Response of Soil Microbial Communities to Warming and Clipping in Alpine Meadows in Northern Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis and Plotting
3. Results
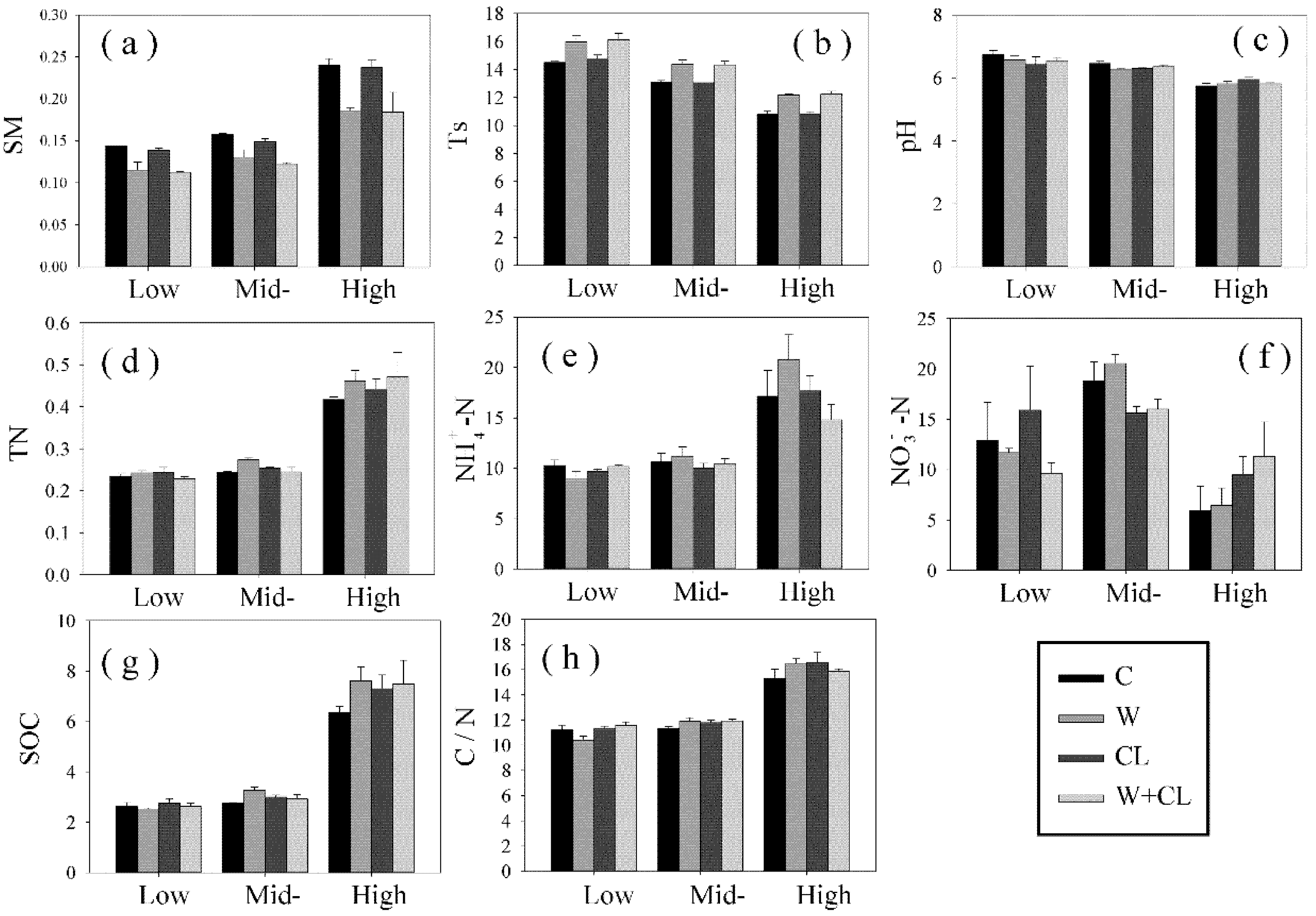
3.1. Impact of Warming and Clipping on Environmental Factors
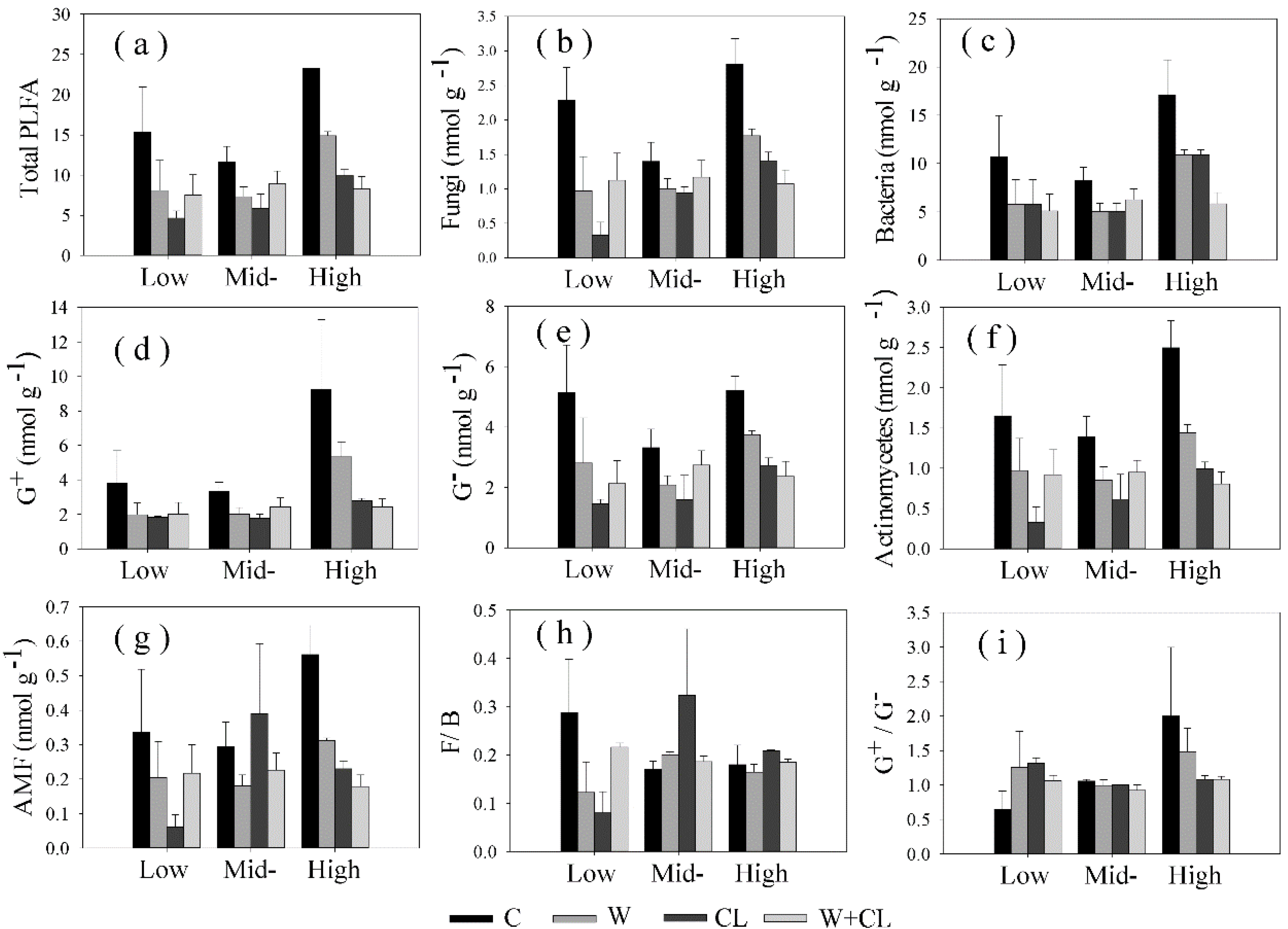
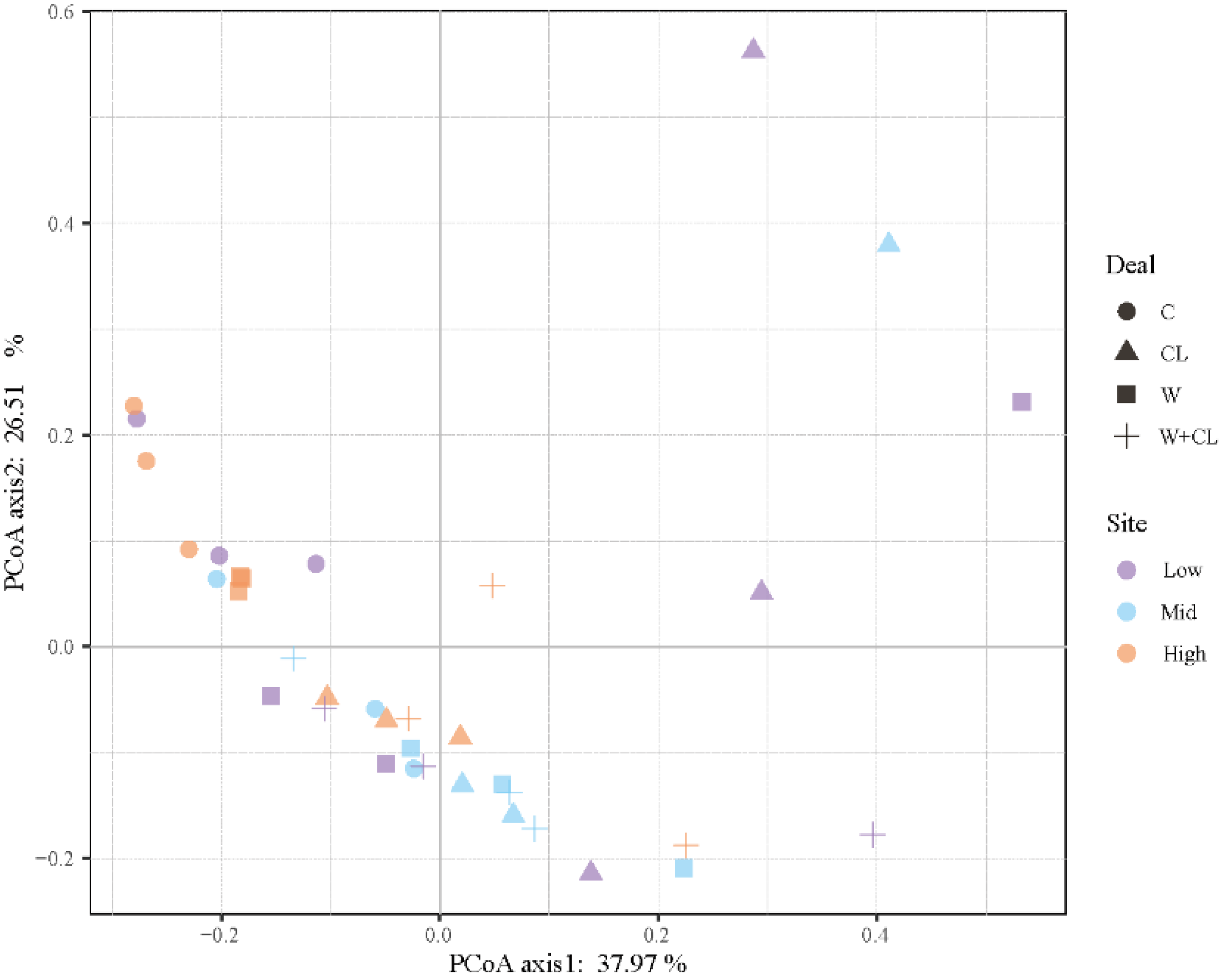
3.2. Effect of Warming and Clipping on Soil Microbial Biomass and Community Composition
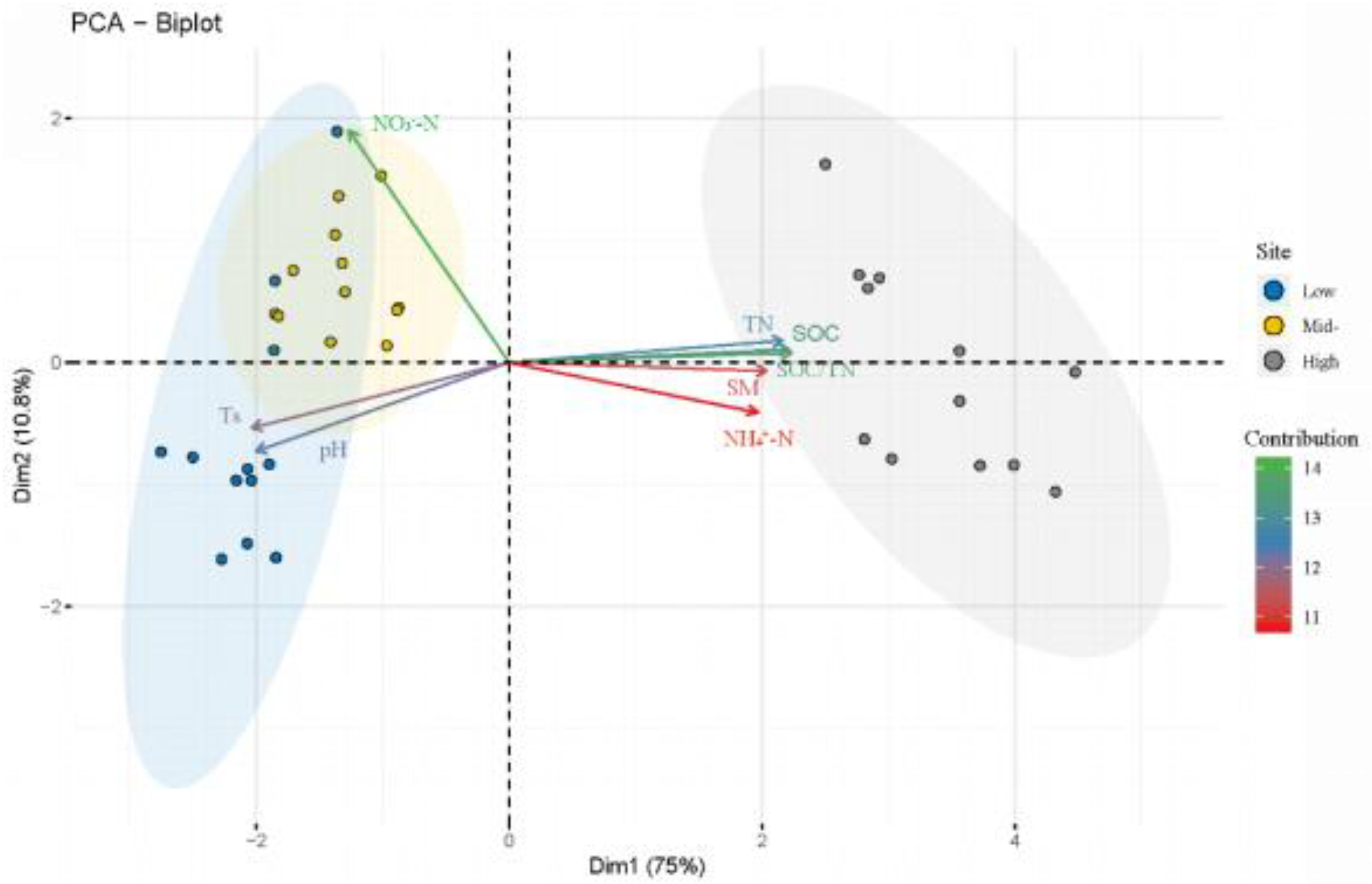
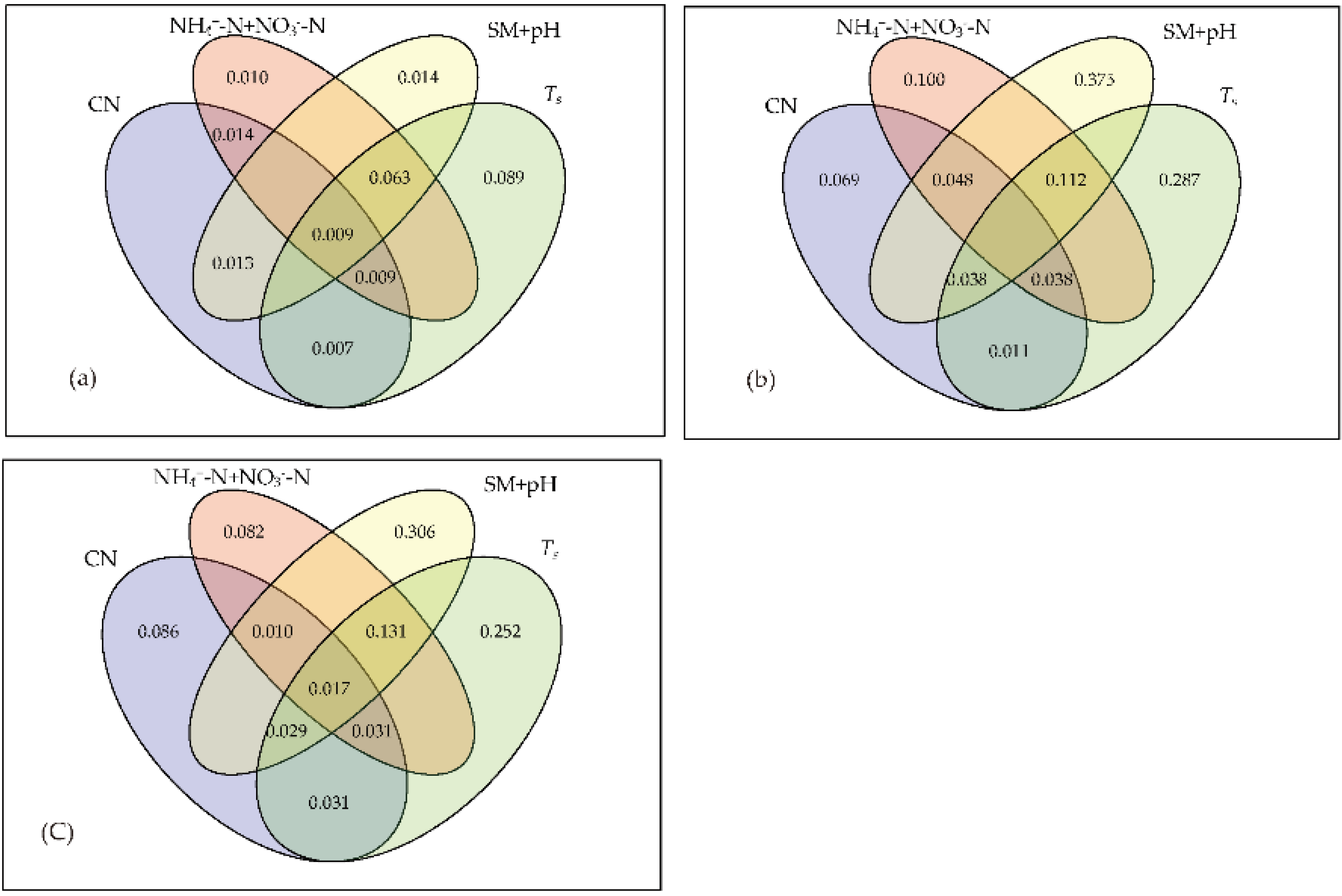
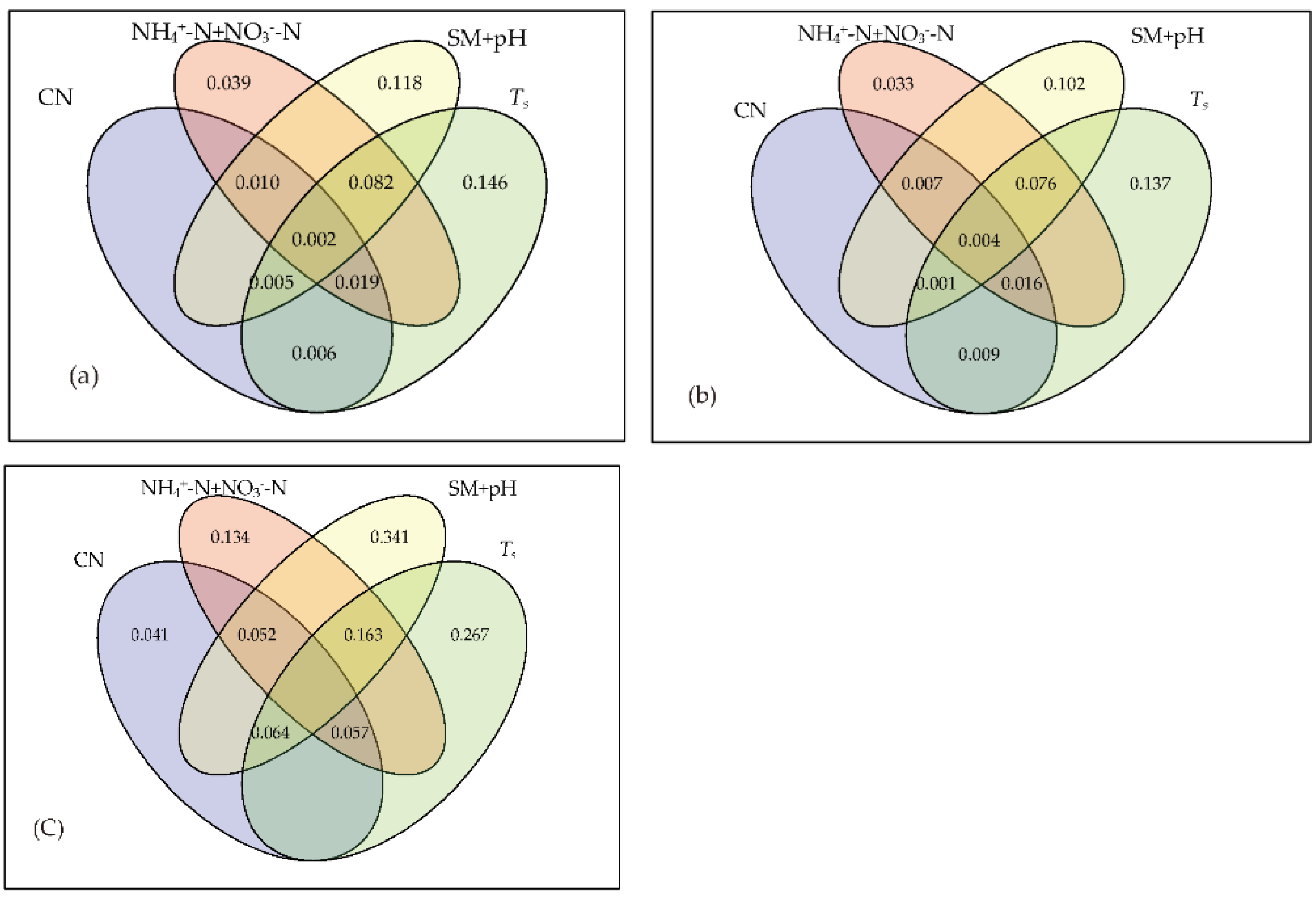
3.3. Relationship between Soil Microbial Community and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| PLFA | Fungi | Bacteria | G+ | G− | Actinomycetes | AMF | F/B | G+/G− | |
|---|---|---|---|---|---|---|---|---|---|
| TN | 0.33 | 0.24 | 0.33 | 0.39 | 0.23 | 0.20 | 0.18 | 0.04 | 0.24 |
| SOC | 0.25 | 0.22 | 0.25 | 0.33 | 0.14 | 0.11 | 0.10 | 0.13 | 0.25 |
| SOC/TN | 0.30 | 0.30 | 0.30 | 0.38 | 0.18 | 0.16 | 0.19 | 0.20 | 0.24 |
| NH4+-N | 0.62 ** | 0.48 | 0.63 ** | 0.70 *** | 0.51 * | 0.51 * | 0.36 | 0.01 | 0.19 |
| NO3−-N | −0.38 | −0.42 | −0.38 | −0.39 | −0.38 | −0.32 | −0.31 | −0.12 | −0.18 |
| pH | −0.29 | −0.20 | −0.30 | −0.38 | −0.17 | −0.17 | −0.23 | −0.05 | −0.32 |
| Ts | −0.40 | −0.37 | −0.40 | −0.48 | −0.31 | −0.27 | −0.30 | −0.09 | −0.24 |
| SM | 0.39 | 0.36 | 0.40 | 0.47 | 0.33 | 0.27 | 0.27 | 0.04 | 0.22 |
| Taxa | Treatment | Low Altitude | Middle Altitude | High Altitude | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | p | R2 | F | p | R2 | F | p | ||
| PLFA | W | 0.06 | 0.80 | 0.457 | 0.06 | 0.80 | 0.424 | 0.06 | 0.80 | 0.433 |
| CL | 0.20 | 2.72 | 0.097 | 0.20 | 2.72 | 0.100 | 0.20 | 2.72 | 0.096 | |
| W × CL | 0.17 | 2.35 | 0.129 | 0.17 | 2.35 | 0.118 | 0.17 | 2.35 | 0.117 | |
| G+ | W | 0.10 | 1.11 | 0.354 | 0.06 | 0.81 | 0.403 | 0.05 | 0.56 | 0.571 |
| CL | 0.08 | 0.86 | 0.423 | 0.11 | 1.65 | 0.216 | 0.21 | 2.45 | 0.02 * | |
| W × CL | 0.09 | 0.98 | 0.365 | 0.28 | 4.15 | 0.043 * | 0.04 | 0.49 | 0.629 | |
| G− | W | 0.09 | 1.33 | 0.272 | 0.01 | 0.17 | 0.798 | 0.19 | 7.19 | 0.021 * |
| CL | 0.21 | 3.15 | 0.068 | 0.06 | 0.77 | 0.433 | 0.57 | 22.03 | 0.001 *** | |
| W × CL | 0.16 | 2.35 | 0.132 | 0.35 | 4.92 | 0.039 * | 0.03 | 1.27 | 0.302 | |
