Investigating the Effect of Processing Parameters on the Products of Hydrothermal Carbonization of Corn Stover
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Carbonization of Corn Stover
2.2. Characterization of Raw Corn Stover and Products of HTC
2.3. Experimental Design, Response Surface Methodology Development and Hydrothermal Process Optimization
3. Results and Discussion
3.1. Characterization of Solid Fuel, Liquid and Gas Fraction from Corn Stover
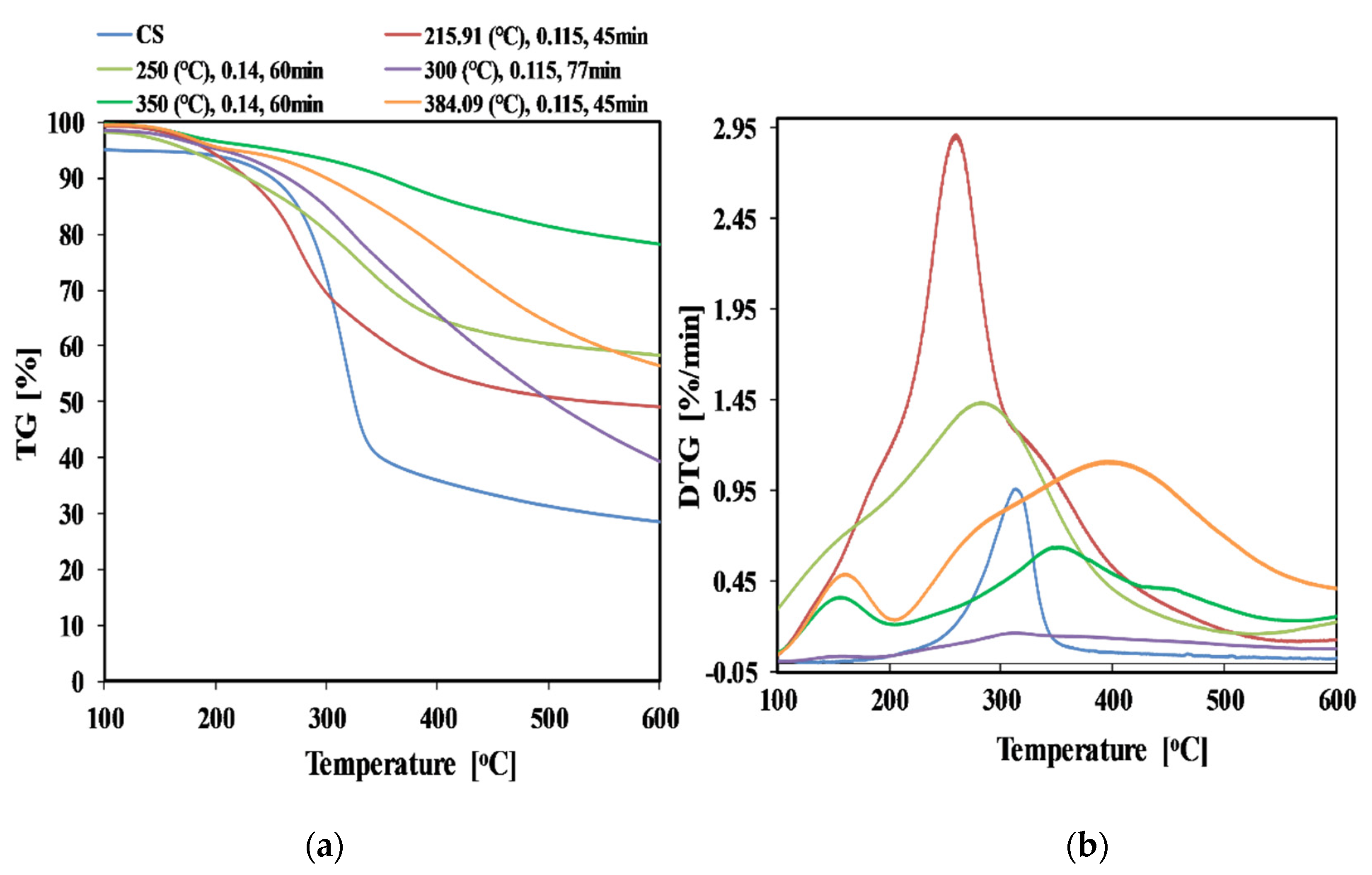
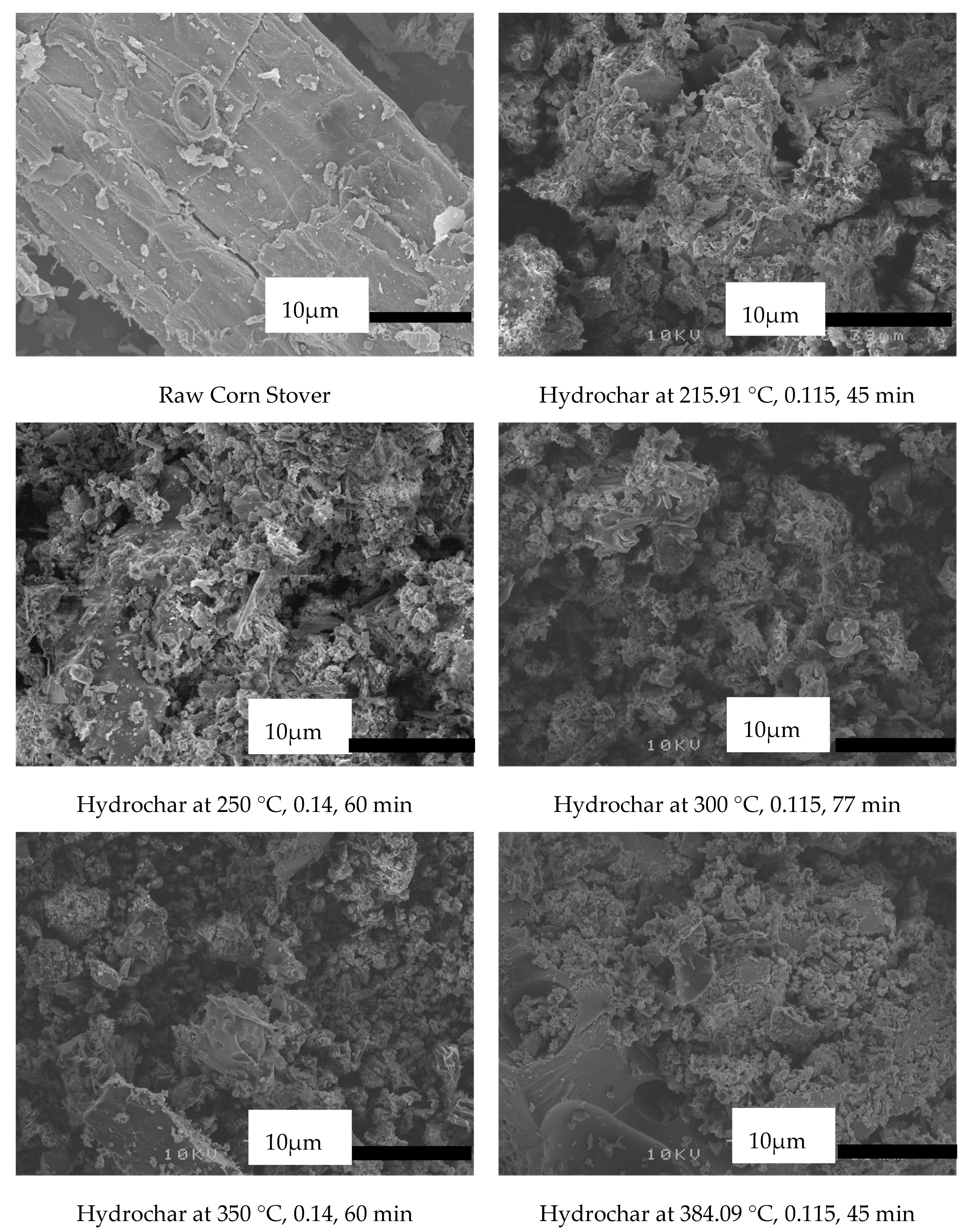
3.1.1. Solid Fuel
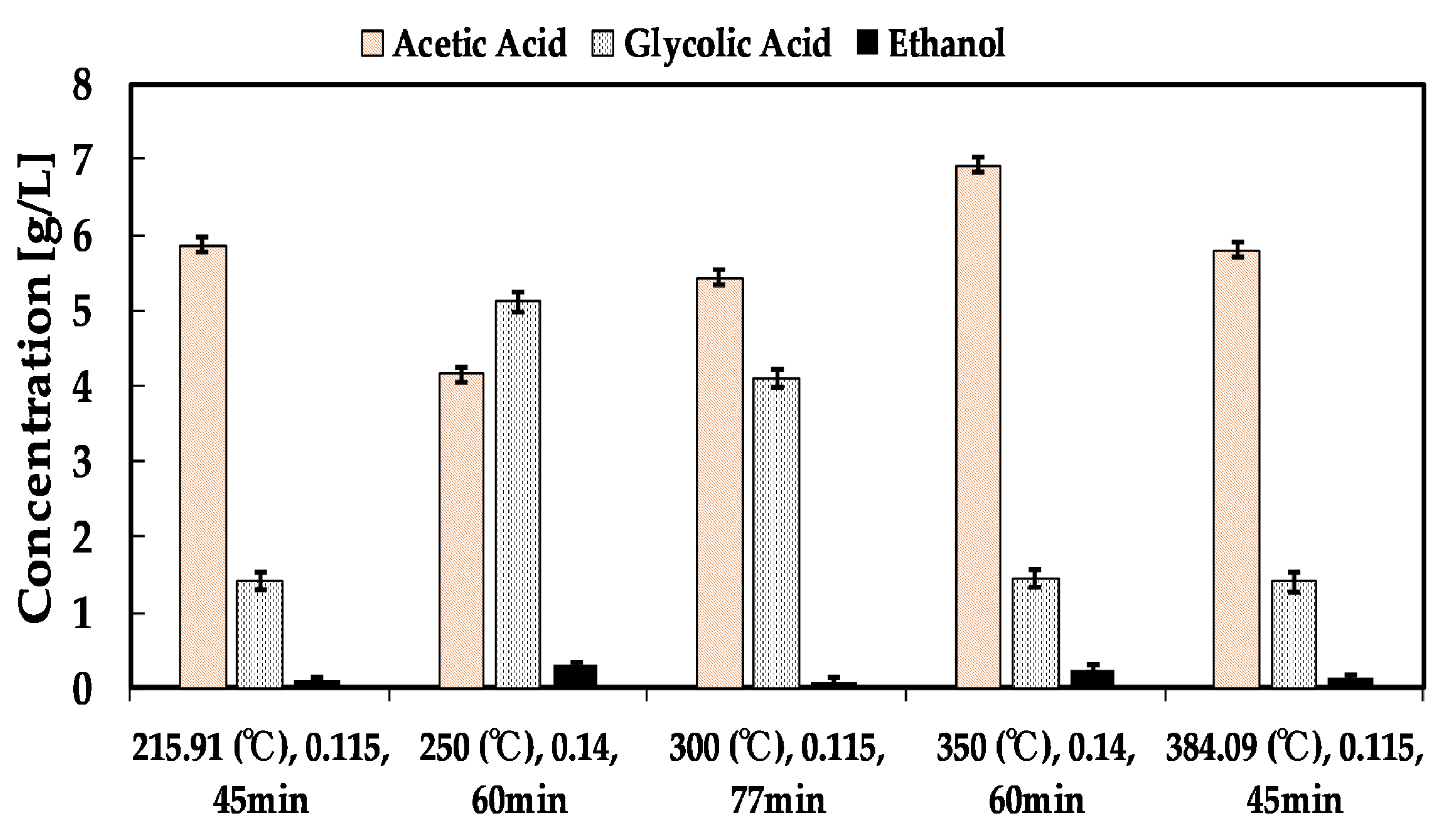
3.1.2. Liquid Fraction
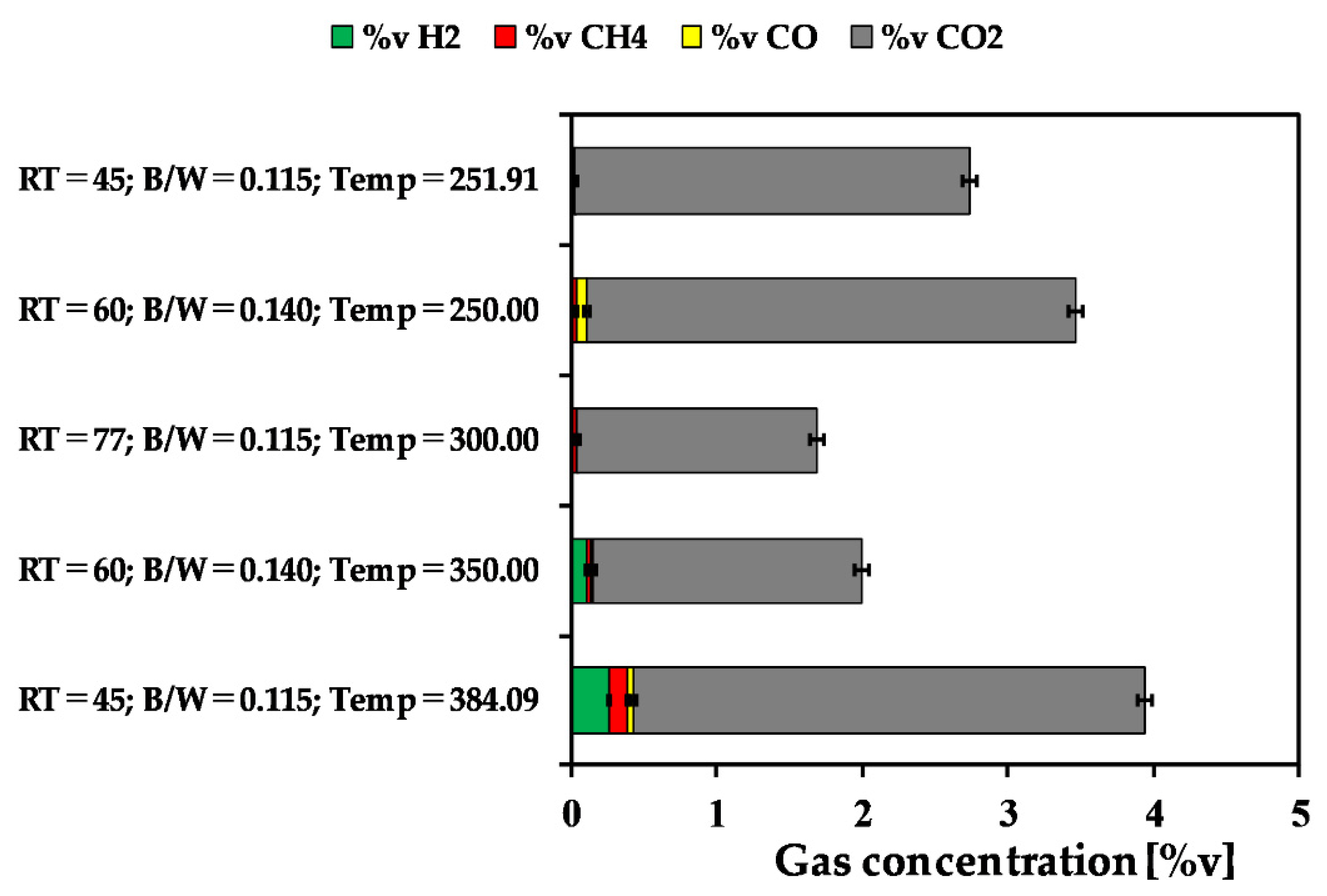
3.1.3. Gas Fraction
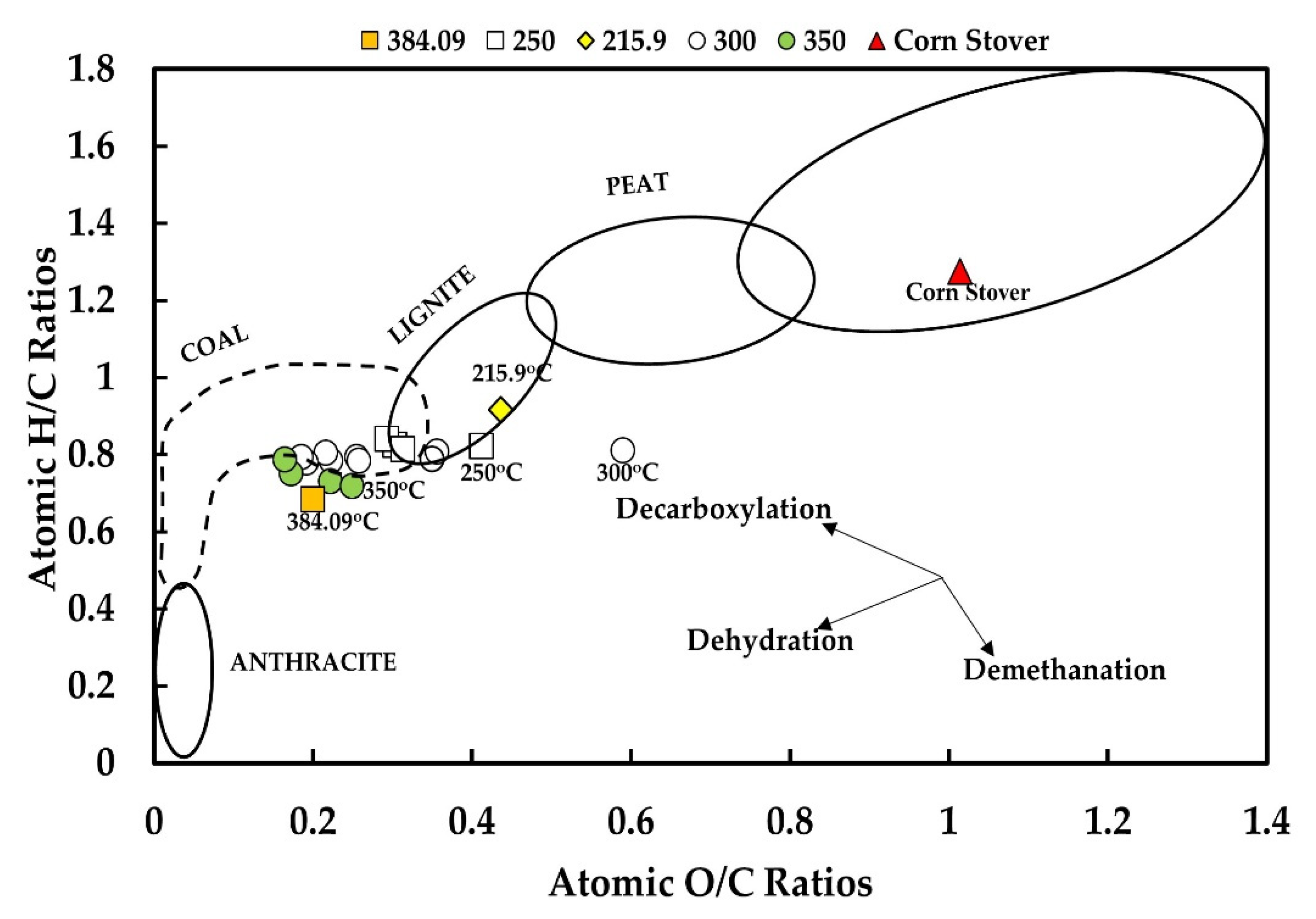
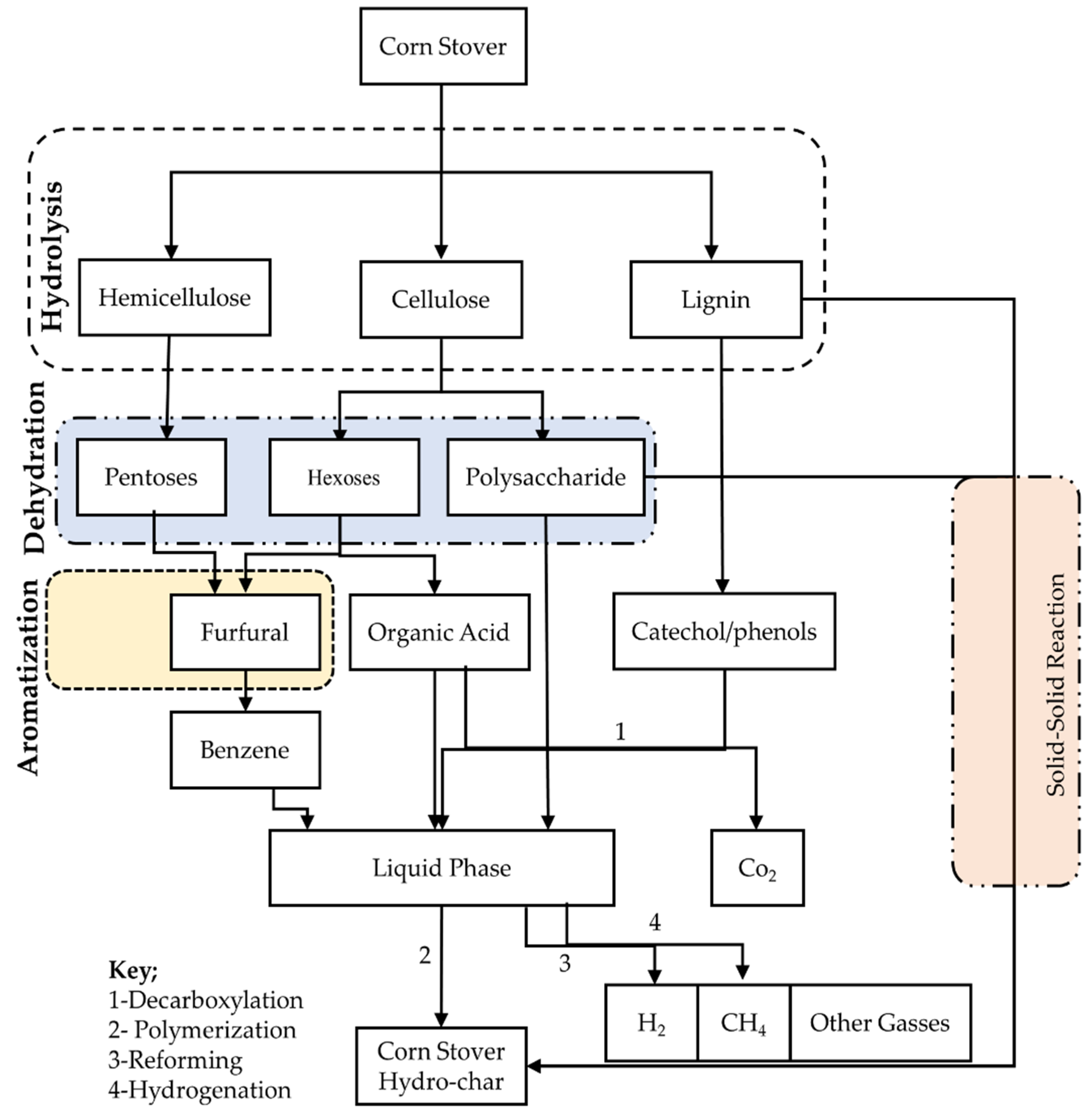
3.2. Mechanism of Hydrothermal Carbonization
3.3. Effect of Temperature, Residence Time, Biomass/Water Ratio on Corn Stover Hydrochar
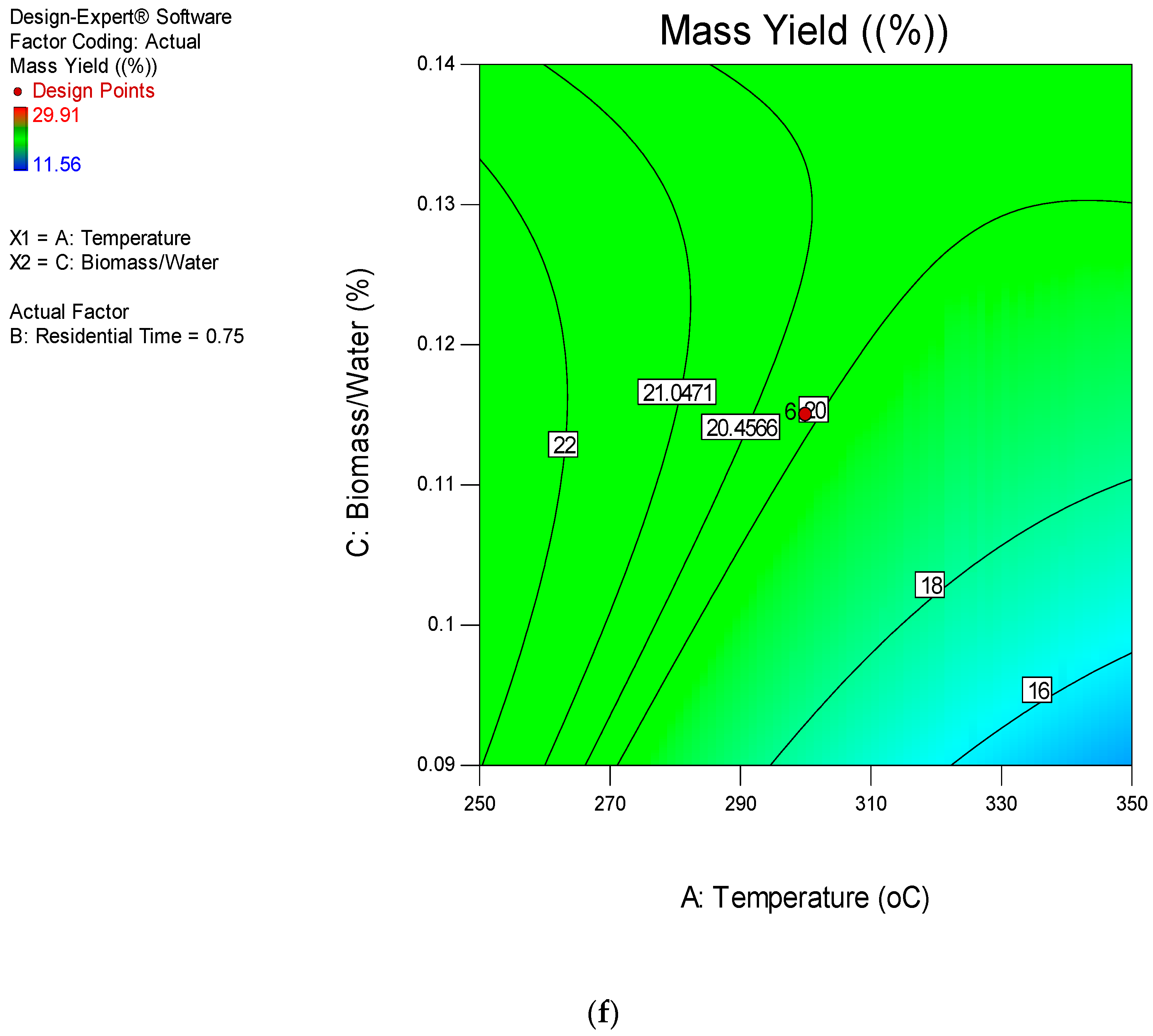
3.4. Hydrothermal Process Modelling and Optimization of Corn Stover Using RSM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Future Work
References
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment–Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Hastings, A.; Clifton-Brown, J.; Wattenbach, M.; Mitchell, C.P.; Stampfl, P.; Smith, P. Future energy potential of Miscanthusin Europe. GCB Bioenergy 2009, 1, 180–196. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Aliyu, M.; Abdullahi, N.A.; Alhaji, I.A. Production of bioenergy from rice-melon co-digested with cow dung as inoculant. Agri. Eng. Int. CIGR J. 2020, 22, 108–117. [Google Scholar]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Dutta, A.; Defersha, F. Biocarbon, biomethane and biofertilizer from corn residue: A hybrid thermo-chemical and biochemical approach. Energy 2018, 165, 370–384. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonization: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wikberg, H.; Ohra-aho, T.; Honkanen, M.; Kanerva, H.; Harlin, A.; Vippola, M.; Laine, C. Hydrothermal carbonization of pulp mill streams. Bioresour. Technol. 2016, 212, 236–244. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Gao, Y.; Xian-Hua, W.; Hai-Ping, Y.; Han-Ping, C. Characterization of products from hydrothermal treatments of cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Kang, S.; Ye, J.; Zhang, Y.; Chang, J. Preparation of biomass hydrochar derived sulfonated catalysts and their catalytic effects for 5-hydroxymethylfurfural production. RSC Adv. 2013, 3, 7360–7366. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.S.; Aliyu, M.; Dauda, S.M.; Balami, A.A.; Yunusa, B.K. Synthesis and optimization process of ethylene glycol-based biolubricant from palm kernel oil (PKO). JREE Spring 2018, 5, 2–8. [Google Scholar]
- Zhu, Z.; Zhidan, L.; Yuanhui, Z.; Baoming, L.; Haifeng, L.; Na, D.; Buchun, S.; Ruixia, S.; Jianwen, L. Recovery of reducing sugars and volatile fatty acids from cornstalk at different hydrothermal treatment severity. Bioresour. Technol. 2016, 199, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.T.; de Castro, D.A.R.; Santos, M.C.; Araújo, M.E.; Lüder, U.; Herklotz, L.; Werner, M.; Mumme, J.; Hoffmann, T. Process analysis of hydrothermal carbonization of corn Stover with Subcritical water. J. Supercrit. Fluids 2018, 136, 110–122. [Google Scholar] [CrossRef]
