:1, 0.8:1, and 1:1). Among them, the surface area and the total pore volume of the biochar increased with increasing pyrolyzing temperature from 500 to 700 °C. In contrast, the surface area and total pore volume of the biochar pyrolyzed at 800 °C decreased due to the destruction of the mesoporous structure. In addition, depending on the composition of biochar toward TiO2, the photocatalyst with a ratio of 0.2:1 showed the highest efficiency for the removal of methyl orange. The activity of the TiO2/biochar photocatalyst with the optimized composition on the decolorization and mineralization of methyl orange was excellent, as illustrated by the 96.88% and 83.23% decrease, respectively. Further increases in the amount of biochar resulted in a decrease in decolorization and mineralization efficiency. The decolorization and mineralization efficiency of methyl orange decreased to 87.23% and 73.23%, respectively, when a 0.5:1 catalyst was employed. In addition, the removal efficiencies of methyl orange using the 1:1 catalyst were 63.11% and 43.35% for decolorization and mineralization, respectively. They reported that the overloaded biochar on the TiO2 surface might block the interaction of biochar and TiO2, e.g., electron transfer [50]. The decolorization and mineralization of methyl orange were negligible in the absence of a biochar catalyst: 2.24% and 1.20%, respectively. The removal efficiencies of methyl orange using walnut shells biochar only were 3.43% and 1.40% for decolorization and mineralization, respectively. When only bare TiO2 was employed, the decolorization and mineralization of methyl orange were relatively higher than that by the biochar only, 76.69% and 55.12%, respectively. The major species involving the degradation of methyl orange were determined to be superoxide anions and hydroxyl radicals. In addition, they examined the kinetic study on the removal of methyl orange by photocatalysis using TiO2 with the biochar support and reported that the photocatalytic oxidation of methyl orange followed the first-order kinetics.
In addition, the TiO2-coated biochar (TBCs) was used for the removal of safranine T in aqueous solution and a higher photocatalytic efficiency was observed compared to the bare TiO2 [53]. The biochar was prepared by pyrolyzing ramie bars at 500 °C and the prepared biochar was added to the TiO2 precursor solution at different amounts (1, 1.5, 2, and 2.5 g) to synthesize TBC-1, TBC1.5, TBC-2, and TBC-2.5, respectively. Among these four samples, TBC-2 showed the best efficiency for the decolorization of safranine T. The synergetic effects of adsorption through electrostatic interactions, π- π interactions, H-bonding between safranine T and functional groups on the biochar surface, and photocatalytic degradation by TiO2 contributed to the removal of the contaminant. The TiO2-coated biochar showed improved adsorption capacity compared to the bare biochar.
Table 2 lists the biochar based TiO2 photocatalysts for the removal of contaminants.

Table 2.
The biochar-based TiO2 photocatalysts for the removal of contaminants.
5. Applications of Biochar for the Remediation of Eutrophic Water
As the increase of discharge of nutrients containing wastewater, especially for the disposal of nitrogen and phosphorus included compounds, there are many treatments to recover deteriorated water. Among them, biochar or modified biochar materials were utilized for the removal of phosphate (PO43−) and nitrate (NO3−) to cause the eutrophication of water in the wake of phytoplankton blooming and rapid decline of dissolved oxygen in water system.
The biochar prepared from the pyrolysis of poplar chips at 550 °C was used for the adsorption of phosphate and nitrate after Al preloading [54]. The Al-biochar samples were synthesized with different content of Al (5, 10, 15, 20 wt%) to compare the adsorption capacity according to the Al loading on the biochar. The concentration of nitrate and phosphate were adjusted at 50 mg/L by addition of NaNO3 and KH2PO4, respectively. The adsorption of phosphate and nitrate by the Al-biochar showed differences according to pH and Al loading. The adsorption of nitrate showed the best activity (89.58 mg/g) by 15 wt% Al-biochar at pH = 6 while that of phosphate showed the highest value (57.49 mg/g) by 20 wt% Al-biochar under pH < 6. However, for the simultaneous adsorption of two contaminants, the nitrate adsorption was affected more than phosphate. The adsorption of nitrate by Al-biochar reduced significantly compared to that of phosphate in coexisting solution. These two anions may compete toward limited active sites on the Al-biochar surface and phosphate can be more favorable to adsorb on the biochar under the condition. They also studied on the kinetics for the adsorption of nitrate and phosphate by Al-biochar, which was ascribed to follow the pseudo-second-order model well. In addition, based on the experimental results, the adsorption of nitrate and phosphate by 15 wt% Al-biochar and 20 wt% Al-biochar, respectively, were fitted well to the Langmuir–Freundlich mechanism.
