Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Satellite Telemetry Data
2.2. Tagging Operation
2.3. Data Analysis
3. Results
4. Discussion
4.1. Short-Term Movement Patterns
4.2. Vertical Distribution
4.3. Future Research and Management
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vladykov, V.D.; McKenzie, R.A. The marine fishes of Nova Scotia. Proc. Nova Scotian Inst. Sci. 1935, 19, 17–113. [Google Scholar]
- Shcherbachev, Y.N. The biology and distribution of the dolphins (Pisces, Coryphaenidae). J. Ichthyol. 1973, 13, 182–191. [Google Scholar]
- Palko, B.J.; Beardsley, G.L.; Richards, W.J. Synopsis of biological data on dolphin-fishes, Coryphaena hippurus Linnaeus and Coryphaena equiselis Linnaeus. FAO Fish. Synop. 1982, 130, 1–28. [Google Scholar]
- Norton, J.G. Apparent habitat extensions of dolphinfish (Coryphaena hippurus) in response to climate transients in the California Current. Sci. Mar. 1999, 63, 239–260. [Google Scholar] [CrossRef]
- Oxenford, H.A.; Hunte, W. Feeding habits of the dolphinfish (Coryphaena hippurus) in the eastern Caribbean. Sci. Mar. 1999, 63, 303–315. [Google Scholar] [CrossRef]
- Rivera, G.A.; Appeldoorn, R.S. Age and growth of dolphinfish, Coryphaena hippurus, off Puerto Rico. Fish. Bull. 2000, 98, 345–352. [Google Scholar]
- Alejo-Plata, C.; Díaz-Jaimes, P.; Salgado-Ugarte, I.H. Sex ratios, size at sexual maturity, and spawning seasonality of dolphinfish (Coryphaena hippurus) captured in the Gulf of Tehuantepec, Mexico. Fish. Res. 2011, 110, 207–216. [Google Scholar] [CrossRef]
- Farrell, E.R.; Boustany, A.M.; Halpin, P.N.; Hammond, D.L. Dolphinfish (Coryphaena hippurus) distribution in relation to biophysical ocean conditions in the northwest Atlantic. Fish. Res. 2014, 151, 177–190. [Google Scholar] [CrossRef]
- Kraul, S. Seasonal abundance of dolphinfish, Coryphaena hippurus, in Hawaii and the tropical Pacific Ocean. Sci. Mar. 1999, 63, 261–266. [Google Scholar] [CrossRef]
- Zúñiga-Flores, M.S.; Ortega-García, S.; Klett-Trauslen, A. Interannual and seasonal variation of dolphinfish (Coryphaena hippurus) catch rates in the southern Gulf of California, Mexico. Fish. Res. 2008, 94, 13–17. [Google Scholar] [CrossRef]
- Schwenke, K.L.; Buckel, J.A. Age, growth, and reproduction of dolphinfish (Coryphaena hippurus) caught off the coast of North Carolina. Fish. Bull. 2008, 106, 82–92. [Google Scholar]
- Torrejón-Magallanes, J.; Grados, D.; Lau-Medrano, W. Spatio-temporal distribution modeling of dolphinfish (Coryphaena hippurus) in the Pacific Ocean off Peru using artisanal longline fishery data. Deep-Sea Res. Part II 2019, 169–170, 104665. [Google Scholar] [CrossRef]