| Actinomycetes | W | 0.00 | 0.03 | 0.947 | 0.02 | 0.29 | 0.599 | 0.25 | 15.97 | 0.005 ** |
| CL | 0.15 | 1.66 | 0.228 | 0.17 | 2.57 | 0.143 | 0.56 | 36.24 | 0.001 *** | |
| W × CL | 0.13 | 1.50 | 0.242 | 0.28 | 4.15 | 0.057 | 0.06 | 4.15 | 0.088 | |
| Bacteria | W | 0.08 | 1.09 | 0.369 | 0.03 | 0.41 | 0.601 | 0.06 | 0.79 | 0.49 |
| CL | 0.17 | 2.15 | 0.130 | 0.09 | 1.34 | 0.288 | 0.25 | 3.04 | 0.007 ** | |
| W × CL | 0.14 | 1.76 | 0.184 | 0.32 | 4.57 | 0.044 * | 0.04 | 0.54 | 0.655 | |
| Fungal | W | 0.04 | 0.99 | 0.364 | 0.04 | 0.46 | 0.739 | 0.23 | 6.64 | 0.006 ** |
| CL | 0.27 | 6.67 | 0.020 * | 0.07 | 0.80 | 0.446 | 0.47 | 13.83 | 0.001 *** | |
| W × CL | 0.36 | 8.92 | 0.010 ** | 0.14 | 1.56 | 0.217 | 0.03 | 0.92 | 0.391 | |
| TN | SOC | SOC/TN | NH4+-N | NO3−-N | pH | Ts | SM | |
|---|---|---|---|---|---|---|---|---|
| G+ | 0.14 * | 0.11 | 0.06 | 0.07 | 0.00 | 0.17 * | 0.16 | 0.14 |
| G− | −0.06 | −0.06 | 0.00 | 0.04 | 0.06 | 0.10 | 0.03 | 0.01 |
| Actinomycetes | 0.00 | −0.01 | −0.01 | 0.12 | 0.11* | 0.16 ** | 0.07 | 0.16 * |
| AMF | −0.01 | −0.01 | 0.00 | 0.17 * | 0.02 | 0.10 | 0.03 | 0.06 |
| Bacteria | 0.11 | 0.09 | 0.05 | 0.08 | 0.01 | 0.18 * | 0.16 * | 0.14 |
| Fungi | 0.00 | −0.01 | −0.03 | 0.12 | 0.13 * | 0.16 ** | 0.04 | 0.10 |
| F/B | −0.13 | −0.13 | −0.14 | −0.10 | −0.08 | 0.08 | −0.10 | −0.14 |
| G+/G− | 0.09 | 0.07 | 0.03 | 0.01 | −0.07 | 0.18 * | 0.12 | 0.06 |
References
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Martensson, L.M.; Olsson, P.A. Reductions in microbial biomass along disturbance gradients in a semi-natural grassland. Appl. Soil Ecol. 2012, 62, 8–13. [Google Scholar] [CrossRef]
- Zhang, W.; Parker, K.M.; Luo, Y.; Wan, S.; Wallace, L.L.; Hu, S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Chang. Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Wu, J.S.; Fu, G. Modelling aboveground biomass using MODIS FPAR/LAI data in alpine grasslands of the Northern Tibetan Plateau. Remote Sens. Lett. 2018, 9, 150–159. [Google Scholar] [CrossRef]
- Fu, G.; Wu, J.S. Validation of MODIS collection 6 FPAR/LAI in the alpine grassland of the Northern Tibetan Plateau. Remote Sens. Lett. 2017, 8, 831–838. [Google Scholar] [CrossRef]
- Luo, J.; Xu, D.-Y.; Ren, H.-Y. The desertification dynamics in Ordos from 2000 to 2010 and their relationship with climate change and human activities. J. Glaciol. Geocryol. 2013, 35, 48–56. [Google Scholar]