- Mosier, N.; Richard, H.; Nancy, H.; Miroslav, S.; Michael, R.L. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Camps, A.M.; Sevilla, M.; Maciá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macias, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonization of corn stover. Aust. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Xiao, L.; Zheng-Jun, S.; Feng, X.; Run-Cang, S. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 18, 619–623. [Google Scholar] [CrossRef]
- Volpe, M.; Jillian, L.G.; Luca, F. Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef]
- Kang, K.; Sonil, N.; Guotao, S.; Ling, Q.; Yongqing, G.; Tianle, Z.; Mingqiang, Z.; Runcang, S. Microwave-assisted hydrothermal carbonizatijon of corn stalk for solid biofuel production: Optimization of process parameters and characterization of hydrochar. Energy 2019, 186, 115–125. [Google Scholar] [CrossRef]
- Ushiyama, T.; Shimizu, N. Microencapsulation using spray-drying: The use of fine starch solution for the wall material. Food Sci. Technol. Res. 2018, 24, 653–659. [Google Scholar] [CrossRef]
- Shimizu, N.; Abea, A.; Ushiyama, T.; Toksoy Öner, E. Effect of temperature on the hydrolysis of levan treated with compressed hot water fluids. Food Sci. Nutr. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Athika, C.; Tau, L.Y.; Shigeru, M.; Yukihiko, M. Behavior of 5-HMF in Subcritical and Supercritical Water. Ind. Eng. Chem. Res. 2008, 47, 2956–2962. [Google Scholar]