Compared to the bare biochar, which has negligible adsorption capacity for nitrate and phosphate, Al-loaded biochar showed significantly improved activity for the removal of contaminants, which would be carried out via the contributions of interaction of contaminants and Al such as electrostatic interactions of cationic charged Al and anionic charged nitrate or phosphate and formation of partial bond of Al and oxygen atom in contaminants.
Because phosphorus is an essential nutrient for plant growing or seedling, phosphate-adsorbed biochar can be reused as a P-abundant fertilizer for the remediation of soil [55,56]. The Al-doped biochar was employed for the removal of phosphate [55]. Two types of biochar were derived from poultry sewage and sugarcane straw pyrolyzed at low and high temperature, 350 and 650 °C, respectively. However, the prepared biochar only pyrolyzed at 350 °C was applied to the removal of phosphate to avoid the loss of CO2 leading to reducing activity. The biochar was added into AlCl3 solution to fabricate Al-biochar with different content of Al loading. The optimized Al-biochar removed phosphate in the presence of nitrate, sulfate, and chloride ions without any significant influence. The removal efficiency by Al-biochar fabricated with sugarcane straw more than 90% showed slightly better than that by Al-biochar with poultry manure more than 85% although the non-Al biochar showed no adsorption for phosphate. The adsorption results of phosphate on the biochar were described well to the Langmuir model for both Al-biochar adsorbents. For the reuse of phosphate adsorbed Al-biochar as P-fertilizer, the extraction method should be significantly considered regarding releasing rate and quantity of phosphorus. Several extractor solutions such as water, sulfuric acid, and sodium bicarbonate, were evaluated for the extraction of phosphate adsorbed Al-doped biochar. Among them, the extraction with sodium bicarbonate was suitable for phosphate extractor from Al-biochar. With respect to the remediation of phosphorus-deficient soil, rather than extracting phosphate completely at once in the case of sulfuric acid, it is more beneficial for seedling growing to release certain amount of phosphate at an interval of time.
Besides Al, calcium peroxide (CaO2) fabricated with biochar can also be used for the removal of phosphate and reused as P-fertilizer back to soil. In general, calcium peroxide causes precipitation via reaction with anions dissolved in aqueous environment and affects the acidity of solution by producing OH− anions. However, nanosized particles of calcium peroxide can overcome these kinds of problems and induces the synergetic effects attached on the biochar surface. The biochar pyrolyzed the wheat straw at 600 °C and nanosized CaO2 were used to fabricate nano-CaO2/biochar for the removal of phosphate [56]. The adsorption of phosphate on the nano-CaO2/biochar showed quite different tendency according to the initial concentration of phosphate. The kinetic study on the adsorption of phosphate on the nano-CaO2/biochar could be ascribed well to the pseudo-second-order mechanism among the nonlinear pseudo-first-order model, the pseudo-second-order model, and the Elovich model. Namely, at low concentration level, the adsorption of phosphate reached the equilibrium state very fast while as increasing the initial concentration of phosphate, it took much longer time to reach the equilibrium state. They suggested that the chemisorption of phosphate on the nano-CaO2/biochar might be the rate-determining step. The adsorption of phosphate on the nano-CaO2/biochar was also performed at 298, 318, and 338 K to study the adsorption mechanism. Among the Langmuir, the Freundlich, the Langmuir–Freundlich, and the Redlich–Peterson model, the phosphate adsorption was described well following the Langmuir–Freundlich and the Redlich–Peterson model with the values of R2 = 0.999 for both models. The estimated maximum value of adsorption of phosphate was 213 mg/g by the nano-CaO2/biochar at 298 K. Also, the phosphate adsorbed on thenano-CaO2/biochar was applied to the tomato seedling growth with different amounts of phosphate. Compared to the blank and only biochar-loaded samples, the phosphate adsorbed nano-CaO2/biochar applied to several tomato seedlings became stronger and greener leaves.