- Klein, J.A.; Harte, J.; Zhao, X.Q.J. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett. 2010, 7, 1170–1179. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Xu, M.; Zhu, J.; Chen, N.; Jiang, Y.; Huang, K.; Zu, J.; Liu, Y.; Yu, G. Water availability is more important than temperature in driving the carbon fluxes of an alpine meadow on the Tibetan Plateau. Agric. For. Meteorol. 2018, 256, 22–31. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, M.; Zhang, Y. Effect of short-term warming on plant community features of alpine meadow in Northern Tibet. Chin. J. Ecol. 2017, 36, 616–622. [Google Scholar]
- Hiltbrunner, D.; Schulze, S.; Hagedorn, F.; Schmidt, M.W.; Zimmmermann, S.J.G. Cattle trampling alters soil properties and changes soil microbial communities in a Swiss sub-alpine pasture. Geoderma 2012, 170, 369–377. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z. Grazing alters soil microbial community in alpine grasslands of Northern Tibet. Acta Prataculturae Sin. 2017, 26, 170–178. [Google Scholar]
- Weedon, J.T.; Kowalchuk, G.A.; Aerts, R.; van Hal, J.; van Logtestijn, R.; Tas, N.; Roling, W.F.M.; van Bodegom, P.M. Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob. Chang. Biol. 2012, 18, 138–150. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Li, F.; Peng, Y.; Yang, G.; Yu, J.; Zhou, G.; Yang, Y. Effects of short-term experimental warming on soil microbes in a typical alpine steppe. Chin. J. Plant Ecol. 2018, 42, 116–125. [Google Scholar]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Yukun, Z.O.U.; Jingni, Z.; Dianlin, Y.; Xiurong, C.; Tianrui, Z.; Jianning, Z.; Shuai, Z. Phospholipid fatty acid analysis of microbial community structure under different land use patterns in soil ecosystems of Leymus chinensis steppes. Acta Prataculturae Sin. 2011, 20, 27–33. [Google Scholar]
- Shuai, Z.; Jingni, Z.; Xin, L.A.I.; Dianlin, Y.; Jianning, Z.; Gang, L.I.; Yukun, Z.O.U. Analysis of microbial biomass C,N and soil microbial community structure of Stipa steppes using PLFA at grazing and fenced in inner Mongolia, China. J. Agro-Environ. Sci. 2011, 30, 1126–1134. [Google Scholar]
- Qiu, J. China: The third pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef]