- Furukawa, S.; Ohshimo, S.; Tomoe, S.; Shiraishi, T.; Nakatsuka, N.; Kawabe, R. Age, growth, and reproductive characteristics of dolphinfish Coryphaena hippurus in the waters off west Kyushu, northern East China Sea. Fish. Sci. 2012, 78, 1153–1162. [Google Scholar] [CrossRef]
- Trippel, E.A. Age at maturity as a stress indicator in fisheries. BioScience 1995, 45, 759–771. [Google Scholar] [CrossRef]
- Beardsley, G.L., Jr. Age, growth and reproduction of the dolphin, Coryphaena hippurus, in the Straits of Florida. Copeia 1967, 1967, 441–451. [Google Scholar] [CrossRef]
- Rodriguez-Ferrer, G.; Rodriguez-Ferrer, Y.; Matos-Caraballo, D.; Yestrom, C.L. Comparison of dolphinfish (Coryphaena hippurus) commercial and recreational fisheries in Puerto Rico during 2000–2003. Proc. Gulf Caribb. Fish. Inst. 2004, 57, 297–316. [Google Scholar]
- Sakamoto, R.; Kojima, S. Review of dolphinfish biological and fishing data in Japanese waters. Sci. Mar. 1999, 63, 375–385. [Google Scholar] [CrossRef]
- Díaz-Jaimes, P.; Uribe-Alcocer, M.; Ortega-García, S.; Durand, J.D. Spatial and temporal mitochondrial DNA genetic homogeneity of dolphinfish populations (Coryphaena hippurus) in the eastern central Pacific. Fish. Res. 2006, 80, 333–338. [Google Scholar] [CrossRef]
- Tripp-Valdez, M.A.; de García León, F.J.; Ortega-García, S.; Lluch-Cota, D.; López-Martínez, J.; Cruz, P. Population genetic structure of dolphinfish (Coryphaena hippurus) in the Gulf of California, using microsatellite loci. Fish. Res. 2010, 105, 172–177. [Google Scholar] [CrossRef]
- Chang, S.K.; DiNardo, G.; Farley, J.; Brodziak, J.; Yuan, Z.L. Possible stock structure of dolphinfish (Coryphaena hippurus) in Taiwan coastal waters and globally based on reviews of growth parameters. Fish. Res. 2013, 147, 127–136. [Google Scholar] [CrossRef]
- SFP Urges Stock-Wide Assessments of Pacific Mahi in First Sustainability Overiew Report. Available online: https://www.sustainablefish.org/News/SFP-Urges-Stock-Wide-Assessments-of-Pacific-Mahi-in-First-Sustainability-Overview-Report (accessed on 28 March 2020).
- Chang, S.K.; Yuan, T.L.; Wang, S.P.; Chang, Y.J.; DiNardo, G. Deriving a statistically reliable abundance index from landings data: An application to the Taiwanese coastal dolphinfish fishery with a multispecies feature. Trans. Am. Fish. Soc. 2019, 148, 106–122. [Google Scholar] [CrossRef]
- Whitney, N.M.; Taquet, M.; Brill, R.W.; Girard, C.; Schwieterman, G.D.; Dagorn, L.; Holland, K.N. Swimming depth of dolphinfish (Coryphaena hippurus) associated and unassociated with fish aggregating devices. Fish. Bull. 2016, 114, 426–434. [Google Scholar] [CrossRef]
- Furukawa, S.; Kawabe, R.; Ohshimo, S.; Fujioka, K.; Nishihara, G.N.; Tsuda, Y.; Aoshima, T.; Kanehara, H. Vertical movement of dolphinfish Coryphaena hippurus as recorded by acceleration data-loggers in the northern East China Sea. Environ. Biol. Fish. 2011, 92, 89–99. [Google Scholar] [CrossRef]