In Table 3, the removals of pollutants including phosphate and nitrate discussed in this section using biochar-based materials are summarized.

Table 3.
The summarized removals of environmental pollutants by the modified biochar-based materials.
6. Removal of N-Pollutants and Tar
Activated biochar can be used for the removal of NOx and ammonia [57,58]. The activated biochar derived from rice straws and sewage sludge showed comparable activity for the removal of NOx with 86% and 46%, respectively due to the increased surface area and pore volume and the oxygen-containing functional groups [57]. Also, transition metal added biochar showed activity for the removal of NOx. Manganese oxides impregnated rice straws biochar showed similar activity for the removal of NOx as the alkali-activated rice straws biochar [57].
Furthermore, the biochar obtained from the pyrolysis of peanut hulls was used for the removal of ammonia in the presence of ozone and showed 65% conversion of ammonia under the ozone concentration of about 900 ppm [58].
On the other hand, modified biochar could be utilized for the removal of tar, which was produced from the generation of energy and fuel regarding transformation or dealing with syngas [59,60]. Compared to thermal cracking, biochar derived from the pyrolysis of pink bark showed 94% removal efficiency for toluene, which one of the major components of tar owing to significantly lowed activation energy [59]. Also, Fe or alkali metal-loaded biochar was effective for the removal of tar [59,60].
7. Conclusions and Perspectives
The applications of biochar-based materials as catalysts, catalyst supports, and adsorbent for environmental remediation have increased considerably because biochar can be modified for a specific purpose depending on the preparation methods and feedstock. For the control of environmental pollution, the utilization of biochar is sustainable, eco-friendly, and cost-effective in terms of recycling waste materials into new resources. This paper reassessed the value of biochar derived from various feedstock in terms of the catalytic efficiency, the influences of Fe/FeOx, TiO2, and the adsorption capability for the removal of contaminants.
Because the biochar surface can be modified and activated by various processes and compounds, the applications and utilizations of biochar-based materials may be developed further for the removal of contaminants. Also, modified biochar with conjugated aromatic groups or unsaturated bonds can be utilized as electrode or photoelectrode in the electrochemical or photoelectrochemical reaction for the hydrogen production. Further investigations of the relevant reaction mechanisms of biochar-based materials and co-existing compounds, such as the catalyst or active species, will be needed for biochar-based commercial products and the development of improved technologies for the removal of pollutants.
Author Contributions
Conceptualization, J.E.L. and Y.-K.P.; methodology, J.E.L. and Y.-K.P.; investigation, J.E.L. and Y.-K.P.; writing—original draft preparation, J.E.L. and Y.-K.P.; writing—review and editing, J.E.L. and Y.-K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (NRF-2019R1A4A1027795).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Liu, L.; Ayub, K.S.; Zhang, W.; Shen, G.; Hu, S.; Qian, X. Characterization and adsorption performance of biochars derived from three key biomass constituents. Fuel 2020, 269, 117142. [Google Scholar] [CrossRef]
- Ormsby, R.; Kastner, J.R.; Miller, J. Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal. Today 2012, 190, 89–97. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Entchev, E. Ag/Biochar composite for supercapacitor electrodes. Mater. Today Energy 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Gihoon, K.; Bhatnagar, A.; Wang, H.; Kwon, E.E.; Song, H. A Review of Recent Advancements in Utilization of Biomass and Industrial Wastes into Engineered Biochar. J. Hazard. Mater. 2020, 400, 123242. [Google Scholar] [CrossRef]
- Anuj Kumar, P.; Mondal, M.K. Hazardous As (III) removal using nanoporous activated carbon of waste garlic stem as adsorbent: Kinetic and mass transfer mechanisms. Korean J. Chem. Eng. 2019, 36, 1900–1914. [Google Scholar]