- Feng, S.; Tang, M.C. The Qinghai-Tibet Plateau is the new evidence of China’s climate change initiation zone. Chin. Sci. Bull. 1998, 43, 633–636. [Google Scholar]
- Qiu, J. Double threat for Tibet. Nature 2014, 512, 240–241. [Google Scholar] [CrossRef]
- Yu, C.Q.; Han, F.S.; Fu, G. Effects of 7 years experimental warming on soil bacterial and fungal community structure in the Northern Tibet alpine meadow at three elevations. Sci. Total Environ. 2019, 655, 814–822. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, Y.J.; Zhang, X.Z.; Shi, P.L.; Zhou, Y.T.; Li, Y.L.; Shen, Z.X. Response of ecosystem respiration to experimental warming and clipping in Tibetan alpine meadow at three elevations. Biogeosci. Discuss. 2013, 10, 13015–13047. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.X. Environmental humidity regulates effects of experimental warming on vegetation index and biomass production in an alpine meadow of the Northern Tibet. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Xiong, J.B.; Sun, H.B.; Peng, F.; Zhang, H.Y.; Xue, X.; Gibbons, S.M.; Gilbert, J.A.; Chu, H.Y. Characterizing changes in soil bacterial community structure in response to short-term warming. FEMS Microbiol. Ecol. 2014, 89, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Bing-Ru, L.; Guo-Mei, J.; Jian, C.; Gang, W. A review of methods for studying microbial diversity in soils. Pedosphere 2006, 16, 18–24. [Google Scholar]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Fischer, J.; Schauer, F.; Heipieper, H.J. The trans/cis ratio of unsaturated fatty acids is not applicable as biomarker for environmental stress in case of long-term contaminated habitats. Appl. Microbiol. Biotechnol. 2010, 87, 365–371. [Google Scholar] [CrossRef]
- Ramsey, P.W.; Rillig, M.C.; Feris, K.P.; Holben, W.E.; Gannon, J.E. Choice of methods for soil microbial community analysis: PLFA maximizes power compared to CLPP and PCR-based approaches. Pedobiologia 2006, 50, 275–280. [Google Scholar] [CrossRef]