- Merten, W.; Appeldoorn, R.; Rivera, R.; Hammond, D. Diel vertical movements of adult male dolphinfish (Coryphaena hippurus) in the western central Atlantic as determined by use of pop-up satellite archival transmitters. Mar. Biol. 2014, 161, 1823–1834. [Google Scholar] [CrossRef]
- Lin, S.J.; Musyl, M.K.; Wang, S.P.; Su, N.J.; Chiang, W.C.; Lu, C.P.; Tone, K.; Wu, C.Y.; Sasaki, A.; Nakamura, I.; et al. Movement behaviour of released wild and farm-raised dolphinfish, Coryphaena hippurus, tracked by pop-up satellite archival tags. Fish. Sci. 2019, 85, 779–790. [Google Scholar] [CrossRef]
- Furukawa, S.; Tsuda, Y.; Nishihara, G.N.; Fujioka, K.; Ohshimo, S.; Tomoe, S.; Nakatsuka, N.; Kimura, H.; Aoshima, T.; Kanehara, H.; et al. Vertical movements of Pacific bluefin tuna (Thunnus orientalis) and dolphinfish (Coryphaena hippurus) relative to the thermocline in the northern East China Sea. Fish. Res. 2014, 149, 86–91. [Google Scholar] [CrossRef]
- Musyl, M.K.; Brill, R.W.; Curran, D.S.; Fragoso, N.M.; McNaughton, L.M.; Nielsen, A.; Kikkawa, B.S.; Moyes, C.D. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish. Bull. 2011, 109, 341–368. [Google Scholar]
- Musyl, M.K.; Domeier, M.L.; Nasby-Lucas, N.; Brill, R.W.; McNaughton, L.M.; Swimmer, J.Y.; Lutcavage, M.S.; Wilson, S.G.; Galuardi, B.; Liddle, J.B. Performance of pop-up satellite archival tags. Mar. Ecol. Prog. Ser. 2011, 433, 1–28. [Google Scholar] [CrossRef]
- Lam, C.H.; Nielsen, A.; Sibert, J.R. Improving light and temperature based geolocation by unscented Kalman filtering. Fish. Res. 2008, 91, 15–25. [Google Scholar] [CrossRef]
- Lam, C.H.; Nielsen, A.; Sibert, J.R. Incorporating sea-surface temperature to the light-based geolocation model TrackIt. Mar. Ecol. Prog. Ser. 2010, 419, 71–84. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Brill, R.W.; Holts, D.B.; Chang, R.K.C.; Sullivan, S.; Dewar, H.; Carey, F.G. Vertical and horizontal movements of striped marlin (Tetrapturus audax) near the Hawaiian Islands, determined by ultrasonic telemetry, with simultaneous measurement of oceanic currents. Mar. Biol. 1993, 117, 567–574. [Google Scholar] [CrossRef]
- Nielsen, A.; Bigelow, K.A.; Musyl, M.K.; Sibert, J.R. Improving light-based geolocation by including sea surface temperature. Fish. Oceanogr. 2006, 15, 314–325. [Google Scholar] [CrossRef]
- Kojima, S. On the distribution of the dolphin, Coryphaena hippurus L., in the Pacific Ocean and the Indian Ocean. Bull. Jpn. Soc. Sci. Fish. 1964, 30, 472–477. [Google Scholar] [CrossRef]
- Toba, Y.; Tomizawa, K.; Kurasawa, Y.; Hanawa, K. Seasonal and year-to-year variability of the Tsushima-Tsugaru current systrm with its possible cause. La Mer 1982, 20, 41–51. [Google Scholar]