- Chandra, S.T.; Jason, S.M. Hydrothermal liquefaction of separated dairy manure for production of bio-oils with simultaneous waste treatment. Bioresour. Technol. 2012, 107, 456–463. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Kamonwat, N.; Bunyarit, P.; Vorapot, K.; Nawin, V.; Wasawat, K.; Prasert, P. Characteristics of hydrochar and liquid fraction from hydrothermal carbonization of cassava rhizome. J. Energy Inst. 2018, 91, 184–193. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2015, 25, 1802–1810. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Microwave-assisted and conventional hydrothermal carbonization of lignocellulosic waste material: Comparison of the chemical and structural properties of the hydrochars. J. Anal. Appl. Pyrolysis 2016, 118, 1–8. [Google Scholar] [CrossRef]
- Cantero, D.A.; Martinez, C.; Bermejo, M.D.; Cocero, M.J. Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis. Green Chem. 2015, 17, 610–618. [Google Scholar] [CrossRef]
- Abdullah, R.; Ueda, K.; Saka, S. Hydrothermal decomposition of various crystalline celluloses as treated by semi-flow hot-compressed water. J. Wood Sci. 2014, 60, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Bioref. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrog. Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- De Vlieger, D.J.M.; Chakinala, A.G.; Lefferts, L.; Kersten, S.R.A.; Seshan, K.; Brilman, D.W.F. Hydrogen from ethylene glycol by supercritical water reforming using noble and base metal catalysts. Appl. Catal. B Environ. 2012, 112, 536–544. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent, S.; Hoekman, R.B. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- In-Gu, L.; Mi-Sun, K.; Son-Ki, I. Gasification of Glucose in Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 1182–1188. [Google Scholar]