- Baath, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Kong, A.Y.Y.; Scow, K.M.; Cordova-Kreylos, A.L.; Holmes, W.E.; Six, J. Microbial community composition and carbon cycling within soil microenvironments of conventional, low-input, and organic cropping systems. Soil Biol. Biochem. 2011, 43, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, A.; Tunlid, A.; Baath, E. Phospholipid fatty-acid composition, biomass, and activity of microbial communities from 2 soil types experimentally exposed to different heavy-metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Vestal, J.R.; White, D.C. Lipid analysis in microbial ecology—Quantitative approaches to the study of microbial communities. Bioscience 1989, 39, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A.; Baath, E.; Jacobsen, I.; Soderstrom, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Elias, D.M.; Rowe, R.L.; Pereira, M.G.; Stott, A.W.; Barnes, C.J.; Bending, G.D.; McNamara, N.P. Functional differences in the microbial processing of recent assimilates under two contrasting perennial bioenergy plantations. Soil Biol. Biochem. 2017, 114, 248–262. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Jiang, X.; Han, S. Effects of warming on soil microbial community structure in Changbai Mountain Tundra. Acta Ecol. Sin. 2014, 34, 5706–5713. [Google Scholar]
- Ford, H.; Rousk, J.; Garbutt, A.; Jones, L.; Jones, D.L. Grazing effects on microbial community composition, growth and nutrient cycling in salt marsh and sand dune grasslands. Biol. Fertil. Soils 2013, 49, 89–98. [Google Scholar] [CrossRef]
- Wang, B.; Sun, G.; Luo, P.; Wang, Z.; Zhang, Y.; Wu, N.; Luo, G. Microbial communities of alpine meadow soil in the Eastern Qinghai-Tibetan Plateau subjected to experimental warming and grazing. Chin. J. Appl. Environ. Biol. 2011, 17, 151–157. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Booth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Chen, Y. pH to Uygur biology influence. J. Taiyuan Norm. Univ. 2009, 8, 121–124. [Google Scholar]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Ojeda, G.; Patricio, J.; Navajas, H.; Comellas, L.; Alcaniz, J.M.; Ortiz, O.; Marks, E.; Natal-da-Luz, T.; Sousa, J.P. Effects of nonylphenols on soil microbial activity and water retention. Appl. Soil Ecol. 2013, 64, 77–83. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Schrama, M.J.J.; Cordlandwehr, V.; Visser, E.J.W.; Elzenga, T.M.; de Vries, Y.; Bakker, J.P. Grassland cutting regimes affect soil properties, and consequently vegetation composition and belowground plant traits. Plant Soil 2013, 366, 401–413. [Google Scholar] [CrossRef]