- Li, Y.; Song, N.; Wang, K. Preparation and characterization of a novel graphene/biochar composite and its application as an adsorbent for Cd removal from aqueous solution. Korean J. Chem. Eng. 2019, 36, 678–687. [Google Scholar] [CrossRef]
- Lee, M.E.; Jeon, P.; Kim, J.G.; Baek, K. Adsorption characteristics of arsenic and phosphate onto iron impregnated biochar derived from anaerobic granular sludge. Korean J. Chem. Eng. 2018, 35, 1409–1413. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Cai, W.; Cao, Y.; Sun, Y.; Tan, F. Preparation of corn straw based spongy aerogel for spillage oil capture. Korean J. Chem. Eng. 2018, 35, 1119–1127. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.; Lee, S.M.; Jung, J. Application of iron-modified biochar for arsenite removal and toxicity reduction. J. Ind. Eng. Chem. 2019, 80, 17–22. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Adi, V.S.K.; Huang, H.L.; Lin, H.P.; Huang, Z.H. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: Adsorption isotherm and process simulation. J. Ind. Eng. Chem. 2019, 76, 240–244. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, H.; Kim, Y.M.; Park, R.S.; Park, Y.K. Recent application of biochar on the catalytic biorefinery and environmental processes. Chin. Chem. Lett. 2019, 30, 2147–2150. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical Review on Biochar-Supported Catalysts for Pollutant Degradation and Sustainable Biorefinery. Adv. Sustain. Sys. 2020. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, J.G.; Choi, J.H.; Kwon, E.E.; Baek, K. Photo-induced redox coupling of dissolved organic matter and iron in biochars and soil system: Enhanced mobility of arsenic. Sci. Total Environ. 2019, 689, 1037–1043. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.W.; Tsang, Y.F.; Tsang, D.C.; Kwon, E.E.; Song, H. Synthesis of functionalised biochar using red mud, lignin, and carbon dioxide as raw materials. Chem. Eng. J. 2019, 361, 1597–1604. [Google Scholar] [CrossRef]
- Li, X. Preparation and Adsorption Properties of Biochar/g-C3N4 Composites for Methylene Blue in Aqueous Solution. J. Nanometer. 2019, 2019. [Google Scholar] [CrossRef]
- Caban, M.; Folentarska, A.; Lis, H.; Kobylis, P.; Kumirska, J.; Stepnowski, P.; Ciesielski, W. Valuable polar moieties on cereal-derived biochars. Colloids Surf. A Phys. Eng. Asp. 2019, 561, 275–282. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Yang, H.S.; Zhang, M.; Ju, M.; Liu, L. Preparation and Modification of Biochar Materials and their Application in Soil Remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Ding, Y.; Xiong, J.; Zhou, B.; Wei, J.; Qian, A.; Zhang, H.; Zhu, W.; Zhu, J. Odor removal by and microbial community in the enhanced landfill cover materials containing biochar-added sludge compost under different operating parameters. Waste Manag. 2019, 87, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.; Lee, S.R.; Cho, S.; Ro, K.S.; Spidehs, M.; Woodbury, B.; Silva, P.J.; Han, D.W.; Choi, H.; Kim, K.Y.; et al. Efficacy of Different Biochars in Removing Odorous Volatile Organic Compounds (VOCs) Emitted from Swine Manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 1248422. [Google Scholar] [CrossRef]
- Cao, L.; Iris, K.M.; Chen, S.S.; Tsang, D.C.; Wang, L.; Xiong, X.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Li, R. Characterization, Modification and Application of Biochar for Energy Storage and Catalysis: A Review. Trends Renew. Energy 2017, 3, 86–101. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Vochozka, M.; Maroušková, A.; Váchal, J.; Straková, J. The economic impact of biochar use in Central Europe. Energ. Sour. Part. A 2016, 38, 2390–2396. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: Performance and mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Q.; Sun, M.; Sun, P.; Shen, G. Pyrolysis temperature-induced changes in the catalytic characteristics of rice husk-derived biochar during 1.3-dichloropropene degradation. Chem. Eng. J. 2017, 330, 804–812. [Google Scholar] [CrossRef]