- Kumar, S. Sub-and supercritical water technology for biofuels. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 147–183. [Google Scholar]
- Falco, C.N.B.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sato, T.; Smith, R.L.; Inomata, H.; Arai, K.; Kozinski, J.A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. [Google Scholar] [CrossRef]
- Tengfei, W.; Yunbo, Z.; Yun, Z.; Caiting Li Guangming, Z. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Fan, J.; De bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S. Direct microwave-assisted hydrothermal depolymerization of cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]



| Previous Work | HTC Parameters Using CED | HTC Parameters by RSM | Solid fuel Produced under SCW Conditions from Corn Stover |
|---|---|---|---|
| Zhu et al. [23] | Temperature (190–320 °C) and hydrothermal treatment severity (4.17–8.28 min) | NA | NA |
| Machado et al. [24] | Temperature (175–250 °C) | NA | NA |
| Mosier et al. [25] | Temperature (170–200 °C) and residence time (5–20 min) | NA | NA |
| Fuertes et al. [26] | Temperature (250 °C) | NA | NA |
| Xiao et al. [27] | Temperature (250 °C), | NA | NA |
| Volpe et al. [28] | Temperature (180–250 °C), residence time (0.5–3 h) and biomass/water ratio (0.07–0.30). | NA | NA |
| Kang et al [29] | NA | Temperature (122.7–257.3 °C), residence time (4.8–55.2 min) and biomass (0.98–6.02 g/50 mL H2O) | NA |
| This Study | NA | Temperature (215.91–384.09 °C), residence time (19.8–77 min) and Biomass/water ratio (0.073–0.157) | Solid fuel was produced at SCW conditions |
| Factors | Unit | Code Factor Level | ||||
|---|---|---|---|---|---|---|
| (-α) | (-1) | (0) | (+1) | (+α) | ||