- Ohshima, K.I. The flow system in the Japan sea caused by a sea level difference through shallow straits. J. Geophys. Res. 1994, 99, 9925–9940. [Google Scholar] [CrossRef]
- Takikawa, T.; Yoon, J.H.; Cho, K.D. The Tsushima warm current through Tsushima straits estimated from ferryboat ASCP data. J. Phys. Oceanogr. 2005, 35, 1154–1168. [Google Scholar] [CrossRef]
- Watanabe, T.; Katoh, O.; Yamada, H. Structure of the Tsushima Warm Current in the Northeastern Japan Sea. J. Oceanogr. 2006, 62, 527–538. [Google Scholar] [CrossRef]
- Kojima, S. Studies on fishing conditions of the dolphin, Coryphaena hippurus, in the western regions of the Sea of Japan-XI. School of dolphins accompanying various kinds of flotages. Bull. Jpn. Soc. Sci. Fish. 1966, 32, 647–651. [Google Scholar] [CrossRef]
- Carey, F.G.; Scharold, J.V.; Kalmijn, A.J. Movements of blue sharks (Prionace glauca) in depth and course. Mar. Biol. 1990, 106, 329–342. [Google Scholar] [CrossRef]
- Holland, K.; Brill, R.; Chang, R.K.C. Horizontal and vertical movements of Pacific blue marlin captured and released using sportfishing gear. Fish. Bull. 1990, 88, 397–402. [Google Scholar]
- Block, B.A.; Booth, D.T.; Carey, F.G. Depth and temperature of the blue marlin, Makaira nigricans, observed by acoustic telemetry. Mar. Biol. 1992, 114, 175–183. [Google Scholar] [CrossRef]
- Musyl, M.K.; Brill, R.W.; Boggs, C.H.; Curran, D.S.; Kazama, T.K.; Seki, M.P. Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fish. Oceanogr. 2003, 12, 152–169. [Google Scholar] [CrossRef]
- Massuti, E.; Deudero, S.; Sanchez, P.; Morales-Nin, B. Diet and feeding of dolphin (Coryphaena hippurus) in western Mediterranean waters. Bull. Mar. Sci. 1998, 63, 329–341. [Google Scholar]
- Rothschild, R.J. Observations on dolphins (Coryphaena spp.) in the central Pacific Ocean. Copeia 1964, 1964, 375–385. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, W.Y.; Chen, Y.K.; Kuo, Y.C.; Lee, M.A. Winter abundance and species composition of anchovy larvae associated with hydrological conditions in the coastal waers of Tanshul, Taiwan. J. Mar. Sci. Technol. 2018, 26, 455–474. [Google Scholar] [CrossRef]
- Gleiss, A.C.; Wright, S.; Liebsch, N.; Wilson, R.P.; Norman, B. Contrasting diel patterns in vertical movement and locomotor activity of whale sharks at Ningaloo Reef. Mar. Biol. 2013, 160, 2981–2992. [Google Scholar] [CrossRef]
- Sims, D.W.; Southall, E.J.; Tarling, G.A.; Metcalfe, J.D. Habitat-specific normal and reverse diel vertical migration in the plankton feeding basking shark. J. Anim. Ecol. 2005, 74, 755–761. [Google Scholar] [CrossRef]
- Saunders, R.A.; Royer, F.; Clarke, M.W. Winter migration and diving behavior of porbeagle shark, Lamna nasus, in the northeast Atlantic. ICES J. Mar. Sci. 2011, 68, 166–174. [Google Scholar] [CrossRef]