- Zhao, N.; Yin, Z.; Liu, F.; Zhang, M.; Lv, Y.; Hao, Z.; Pan, G.; Zhang, J. Environmentally persistent free radicals mediated removal of Cr (VI) from highly saline water by corn straw biochars. Bioresour. Technol. 2018, 260, 294–301. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Leandro, L.; Aller, D. Biochar effect on severity of soybean root disease caused by Fusarium Virguliforme. Plant. Soil 2017, 413, 111–126. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanism. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, T.; Shen, M.; Huang, Z.; Chong, Y.; Cui, L. Coagulation treatment of swine wastewater by the method of in-situ forming layered double hydroxides and sludge recycling for preparation of biochar composite catalyst. Chem. Eng. J. 2019, 369, 784–792. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Fan, X.; Wang, T. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: Roles of soil characteristics and ambient conditions. J. Hazard. Mat. 2019, 362, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Criquet, J.; Leitner, N.K.V. Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere 2009, 77, 194–200. [Google Scholar] [CrossRef]
- Zhang, S.; Lyu, H.; Tang, J.; Song, B.; Zhen, M.; Liu, X. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 2019, 217, 686–694. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Tsai, M.L.; Chang, J.H.; Lyu, S.Y.; Hung, C.M. Degradation of 4-nonylphenol in marine sediments by persulfate over magnetically modified biochars. Bioresour. Technol. 2019, 281, 143–148. [Google Scholar] [CrossRef]
- Cho, D.W.; Yoon, K.; Ahn, Y.; Sun, Y.; Tsang, D.C.W.; Hou, D.; Ok, Y.S.; Song, H. Fabrication and environmental applications of multifunctional mixed metal-biochar composites (MMBC) from red mud and lignin wastes. J. Hazard. Mater. 2019, 374, 412–419. [Google Scholar] [CrossRef]
- Yoon, K.; Jung, J.M.; Cho, D.W.; Tsang, D.C.; Kwon, E.E.; Song, H. Engineered biochar composite fabricated from red mud and lipid waste and synthesis of biodiesel using the composite. J. Hazard. Mater. 2019, 366, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Jiang, S.F.; Xi, K.F.; Yang, J.; Jiang, H. Biochar-supported magnetic noble metallic nanoparticles for the fast recovery of excessive reductant during pollutant reduction. Chemosphere 2019, 227, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yongchun, H.; Zhang, H.; Sun, J.; Ayman, K.M.; Hensley, A.J.R.; Gu, M.; Engelhard, M.H.; McEwen, J.-S.; Wang, Y. Synergistic catalysis between Pd and Fe in gas phase hydrodeoxygenation of m-cresol. ACS Catal. 2014, 4, 3381–3392. [Google Scholar]
- Garg, A.; Milina, M.; Ball, M.; Zanchet, D.; Hunt, S.T.; Dumesic, J.A.; Román-Leshkov, Y. Transition-Metal Nitride Core@Noble-Metal Shell Nanoparticles as Highly CO Tolerant Catalysts. Angew. Chem. Int. Ed. 2017, 56, 8828–8833. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Ma, M.; Guo, W.; Yang, Z.; Huang, S.; Wang, G. Preparation and Photocatalytic Activity of TiO2/Fine Char for Removal of Rhodamine B. J. Nanometer. 2015, 2015. [Google Scholar] [CrossRef]
- Mao, C.C.; Weng, H.S. Promoting effect of adding carbon black to TiO2 for aqueous photocatalytic degradation of methyl orange. Chem. Eng. J. 2009, 155, 744–749. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Novais, S.V.; Zenero, M.D.O.; Barreto, M.S.C.; Montes, C.R.; Cerri, C.E.P. Phosphorus removal from eutrophic water using modified biochar. Sci. Total Environ. 2018, 633, 825–835. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Jiang, F.; Wang, B.; Hu, Q.; Tang, Y.; Luo, T.; Wu, T. Enhanced phosphate removal from aqueous solution using resourceable nano-CaO2/BC composite: Behaviors and mechanisms. Sci. Total Environ. 2020, 709, 136123. [Google Scholar] [CrossRef]
- Cha, J.S.; Choi, J.C.; Ko, J.H.; Park, Y.K.; Park, S.H.; Jeong, K.E.; Kim, S.S.; Jeon, J.K. The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Kolar, P.; Das, K.C. Catalytic ozonation of ammonia using biomass char and wood fly ash. Chemosphere 2009, 75, 739–744. [Google Scholar] [CrossRef]
- Mani, S.; Kastner, J.R.; Juneja, A. Catalytic decomposition of toluene using a biomass derived catalyst. Fuel Process. Technol. 2013, 114, 118–125. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, Y. Advances in in situ and ex situ tar reforming with biochar catalysts for clean energy production. Sustain. Energy Fuels 2018, 2, 326. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