| Temperature | °C | 215.91 | 250 | 300 | 350 | 384.09 |
| Residential Time | h | 0.33 | 0.5 | 0.75 | 1 | 1.17 |
| Biomass/water Ratio | % | 0.073 | 0.09 | 0.115 | 0.14 | 0.157 |
| Properties | Raw Corn Stover [This Study] | Hydrochars | ||||||
|---|---|---|---|---|---|---|---|---|
| 215.9 °C, 0.115, 45 min | 250 °C, 0.14, 60 min | 300 °C, 0.115, 77 min | 350 °C, 0.14 60 min | 384.09 °C, 0.115, 45 min | CS [29] | OC [28] | ||
| Proximate Analysis (SD ≤ 1.25) | ||||||||
| Volatile Matter (%) | 71.34 | 57.28 | 60.72 | 54.47 | 42.83 | 40 | 74.32 | 56.88 |
| Fixed Carbon (%) | 17.67 | 24.72 | 19.52 | 26.58 | 31.30 | 30.89 | 18 | 28.17 |
| Ash Content (%) | 11.05 | 18 | 19.75 | 18.95 | 25.87 | 29.11 | 3.54 | 14.95 |
| Ultimate Analysis (SD ≤ 1.09) | ||||||||
| Carbon (%) | 40.83 | 52.39 | 56.67 | 62.07 | 57.60 | 54.18 | 53.44 | 50.48 |
| Hydrogen (%) | 5.21 | 4.8 | 4.76 | 4.93 | 4.33 | 3.7 | 5.67 | 4.83 |
| Oxygen (%) | 41.38 | 22.86 | 16.62 | 11.52 | 9.95 | 10.85 | 39.64 | 28.94 |
| Nitrogen (%) | 1.54 | 1.95 | 2.2 | 2.53 | 2.25 | 2.16 | 1.12 | 0.81 |
| O/C | 1.01 | 0.44 | 0.29 | 0.19 | 0.17 | 0.2 | 0.74 | 0.57 |
| H/C | 1.28 | 0.92 | 0.84 | 0.79 | 0.75 | 0.68 | 0.106 | 0.095 |
| Energy Properties (SD ≤ 1.67) | ||||||||
| HHV (MJ/kg) | 16.16 | 22.30 | 24.20 | 27.47 | 25.37 | 23.75 | 22.82 | 22.39 |
| HHV (MJ/kg) a | 14.72 | 20.86 | 23.20 | 26.03 | 23.93 | 21.75 | ||
| Energy Yield (%) | 1 | 42.38 | 22.88 | 40.90 | 32.97 | 29.88 | 55.70 | 83 |
| Source | Sum of Squares | df | Mean Square | F-Value | P-Value | |
|---|---|---|---|---|---|---|
| Model | 101.72 | 9 | 11.30 | 3.32 | 0.02 | significant |
| X1 -Temperature | 5.63 | 1 | 5.63 | 1.65 | 0.03 | |