- Weng, K.C.; Boustany, A.M.; Pyle, P.; Anderson, S.C.; Brown, A.; Block, B.A. Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar. Biol. 2007, 152, 877–894. [Google Scholar] [CrossRef]
- Stramma, L.; Prince, E.D.; Schmidtko, S.; Luo, J.; Hoolihan, J.P.; Visbeck, M.; Wallace, D.W.R.; Brandt, P.; Kortzinger, A. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat. Clim. Chang. 2012, 2, 33–37. [Google Scholar] [CrossRef]
- Prince, E.D.; Lou, J.; Goodyear, C.P.; Hoolihan, J.P.; Snodgrass, D.; Orbesen, E.S.; Serafy, J.E.; Ortiz, M.; Schirripa, M.J. Ocean scale hypoxiabased habitat compression of Atlantic istiophorid billfishes. Fish. Oceanogr. 2010, 19, 448–462. [Google Scholar] [CrossRef]
- Val, A.L.; Almeida-Val, V.M.F.; Randall, D.J. Fish Physiology. In The Physiology of Tropical Fishes; Elsevier/Academic Press: London, UK, 2006; Volume 21. [Google Scholar]
- Salvadeo, C.; Auliz-Ortiz, D.M.; Petatán-Ramírez, D.; Reyes-Bonilla, H.; Ivanova-Bonchera, A.; Juárez-León, E. Potential poleward distribution shift of dolphinfish (Coryphaena hippurus) along the southern California Current System. Environ. Biol. Fish. 2020, 103, 973–984. [Google Scholar] [CrossRef]
- Furukawa, S.; Chiang, W.C.; Watanabe, S.; Hung, H.M.; Lin, H.C.; Yeh, H.M.; Wang, S.P.; Tone, K.; Kawabe, R. The first record of peritoneal cavity temperature in free-swimming dolphinfish Coryphaena hippurus by using archival tags, on the East Coast of Taiwan. J. Aquacult. Mar. Biol. 2015, 2, 1–7. [Google Scholar] [CrossRef]





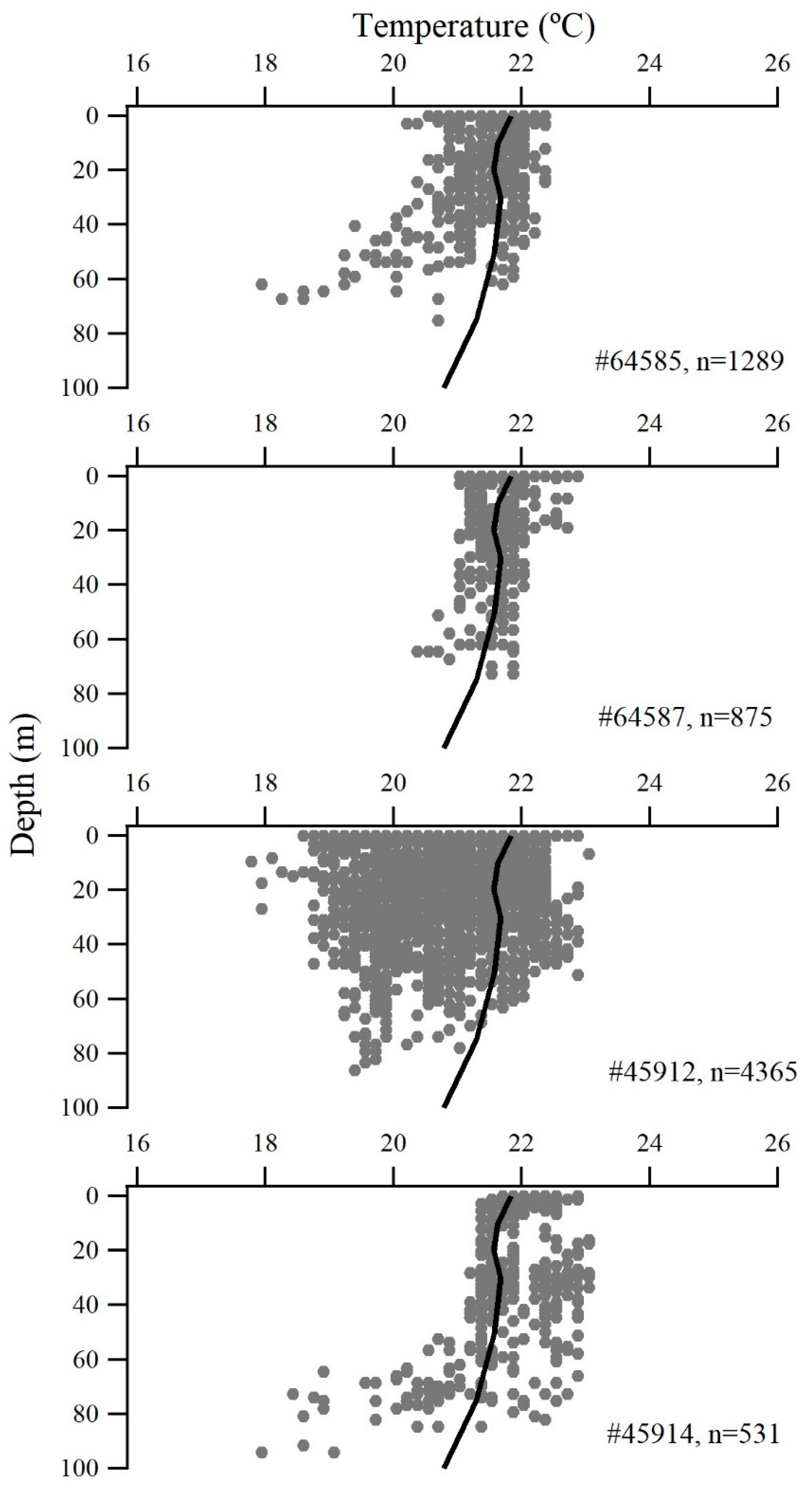


| PSAT# | FL (cm) | Deployment Date | Reporting Date | DAL (Days) | Straight-Line Distance (km) | Average Daily Displacements (km/Day) | Data Return Rate (%) | Pop-off Reason 1 | Tag Type |
|---|---|---|---|---|---|---|---|---|---|
| 64585 | 96 | 31 October 2018 | 20 November 2018 | 21 | 82.92 | 3.95 | 77 | Constant Pressure | X-tag |
| 64587 | 101 | 1 November 2018 | 12 November 2018 | 12 | 97.27 | 8.4 | 89 | Constant Pressure | X-tag |
| 45912 | 94 | 1 November 2018 | 1 December 2018 | 31 | 203.67 | 6.57 | 48 | Memory Full | HR X-tag |
| 45914 | 102 | 1 November 2018 | 5 November 2018 | 5 | 62.64 | 12.4 | 36 | Constant Pressure | HR X-tag |
| PSAT# | △SST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | 0 | −1 | −2 | −3 | −4 | −5 | −6 | −7 | −8 | |
| 64585 | Day | 16.5 | 97.7 | 100 | ||||||
| Night | 18.6 | 91.0 | 98.6 | 99.4 | 100 | |||||
| 64587 | Day | 21.6 | 89.4 | 100 | ||||||
| Night | 15.5 | 87.5 | 99.0 | 100 | ||||||
| 45912 | Day | 22.2 | 93.0 | 99.4 | 100 | |||||
| Night | 13.0 | 84.5 | 97.5 | 99.5 | 100 | |||||
| 45914 | Day | 31.9 | 100 | |||||||
| Night | 15.7 | 76.8 | 89.3 | 95.7 | 99.3 | 100 | ||||