- Shao, C.; Chen, J.; Li, L.; Zhang, L. Ecosystem responses to mowing manipulations in an arid Inner Mongolia steppe: An energy perspective. J. Arid Environ. 2012, 82, 1–10. [Google Scholar] [CrossRef]
- Sorensen, L.I.; Kytoviita, M.-M.; Olofsson, J.; Mikola, J. Soil feedback on plant growth in a sub-arctic grassland as a result of repeated defoliation. Soil Biol. Biochem. 2008, 40, 2891–2897. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.X. Clipping has stronger effects on plant production than does warming in three alpine meadow sites on the Northern Tibetan Plateau. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yan, Q.; Yang, S.; Van Nostrand, J.D.; Wang, Z.; He, Z.; Zhou, J.; Jiang, Y.; Deng, Y. Soil microbial beta-diversity is linked with compositional variation in aboveground plant biomass in a semi-arid grassland. Plant Soil 2018, 423, 465–480. [Google Scholar] [CrossRef]
- Yang, F.; Wu, J.; Zhang, D.; Chen, Q.; Zhang, Q.; Cheng, X. Soil bacterial community composition and diversity in relation to edaphic properties and plant traits in grasslands of southern China. Appl. Soil Ecol. 2018, 128, 43–53. [Google Scholar] [CrossRef]
- Xu, M.; Liu, M.; Xue, X.; Zhai, D.; Peng, F.; You, Q.; Liu, Y. Effects of warming and clipping on the growth of aboveground vegetation in an alpine meadow. Ecol. Environ. Sci. 2015, 24, 231–236. [Google Scholar]
- Ju, Z.; Ting, L. Review and prospects on methodology and affecting factors of soil microbial diversity. Biodivers. Sci. 2007, 15, 306–311. [Google Scholar] [CrossRef]
- Guo, A.; Zhou, R.; Song, Y.; Ma, H. Relationship between physiological protection mechanisms and the compensatory growth of Lolium perenne L. at different cutting treatment levels. Acta Ecol. Sin. 2018, 38, 3495–3503. [Google Scholar]
- Egan, G.; Zhou, X.; Wang, D.; Jia, Z.; Crawley, M.; Fornara, D.A. Long-term effects of grazing, liming and nutrient fertilization on the nitrifying community of grassland soils. Soil Biol. Biochem. 2018, 118, 97–102. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Song, Z.; Wang, J.; Guo, L. Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil Biol. Biochem. 2018, 124, 47–58. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Couper, J.; Parkinson, R.; Schon, N.L.; Stevenson, B.A. Effect of nitrogen fertilizer on nitrogen pools and soil communities under grazed pastures. New Zealand J. Agric. Res. 2012, 55, 217–233. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, H.; Li, S. A meta-analysis of the effects of warming and elevated CO2 on soil microbes. J. Resour. Ecol. 2019, 10, 69–76. [Google Scholar]
- Fu, G.; Shen, Z.; Zhang, X.; Zhou, Y.; Zhang, Y. Response of microbial biomass to grazing in an alpine meadow along an elevation gradient on the Tibetan Plateau. Eur. J. Soil Biol. 2012, 52, 27–29. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Shen, Z.X.; Fu, G. A meta-analysis of the effects of experimental warming on soil carbon and nitrogen dynamics on the Tibetan Plateau. Appl. Soil Ecol. 2015, 87, 32–38. [Google Scholar] [CrossRef]
- Fu, G.; Zhou, Y.; Shen, Z.; Zhang, X.; Shi, P.; He, Y.; Wu, J. Relationships between ecosystem respiration and environmental factors of alpine grazing meadows along an altitudinal gradient (4300~4700 m). Ecol. Environ. Sci. 2010, 19, 2789–2794. [Google Scholar]
- Hartley, I.P.; Heinemeyer, A.; Ineson, P. Effects of three years of soil warming and shading on the rate of soil respiration: Substrate availability and not thermal acclimation mediates observed response. Glob. Chang. Biol. 2007, 13, 1761–1770. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.; Zhang, X.; Zhou, Y. Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Appl. Soil Ecol. 2012, 61, 158–160. [Google Scholar] [CrossRef]

| Microbial Type | PLFA Biomarkers |
|---|---|
| Fungi | 16:1ω5c, 18:1ω9c, 18:2ω6c |
| Bacteria | 14:0, i14:0, 15:0, i15:0, a15:0, i16:0, a16:0, 10Me16:0, 16:1ω7c, 17:0, 10Me17:0, i17:0, a17:0, cy17:0ω7c, 17:1ω8c, 10Me18:0, 18:1ω7c and cy19:0ω7c |
| Gram-positive bacteria (G+) | i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 |
| Gram-negative bacteria (G−) | 16:1ω7c, cy17:0ω7c, 17:1ω8c, 18:1ω7c and cy19:0ω7c |
| Actinomycetes | 10Me16:0, 10Me17:0 and 10Me18:0 |
| Arbuscular mycorrhizal fungi (AMF) | 16:1ω5c |
| Others | 6:0, 18:0, 22:0, 24:0 |
| Variables | Low | Mid | High | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W | CL | W × CL | W | CL | W × CL | W | CL | W × CL | |
| TN | 0.11 | 0.1 | 2.27 | 2.94 | 2.36 | 9.61 * | 1.18 | 0.24 | 0.03 |