| X2 -Residential Time | 54.19 | 1 | 54.19 | 15.91 | 0.00 | |
| X3 -Biomass/Water | 0.65 | 1 | 0.65 | 0.19 | 0.02 | |
| X1X2 | 0.49 | 1 | 0.49 | 0.14 | 0.01 | |
| X1X3 | 1.08 | 1 | 1.08 | 0.32 | 0.05 | |
| X2X3 | 0.10 | 1 | 0.10 | 0.03 | 0.02 | |
| X12 | 21.91 | 1 | 21.91 | 6.43 | 0.03 | |
| X22 | 18.05 | 1 | 18.05 | 5.30 | 0.01 | |
| X32 | 6.66 | 1 | 6.66 | 1.96 | 0.03 | |
| Residual | 34.05 | 10 | 3.41 | |||
| Lack of Fit | 27.86 | 5 | 5.57 | 4.50 | 0.06 | Not significant |
| Pure Error | 6.19 | 5 | 1.24 | |||
| Cor Total | 135.78 | 19 | ||||
| Std Dev. | 1.85 | R2 | 0.85 | |||
| Mean | 19.15 | AdJ-R2 | 0.80 | |||
| C.V. % | 9.63 | Pred-R2 | 0.70 | |||
| PRESS | 231.49 | Adeq-Precision | 5.21 | |||
| -2Log Likelihood | 67.40 | BIC | 97.36 | |||
| AICc | 111.85 |
| Run | Temperature | Residence Time (h) | Biomass/Water Ratio | Final Pressure (Mpa) | HHV (MJ/kg) | Energy Yield (%) | Mass Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 300 | 0.75 | 0.157 | 14.95 | 20.73 | 28.48 | 20.23 |
| 2 | 300 | 0.75 | 0.115 | 15.95 | 25.85 | 31.45 | 17.91 |
| 3 | 300 | 0.75 | 0.115 | 14.99 | 23.70 | 31.26 | 19.42 |
| 4 | 350 | 1 | 0.09 | 22.45 | 21.95 | 19.21 | 12.89 |
| 5 | 300 | 0.75 | 0.115 | 15.75 | 24.49 | 41.46 | 24.93 |
| 6 | 300 | 0.75 | 0.073 | 12.25 | 23.23 | 31.03 | 19.66 |
| 7 | 384.09 | 0.75 | 0.115 | 26 | 21.75 | 29.88 | 20.23 |
| 8 | 300 | 0.33 | 0.115 | 15.95 | 16.37 | 28.36 | 25.51 |
| 9 | 250 | 0.5 | 0.09 | 10.35 | 19.85 | 31.19 | 23.13 |
| 10 | 250 | 1 | 0.14 | 10.50 | 23.08 | 30.16 | 19.24 |
| 11 | 300 | 0.75 | 0.115 | 15.75 | 24.05 | 27.94 | 17.10 |
| 12 | 215.91 | 0.75 | 0.115 | 8.85 | 20.86 | 42.38 | 29.91 |