| 132762 | Day | 21.2 | 90.6 | 98.8 | 100 | |||||
| Night | 9.96 | 80.1 | 93.1 | 99.6 | 100 | |||||
| 157954 | Day | 20.0 | 89.4 | 98.4 | 100 | |||||
| Night | 20.5 | 82.7 | 92.9 | 99.0 | 100 | |||||
| 157963 | Day | 15.8 | 69.6 | 76.5 | 84.2 | 91.5 | 97.2 | 100 | ||
| Night | 11.2 | 64.4 | 76.8 | 89.6 | 94.4 | 98.8 | 100 | |||
| 034331 | Day | 3.3 | 7.4 | 15.5 | 24.0 | 45.1 | 67.9 | 90.1 | 96.8 | 99.3 |
| Night | 1.6 | 6.2 | 20.9 | 37.6 | 56.2 | 77.9 | 90.3 | 97.7 | 99.5 | |
| 163106 | Day | 32.2 | 98.0 | 98.0 | 99.3 | 99.3 | 100 | |||
| Night | 4.4 | 99.5 | 100 | |||||||
| 157958 | Day | 34.4 | 100 | |||||||
| Night | 15.3 | 99.9 | 100 | |||||||
| Summary | Day | 20.9 | 81.9 | 87.1 | 88.5 | 90.8 | 93.8 | 97.7 | 99.6 | 100.0 |
| Night | 13.1 | 80.7 | 91.1 | 94.6 | 96.6 | 98.4 | 99.3 | 99.8 | 100 | |
| Total | 17.2 | 81.4 | 89.1 | 91.6 | 93.7 | 96.1 | 98.6 | 99.7 | 100 | |
| Electronic Device Type | Location | N | Length (cm) | Days-at-Liberty (Days) | Max Depth Range (m) | Temperature Range (°C) | Reference |
|---|---|---|---|---|---|---|---|
| PSAT | Northern East China Sea | 4 | 94–102 | 5–30 | 59–94 | 17.8–23.05 | This study |
| PSAT | Southeastern Taiwan | 4 | 99–113 | 7–30 | 40–89 | 21.4–30.1 | Lin et al. (2019) [26] |
| PSAT | Kagoshima Bay | 2 | 81–84 | 20–30 | 24–99 | 17.9–23.6 | Lin et al. (2019) [26] |
| PSAT | Western Central Atlantic | 6 | 97.5–120 | 4–30 | 74–255.5 | 16–30 | Merten et al. (2014) [25] |
| Archival tag | Southeastern Taiwan | 2 | 54–73 | 3–9 | 54.1–62.7 | 19.0–27.9 | Furukawa et al. (2015) [56] |
| Acceleration data-logger | Northern East China Sea | 8 | 67–90 | 3–47 * | 53.1–95.4 | 18.5–28.4 | Furukawa et al. (2011) [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-J.; Chiang, W.-C.; Musyl, M.K.; Wang, S.-P.; Su, N.-J.; Chang, Q.-X.; Ho, Y.-S.; Nakamura, I.; Tseng, C.-T.; Kawabe, R. Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea. Sustainability 2020, 12, 5793. https://doi.org/10.3390/su12145793
Lin S-J, Chiang W-C, Musyl MK, Wang S-P, Su N-J, Chang Q-X, Ho Y-S, Nakamura I, Tseng C-T, Kawabe R. Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea. Sustainability. 2020; 12(14):5793. https://doi.org/10.3390/su12145793
Chicago/Turabian StyleLin, Shian-Jhong, Wei-Chuan Chiang, Michael K. Musyl, Sheng-Ping Wang, Nan-Jay Su, Qi-Xuan Chang, Yuan-Shing Ho, Itsumi Nakamura, Chen-Te Tseng, and Ryo Kawabe. 2020. "Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea" Sustainability 12, no. 14: 5793. https://doi.org/10.3390/su12145793
APA StyleLin, S.-J., Chiang, W.-C., Musyl, M. K., Wang, S.-P., Su, N.-J., Chang, Q.-X., Ho, Y.-S., Nakamura, I., Tseng, C.-T., & Kawabe, R. (2020). Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea. Sustainability, 12(14), 5793. https://doi.org/10.3390/su12145793