| SOC | 0.64 | 0.82 | 0.01 | 3.21 | 0.21 | 5.68 * | 1.31 | 0.42 | 0.74 |
| SOC/TN | 1.07 | 4.63 | 3.05 | 2.44 | 1.27 | 0.98 | 0.17 | 0.33 | 2.71 |
| NH4+-N | 0.64 | 0.33 | 2.83 | 0.42 | 0.72 | 0.01 | 0.02 | 0.91 | 1.29 |
| NO3−-N | 1.63 | 0.03 | 0.75 | 0.73 | 10.73 * | 0.33 | 0.21 | 2.95 | 0.07 |
| pH | 0.07 | 1.49 | 0.84 | 1.16 | 0.17 | 2.86 | 0.14 | 1.67 | 1.67 |
| Ts | 15.42 ** | 0.22 | 0.02 | 35.61 *** | 0.10 | 0.00 | 70.24 *** | 0.05 | 0.02 |
| SM | 30.46 *** | 0.67 | 0.07 | 27.66 *** | 2.51 | 0.01 | 16.29 ** | 0.02 | 0.00 |
| Taxa | Low | Mid | High | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W | CL | W × CL | W | CL | W × CL | W | CL | W × CL | |
| PLFA | 0.36 | 2.37 | 1.91 | 0.14 | 1.54 | 4.79 | 5.52 * | 21.67 ** | 2.46 |
| Fungi | 0.39 | 4.91 | 6.66 * | 0.17 | 0.46 | 2.32 | 9.30 * | 21.65 ** | 2.44 |
| Bacteria | 0.45 | 2.18 | 1.48 | 0.18 | 1.49 | 4.99 | 3.27 | 15.35 ** | 1.81 |
| G+ | 0.63 | 0.82 | 0.90 | 0.59 | 1.71 | 5.34 * | 1.06 | 5.05 | 0.73 |
| G− | 0.54 | 3.55 | 1.53 | 0.01 | 0.79 | 4.32 | 4.69 | 26.20 *** | 1.59 |
| Actinomycetes | 0.01 | 2.75 | 2.36 | 0.20 | 2.23 | 3.66 | 10.12 * | 29.66 *** | 4.92 |
| AMF | 0.01 | 1.31 | 1.58 | 1.55 | 0.40 | 0.05 | 10.01* | 24.03 ** | 4.32 |
| F/B | 0.05 | 0.74 | 5.07 | 0.59 | 1.02 | 1.45 | 0.79 | 1.23 | 0.03 |
| G+/G− | 0.37 | 0.63 | 2.10 | 0.15 | 3.23 | 0.40 | 0.24 | 1.57 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, S.; Zhang, G.; Fu, G. Response of Soil Microbial Communities to Warming and Clipping in Alpine Meadows in Northern Tibet. Sustainability 2020, 12, 5617. https://doi.org/10.3390/su12145617
Zhang H, Li S, Zhang G, Fu G. Response of Soil Microbial Communities to Warming and Clipping in Alpine Meadows in Northern Tibet. Sustainability. 2020; 12(14):5617. https://doi.org/10.3390/su12145617
Chicago/Turabian StyleZhang, Haorui, Shaowei Li, Guangyu Zhang, and Gang Fu. 2020. "Response of Soil Microbial Communities to Warming and Clipping in Alpine Meadows in Northern Tibet" Sustainability 12, no. 14: 5617. https://doi.org/10.3390/su12145617
APA StyleZhang, H., Li, S., Zhang, G., & Fu, G. (2020). Response of Soil Microbial Communities to Warming and Clipping in Alpine Meadows in Northern Tibet. Sustainability, 12(14), 5617. https://doi.org/10.3390/su12145617