| 13 | 300 | 0.75 | 0.115 | 15.25 | 24.19 | 24.67 | 15.01 |
| 14 | 300 | 0.75 | 0.115 | 16 | 23.91 | 40.76 | 25.10 |
| 15 | 250 | 1 | 0.09 | 10.40 | 23.20 | 22.88 | 14.52 |
| 16 | 300 | 1.170 | 0.115 | 16 | 26.03 | 40.90 | 23.13 |
| 17 | 350 | 1 | 0.14 | 23.2 | 23.93 | 32.97 | 20.29 |
| 18 | 250 | 0.5 | 0.14 | 20 | 19.82 | 26.40 | 19.61 |
| 19 | 250 | 0.5 | 0.14 | 10.50 | 21.44 | 29.68 | 20.38 |
| 20 | 350 | 0.5 | 0.09 | 23 | 18.01 | 14.14 | 11.56 |
| Raw Samples | 14.72 | ||||||
| HHV (Optimum) | 305 | 1 | 0.14 | 16.34 | 25.42 | - | - |
| HHV (Validated) | 305 | 1 | 0.14 | 16.34 | 24.45 | - | - |
| Standard deviation (%) | 0.016 | 0.017 | 0.018 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, I.S.; Na, R.; Kushima, K.; Shimizu, N. Investigating the Effect of Processing Parameters on the Products of Hydrothermal Carbonization of Corn Stover. Sustainability 2020, 12, 5100. https://doi.org/10.3390/su12125100
Mohammed IS, Na R, Kushima K, Shimizu N. Investigating the Effect of Processing Parameters on the Products of Hydrothermal Carbonization of Corn Stover. Sustainability. 2020; 12(12):5100. https://doi.org/10.3390/su12125100
Chicago/Turabian StyleMohammed, Ibrahim Shaba, Risu Na, Keisuke Kushima, and Naoto Shimizu. 2020. "Investigating the Effect of Processing Parameters on the Products of Hydrothermal Carbonization of Corn Stover" Sustainability 12, no. 12: 5100. https://doi.org/10.3390/su12125100
APA StyleMohammed, I. S., Na, R., Kushima, K., & Shimizu, N. (2020). Investigating the Effect of Processing Parameters on the Products of Hydrothermal Carbonization of Corn Stover. Sustainability, 12(12), 5100. https://doi.org/10.3390/su12125100





